Abstract
Context: Crataegus songarica K. Koch (Rosaceae) has been used in folk medicine to treat various diseases.
Objective: This study evaluates the effect of C. songarica methanol extract on the kidney and heart tissue damage of albino rats, and to determine cytotoxic activity of various extracts of songarica on various human cancer cell lines.
Materials and methods: Rats were divided into six groups, Group I received water only; Group II received CCl4 (1 mL/kg b wt) intraperitoneal; C. songarica extract (at doses of 100, 200 and 300 mg/kg b wt) orally for 15 days. Cytotoxic activity was determined by SRB method using MCF-7, HeLa, HepG2, SF-295, SW480 and IMR-32 cell lines.
Results: Compared with CCl4 group, administration of C. songarica extract at the dose of 300 mg/kg b wt, significantly decreases serum creatinine (59.74%), urea (40.23%) and cholesterol (54 mg/dL), MDA (0.007 nmol/mg protein) in kidney and (0.025 nmol/mg protein) in heart tissue, along with evaluation of GSH (209.79 ± 54.6), GR (111.45 ± 2.84), GPx (94.01 ± 14.80), GST (201.71) in kidney tissue and GSH (51.47 ± 1.47), GR (45.42 ± 6.69), GPx (77.19 ± 10.94), GST (49.89) in heart tissue. In addition, methanol, ethanol and ethyl acetate extracts exhibited potent anticancer activity on six cancer cell lines with IC50 values ranging from 28.57 to 85.106 µg/mL.
Discussion and conclusion: Crataegus songarica methanol extract has a potential antioxidant effect as it protects the kidney and heart tissue against CCl4-induced toxicity, prevents DNA damage and showed strong anticancer activity.
Introduction
Reactive oxygen species (ROS), such as hydrogen peroxides (H2O2), hydroxyl radicals (OH√), and superoxide anions () are commonly generated as a consequence of aerobic metabolism in mitochondria. Animal tissues have to constantly cope with these highly reactive oxygen species (ROS) and they do so with the help of antioxidants present in their tissues (Cabre et al., Citation2000; Castillo et al., Citation1992; Hartley et al., Citation1999; Melin et al., Citation2000). The generation of small quantities of free radicals appears to have an important biological function. Oxidative stress occurs when there is an imbalance between reactive oxygen (ROS) formation and scavenging by antioxidants (Lequeux et al., Citation2010; Sarkar et al., Citation2006). The overproduction of harmful ROS can cause severe damage to membrane lipids, protein synthesis and DNA (Abraham et al., Citation1999). Many investigators have reported that the industrial solvent, carbon tetrachloride (CCl4) is a potent environmental hepatotoxin (Guven et al., Citation2003; Szymonik-Lesiuk et al., Citation2003). Nevertheless, the CCl4-induced damaged is not confined to liver only as suggested by various studies, CCl4 also causes disorders in kidneys, lungs, testis and brain as well as in blood by generating free radicals (Ahmad et al., Citation1987; Charbonneau et al., Citation1986; Ozturk et al., Citation2003). Reports from Perez et al. (Citation1987), Ogeturk et al. (Citation2005a,Citationb), and Churchill et al. (Citation1983) suggest that acute and chronic renal injuries occur due to the exposure of CCl4. Once inside biological system, Cytochrome P-450 isozyme leads to bioactivation of CCl4 to trichloromethyl free radical √CCl3 and chlorine radical √Cl (Raucy et al., Citation1993). The trichloromethyl radical reacts with oxygen to form the highly toxic, reactive oxygen species (ROS), trichloromethyl peroxy radical which in turn, initiate lipid peroxidation process. Lipid peroxidation is believed to be one of the major causes of cell membrane damage. It has been found that treatment with antioxidants, such as vitamins C and E can ameliorate the toxic effects of CCl4 on liver and kidneys and other tissues as well (Halim et al., Citation1997). Many herbs and medicinal plants in India are rich in natural sources of antioxidants. Studies have shown that the antioxidant activity of plants might be due to their phenolic compounds (Cook & Samman, Citation1996) and the extracts from these herbs and medicinal plants could protect organs against CCl4-induced oxidative stress by altering the levels of increased lipid peroxidation, and enhancing the decreased activities of antioxidant enzymes, like superoxide dismutase (SOD), catalase (CAT) and glutathione-S-transferase (GST), etc. (Ko et al., Citation1995). Our Kashmir valley is well known for a plethora of medicinal plants, which act as antiradicals and DNA cleavage protectors. Crataegus songarica K. Koch (Rosaceae) commonly called hawthorn, or thorn apple, is a large genus of shrubs and trees, native to temperate regions of the North Hemisphere in Europe, Asia and North America. Crataegus songarica is used orally for cardiovascular conditions, such as congestive heart failure, coronary circulation problems and arrhythmias. It is also used to increase cardiac output reduced by hypertension or pulmonary disease, to support hypotension and hypertension, atherosclerosis, hyperlipidemia and Buerger’s disease. Crataegus songarica is also used as a sedative, antispasmodic, astringent and diuretic. The fruits are eaten to keep the heart healthy. The fruits are also eaten against constipation, for asthma, cardio tonic, cough, epilepsy, hemorrhage and hypertensiveness. The plant is also used for gastrointestinal conditions, such as indigestion, enteritis, epigastric distension, diarrhea and abdominal pain. Crataegus songarica fruit may also be effective orally for tapeworm infections, acute bacillary dysentery and amenorrhea. Crataegus songarica fruit liquid preparations are used as washes for sores, itching and frostbite.
No work has been done on this plant to determine its anti-CCl4 mediated kidney and heart damages in experimental animals. Therefore, the current study was undertaken to evaluate the protective effect of C. songarica methanolic extract on CCl4-induced kidney and heart toxicity in comparison with known antioxidant agents.
Materials and methods
Plant material collection and extraction
The fresh leaves of C. songarica was collected from Vanaspati nursery of Ganderbal Srinagar, J&K, India in the month of September and October 2012, identified by the Centre of Plant Taxonomy, Department of Botany, University of Kashmir, and authenticated by Dr Irshad Ahmad Nawchoo (Department of Botany) and Akhter Hussain Malik (Curator, Centre for Plant Taxonomy, University of Kashmir). A reference specimen has been retained in the herbarium of the Department of Botany at the University of Kashmir under reference number KASH-bot/Ku/CS-702-SAG.
The leaves were shade dried at 30 ± 2 °C. The dried material was ground into a powder using mortar and pestle and passed through a sieve of 0.3 mm mesh size. The powder obtained was extracted with methanol for 48 h using a Soxhlet extractor (60–80 °C). The extract was then concentrated with the help of rotary evaporator under reduced pressure and the solid extract was stored in refrigerator for further use.
Animals
All experiments were performed in compliance with the Indian legislation on the use and care of laboratory animals and were approved by the Institutional Animal Ethics Committee (Reg. No 801/03/CA/CPCSEA/2012), University of Kashmir. Male Wistar rats (200 ± 20 g) were grouped (six rats per cage) with free access to food and water, and kept in a regulated environment 25 ± 1 °C under 12/12 h (light/dark) cycle. After 1 week of acclimation to the laboratory, rats were selected randomly into six groups (six animals in each group). The extract was suspended in normal saline at a dose level of 100, 200 and 300 mg/kg body weight. The final volume of extract at each dose was 1 mL which was fed to rats by gavage. Group I: Received water only (Normal control); Group II: Received CCl4 (1 mL/kg b wt); Group III: Were administered with plant extract (100 mg/kg b wt) and CCl4; Group IV: Received CCl4 and plant extract (200 mg/kg b wt); Group V: Received 1 mL of methanolic extract of C. songarica at the concentration of (300 mg/kg b wt) along with CCl4; Group VI: Were administered with vitamin E (50 mg/kg body weight). The experiment continued for a period of 3 weeks. The animals of all groups were sacrificed at the end of the experiment and groups II–VI were administered with CCl4, 48 h prior to sacrifice. Liver kidney and heart tissue was collected and post-mitochondrial supernatant was prepared.
Preparation of tissue and biochemical assays
The tissue like kidney and heart were quickly removed, weighed and homogenized in ice-cold 50 mM phosphate buffer, pH 7. After centrifugation of the homogenates (3000 rpm, 15 min), pellet was discarded and the supernatant was further centrifuged at 10 000 rpm for 20 min, the remaining supernatant was used for biochemical assays.
Measurement of kidney function markers
Serum creatinine, urea and cholesterol levels were evaluated calorimetrically by using commercial available kits.
Estimation of lipid peroxidation
Lipid peroxidation in tissue homogenate was estimated by the formation of thiobarbituric acid reactive substances (TBARS) using the method of Nichans and Samuelson (Citation1968). In brief, 0.1 mL of tissue homogenate (PMS; Tris-HCl buffer, pH 7.5) was treated with 2 mL of (1:1:1 ratio) TBA-TCA-HCl reagent (0.37% thiobarbituric acid, 0.25 mol/L HCl and 15% TCA), placed in boiling water bath for 15 min, cooled and was centrifuged at room temperature for 10 min. The absorbance of the clear supernatant was measured against reference blank at 535 nm.
Determination of total sulphydryl groups
The acid soluble sulphydryl groups (non-protein thiols of which more than 93% was reduced glutathione, GSH) formed a yellow-colored complex with DTNB that showed the absorption maximum at 412 nm. The assay procedure followed was that of Moren et al. (Citation1985). Homogenate (500 µL) precipitated with 100 µL of 25% TCA, was subjected to centrifugation at 3000 rpm for 10 min to settle the precipitate. 100 µL of the supernatant was taken in a test tube containing the 2 mL of 0.6 mmol/L DTNB and 0.9 mL of 0.2 mmol/L sodium phosphate buffer (pH 7.4). The yellow color obtained was measured at 412 nm against the reagent blank which contained 100 µL of 25% TCA in place of the supernatant. Sulphydryl content was calculated using the DTNB molar extension coefficient of 13100.
Glutathione peroxidase
Glutathione peroxidase (GPx) activity was assayed using the method of Sharma et al. (Citation2001). The assay mixture consisted of 1.49 mL of sodium phosphate buffer (0.1 mol/L pH 7.4), 0.1 mL EDTA (1 mmol/L), 0.1 mL sodium azide (1 mmol/L), 0.1 mL GSH (1 mmol/L), 0.1 mL of NADPH (0.02 mmol/L), 0.01 mL of 1 mmol/L H2O2 and 0.1 mL PMS in a total volume of 2 mL. Oxidation of NADPH was recorded spectrophotometrically at 340 nm and the enzyme activity was calculated as nmoles NADPH oxidized/min/mg of protein, using extinction coefficient of 6.22 × 103 L mol−1 cm−1.
Glutathione reductase activity
Glutathione reductase (GR) activity was assayed by the method of Sharma et al. (Citation2001). The assay mixture consisted of 1.6 mL of sodium phosphate buffer (0.1 mol/L pH 7.4), 0.1 mL EDTA (1 mmol/L), 0.1 mL (1 mmol/L) oxidized glutathione, 0.1 mL of NADPH (0.02 mmol/L), 0.01 mL of 1 mmol/L H2O2 and 0.1 mL PMS in a total volume of 2 mL. The enzyme activity measured as absorbance at 340 nm was calculated as nmoles of NADPH oxidized/min/mg of protein using extinction coefficient of 6.22 × 103 L mol−1 cm−1.
Glutathione-S-transferase activity
Glutathione-S-transferase (GST) activity was assayed using the method of Haque et al. (Citation2003). The reaction mixture consisted of 1.67 mL sodium phosphate buffer (0.1 mol/L pH 6.5), 0.2 mL of 1 mmol/L GSH, 0.025 mL of 1 mmol/L CDNB and 0.1 mL of post-mitochondrial supernatant in a total volume of 2 mL. The change in absorbance was recorded at 340 nm and the enzyme activity was calculated as nmoles of CDNB conjugates formed/min/mg protein using extinction coefficient of 9.6 × 103 L mol−1 cm−1.
DNA ladder assay
DNA was isolated by using the methods of Wu et al. (Citation2005) to estimate DNA damages. 5 μg DNA of rats were separately loaded in 1.5% agarose gel containing 1.0 μg/mL ethidium bromide including DNA standards (0.5 μg per well). Electrophoresis was performed for 45 min at 100 V. After electrophoresis gel was studied under gel doc system and was photographed through digital camera.
Estimation of protein
Proteins from each biological sample were estimated by the method of Lowry et al. (Citation1951).
Cell lines and culture medium
Human breast cancer cell line (MCF-7), human cervix carcinoma cell line (HeLa), human liver carcinoma cell line (HepG2), CNS cell lines (SF-295), human colon cancer cell line (SW480), and neuroblastoma cell line (IMR-32) were used in this study. These cells were grown as monolayers in minimum essential medium, glucose concentration (4.5 g/L) with 10% heat inactivated fatal calf serum and 1% penicillin-streptomycin mixture. The cells were maintained at 37 °C and in 5% CO2 atmosphere.
Cytotoxicity assay
The effects of different extracts of the C. songarica on the inhibition of cancer cell growth were evaluated by SRB method as described by Monks et al. (Citation1991). Cells were harvested from culture flasks, and the cells (1 × 104), were seeded in each well of the 96-well plate containing 100 µL fresh growth medium and were permitted to adhere for 24 h. The cells were treated with 200 µL of different concentrations (20–80 µg/mL) of each extract. After 48 h of treatment, the cells were fixed with 10% TCA and stained with 100 µL of SRB solution in 1% acetic acid for 15 min. Unbound dye was removed by using 1% acetic acid. The bound dye was extracted with 10 mM Tris-HCl for determination of optical density at 492 nm. Each test was done in triplicates. The percentage of cell viability was calculated by using the following formula:
Statistical analysis
The values are expressed as mean ± standard deviation (SD). The results were evaluated by using the SPSS (version 12.0) (SPSS Inc., Chicago, IL) and Origin 6 softwares (MicroCal Inc., Northampton, MA) and evaluated by one-way ANOVA followed by Bonferroni’s t-test. Statistical significance was considered when value of p < 0.05.
Results
Effects of C. songarica methanol extract on kidney function
presents the results of kidney function test in rats administered with CCl4. The levels of creatinine and urea were significantly elevated in rats administered CCl4 only. Serum creatinine levels in the CCl4 intoxicated rats were significantly reduced (p < 0.05) by 19.91, 42.85 and 59.74% at 100, 200 and 300 mg/kg b wt of C. songarica extract, respectively, compared to CCl4 only treated group. Vitamin E, a known antioxidant, decreases the serum creatinine levels to 61.47%. Serum urea levels were also decreased by 42.57, 25.78, 31.64 and 40.23% in the Vitamin E, 100, 200 and 300 mg/kg b wt pre-treated groups, respectively (). High levels of cholesterol (132 ± 15.36) were observed in rats treated with CCl4 only. Pre-treatment with Vitamin E and plant extract decreased serum cholesterol levels to 53.5, 83, 68 and 54 as compared to CCl4 group, respectively ().
Table 1. Effects of pre-treatment with C. songarica on serum creatinine, urea and cholesterol levels in rats induced with CCl4 toxicity.
Effects of C. songarica methanol extract on MDA levels
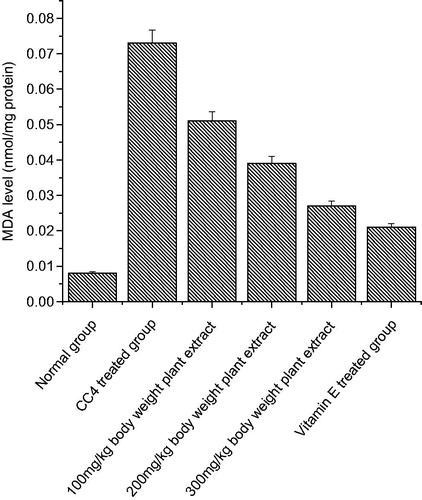
The lipid peroxidation levels significantly increased in both the organs as compared to normal group of animals due to the CCl4 treatment (p < 0.05) ( and ). After CCl4 administration, the MDA levels increased significantly from 0.001 to 0.075 nmol/mg protein in kidney tissue homogenate and in heart tissue homogenate, the MDA level increased from 0.005 to 0.023 nmol/mg proteins. Administration of C. songarica extract showed significant reduction in lipid peroxidation in kidney and heart tissues as compared to CCl4-induced rats. There was a significant increase in TBARS, which is an indirect measure of lipid peroxidation that suggests the possibility of enhanced free radical generation by CCl4.
Figure 1. The effect of C. songarica methanol extract on rat kidney tissue homogenate lipid peroxidation in CCl4-treated rats. The data are presented as means ± SD for six animals in each observation and evaluated by one-way ANOVA followed by Bonferroni’s t-test to detect intergroup differences. Differences are considered to be statistically significant if p < 0.05.
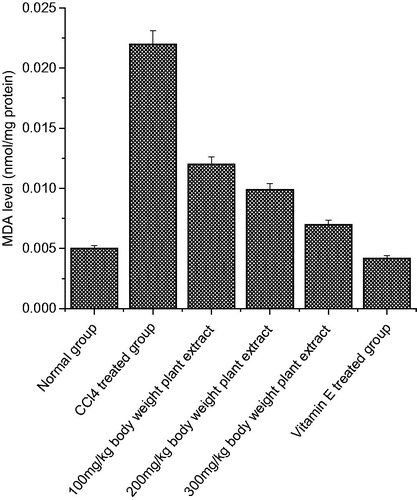
Figure 2. The effect of C. songarica methanol extract on rat heart tissue homogenate lipid peroxidation in CCl4-treated rats. The data are presented as means ± SD for six animals in each observation and evaluated by one-way ANOVA followed by Bonferroni’s t-test to detect inter group differences. Differences are considered to be statistically significant if p < 0.05.
Effect of C. songarica methanol extract and vitamin E on CCl4-induced changes in antioxidant enzyme activities in the kidney tissue (GPx, GR, GST and CAT)
indicates the activities of antioxidant enzymes in the kidney of six experimental groups. Nephrotoxicity induced by CCl4 causes a significant decrease in antioxidant enzyme levels (catalase, glutathione peroxidase, glutathione-S-transferase and glutathione reductase) as compared to the normal control group. Whereas oral administration of C. songarica extract in groups IV, V and VI at the dose level of 100, 200 and 300 mg/kg b wt and its combination with Vitamin E (group III) in rats treated with CCl4 showed recovery of the activities of antioxidant enzymes.
Table 2. The effect of treatment of methanol extract of C. songarica on glutathione and antioxidant enzymes in kidney tissue of CCl4 challenged rats.
CCl4 administration markedly decreased the levels of reduced glutathione in kidneys (control = 90.09 nm/g protein) demonstrating oxidative stress. Crataegus songarica extract at the dose level of 100, 200 and 300 mg/kg b wt increased the levels of reduced glutathione in kidney tissue to 150, 190 and 209 nm/g protein, respectively. Vitamin E enhanced the glutathione levels to 220.33 nmol/g protein as compared to CCl4-treated group.
Effects of C. songarica extract on cardiac enzymatic antioxidant levels
Alteration in antioxidant defense system, such as GPx, GR, GST, CAT and GSH is explained in . CCl4 intoxication markedly lessened the antioxidative status of cardiac tissue in comparison to control group. The present study revealed that methanol extract of C. songarica ameliorated the toxic effects of CCl4 near to control by elevating the activity of suppressed antioxidant enzymes. In the heart tissue (), GR, CAT, GPX and GST activities were reduced to 16.05, 536.1, 22.0 and 15.51 in CCl4-treated animals. Dose-dependent increase in the activity was observed when treated with the different concentrations of C. songarica extract.
Table 3. The effect of treatment of methanol extract of C. songarica on glutathione and antioxidant enzymes in heart tissue of CCl4 challenged rats.
The glutathione levels were examined in this study in order to evaluate endogenous antioxidant system. Effect of methanol extract of C. songarica on GSH level for all experimental groups is shown in . CCl4 treatment caused significant decrease of GSH level in heart tissue homogenates compared to the normal group. When rats were treated with CCl4, GSH decreased from 81.61.14 ± 5.73 nm/g protein (control group) to 27.93 ± 1.94 nm/g protein (CCl4-treated group). Pretreatment of methanol extract for 15 days at the oral doses of 100, 200 and 300 mg/kg b wt followed by 2-day CCl4 treatment enhanced the level of GSH to 31.85, 39.45 and 51.47 nm/g protein, respectively. The heart of vitamin E-treated animals also showed a significant increase in GSH activity as compared to CCl4-treated rats.
Effect of C. songarica methanolic extract on DNA
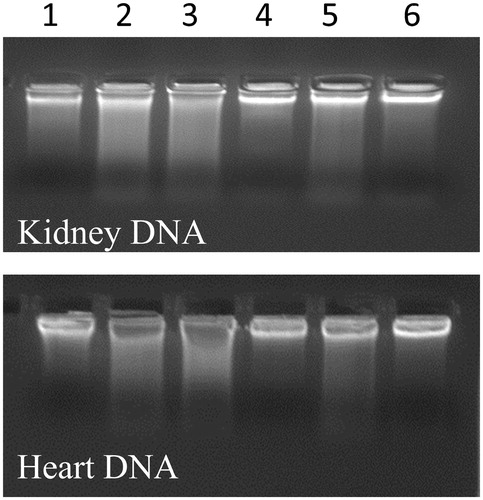
shows the effect of methanolic extract of C. songarica on the genomic DNA integrity of CCl4-treated and untreated kidney and heart tissue of rats. Line 1 represents the genomic DNA of untreated rats (control rats) and seen to intact. But the genomic DNA of lane 2 is disintegrated as it represents the DNA of CCl4-treated animals. The other lanes (i.e. from 3 to 6) represent the DNA of C. songarica and Vitamin E (50 mg/kg b wt) protected animals against CCl4 at the concentration of 100, 200 and 300 mg/kg b wt.
Figure 3. Protective effect of methanol extract of C. songarica on DNA of kidney and heart tissue. Lane 1 – Control, Lane 2 – CCl4-treated rats, Lane 3 – (CCl4 + 100 mg/kg b wt plant extract), Lane 4 – (CCl4 + 200 mg/kg b wt plant extract), Lane 5 – (CCl4 + 300 mg/kg b wt plant extract) and Lane 6 – (CCl4 + vitamin E 50 mg/kg b wt).
Effect of C. songarica different extracts on human cancer cell lines
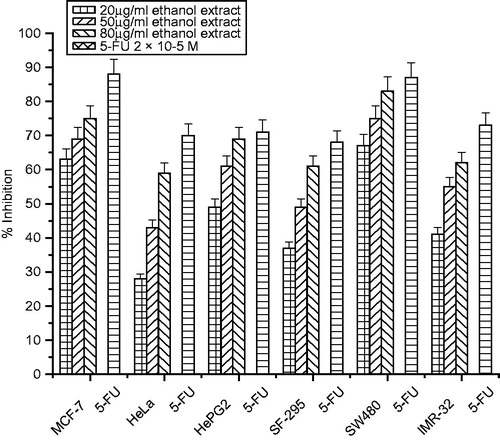
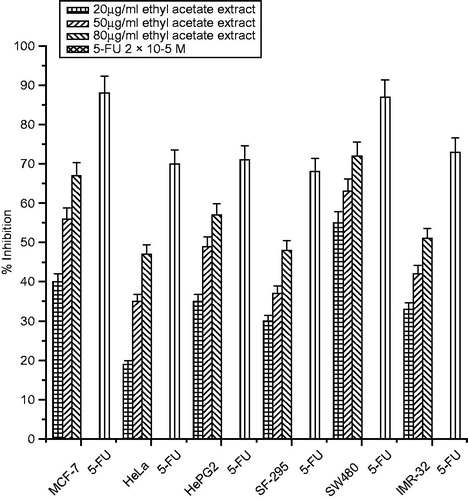
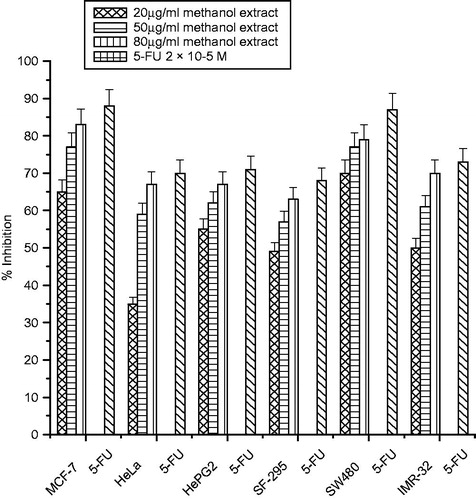
The growth inhibitory effects of different extracts of C. songarica on six human cancer cell lines (MCF-7, HeLa, HepG2, SF-295, SW480 and IMR-32) were tested with the SRB assay. Cells were treated with various concentrations (20–80 µg/mL) of the extracts for 48 h. All the three extracts inhibited cell proliferation in a significant dose-dependent manner (). Among the cancer cells tested, MCF-32 was the most sensitive cancer cell to the extracts with the percentage inhibition of 83% (methanol extract), 75% (ethanol extract) and 67% (ethyl acetate extracts at the concentration of 80 µg/mL, followed by the SW480 cells as shown in ). Most resistant cancer cell to the extracts induced growth inhibition was found to be HeLa and SF-295 with inhibition of 67, 59 and 47% and 68, 61 and 48% at the highest concentration of 80 µg/mL, respectively. Interestingly, the antiproliferation effect of methanolic and ethanolic extracts cells were comparable to that of 5-FU on the MCF-7 and SW480 cell lines as shown in .
Figure 4. Cytotoxicity of methanol extract of C. songarica and 5-fluorouracil on six human cancer cell lines. The data are presented as means ± SD and evaluated by one-way ANOVA followed by Bonferroni’s t-test. Differences are considered to be statistically significant if p < 0.05.
Discussion
Exposure to CCl4 has been reported to induce free radical generation in tissues, such as liver, kidney, heart, lung, testis, brain and blood (Ahmad et al., Citation1987). The reactive metabolite trichloromethyl radical (√CCl3) and trichloromethyl peroxide radical () are generated from the metabolic conversion of CCl4 by cytochrome P-450. As O2 tension rises, a greater fraction of √CCl3 present in the system reacts very rapidly with O2 and more reactive free radicals, like CCl3OO√ are generated from √CCl3. The present study was carried out to evaluate the protective effect of C. songarica methanolic extract on CCl4-induced kidney and heart toxicity. The kidneys are responsible for the elimination of unmodified drugs and metabolites. Additionally, these organs are also capable to realize diffused biotransformation reactions. Ongoing studies demonstrate that nephrotoxicity induced by chemical agents are one of the consequences of the accumulation of certain metabolites in kidneys (Sener et al., Citation2003). Kidney damage is one of the most prominent reasons of death due to CCl4 intoxication. Kidneys have an affinity against CCl4, as they predominantly contain cytochrome P-450 in the cortex and is thus possible that CCl4 may contribute a lot to nephrotoxicity (Ogeturk et al., Citation2005a,Citationb).
The presence of abnormally high levels of urea, creatinine and cholesterol in serum are possible indicators of kidney injuries induced through CCl4 treatment (Ogeturk et al., Citation2005a). The serum creatinine level does not rise until at least half of the kidney nephrons are damaged or destroyed (Bhattacharya et al., Citation2005). As reported previously that chronic renal injuries and urea elevations developed in rats after CCl4 intoxication (Khan & Ahmed, Citation2009). The present study revealed that administration of C. songarica significantly restored the levels of urea, creatinine and cholesterol in serum. Similar investigations were also documented that different plant extracts significantly recovered the renal injuries induced through CCl4 intoxication (Khan et al., Citation2010; Ogeturk et al., Citation2005a,Citationb). Malondialdehyde, a secondary product of lipid peroxidation, is used as an indicator of tissue damage (Ohkawa et al., Citation1979). Elevated levels of MDA following CCl4 administration have been well documented in various organs, such as liver (Lee & Jeong, Citation2002; Shahjahan et al., Citation2004), kidney (Srinivasan et al., Citation2005; Tirkey et al., Citation2005) and heart (Thieophile et al., Citation2006). In this study, increased MDA levels were observed in the kidneys and heart tissue of CCl4-administered (Group II) rats when compared to normal (Group I) rats. The observed reduction in MDA level in these organs of rats following treatment with extract of C. songarica suggests the tissue protective potential of C. songarica.
GSH is involved in several defense processes against oxidative damage protects cells against free radicals, peroxides and other toxic compounds (Sies, Citation1999). Indeed, glutathione depletion increases the sensitivity of cells to various aggressions and also has several metabolic effects. It is widely known that a deficiency of GSH within living organisms can lead to tissue injury (Limon-Pacheco et al., Citation2007). GSH is used to evaluate the non-enzymatic antioxidant capacity of a tissue to prevent the damage associated to free radical production (Halliwell & Gutteridge, Citation2007). It was previously observed that √CCl3 radical can even react with sulfhydryl groups of glutathione and protein thiols to alter the redox status of cells (Sheweita et al., Citation2001). Since GSH is considered as an important defense against lipid oxidative damage in the kidneys eliminating hydrogen peroxide, peroxyl and hydroxyl radicals formed during this process, therefore, GSH-dependent enzymes will be affected when its level is depleted in the cells (Khan & Ahmed, Citation2009). In the present study, decline in GSH content in the kidney and heart tissue of CCl4-intoxicated rats, and its dose-dependent increase in both tissues reveals the antioxidant potential of C. songarica.
Organisms do have an effective mechanism to prevent and neutralize the free radical-induced damage, which is accomplished by a set of endogenous antioxidant enzymes, such as SOD, CAT, GST, GPx and GR. When the balance between ROS production and antioxidant defenses is lost, oxidative stress results, which leads to various pathological situations. Any compound whether natural or synthetic with antioxidant properties may contribute towards the partial or total alleviation of this type of damage. In the present study, we observed a decline in the levels of antioxidant enzymes like CAT, GST, GPx and GR in CCl4-treated rats, which are a clear manifestation of excessive formation of free radicals and tissue damage. Significant dose-dependent increase of antioxidant enzymes were observed in kidney and heart tissue of animals treated with C. songarica and Vitamin E, which indicates the antioxidant potential of C. songarica. Similar results were reported by Szymonik-Lesiuk et al. (Citation2003) that rats exposed to CCl4, would reduce CAT, GST, GPx and GR activities in kidney and heart tissue.
Like other macro-molecules, such as lipids and proteins, nucleic acids are also attacked by free radicals to cause oxidative DNA damage. In the present study, carbon tetrachloride degrades the DNA of kidney and heart tissue of rats by generating free radicals. On the other hand, pretreatment of C. songarica methanolic extract appreciably reduced the DNA damage assayed by DNA ladder assay banding pattern. Similar results were reported by Murugesan et al. (Citation2009), while studying the protective effects of Kombucha tea against CCl4-induced oxidative stress in kidneys of rats.
A large variety of phytochemicals that have been reported from natural product research has been proven successful as anticancerous agents (Androutsopoulos et al., Citation2008). This study was undertaken to prove the anti-cancerous property of C. Songarica, which is to be used to treat various diseases on traditional level. The findings from this study observed that methanolic, ethanolic and ethyl acetate extracts of C. songarica inhibits the proliferation of six human cancer cell lines. The cytotoxicity effect found was highest with MFC-70 and SW480 cell lines. The effect was analyzed at different concentration levels via 20, 50 and 80 μg mL−1 and the inhibitory effect was concentration dependent and cell line specific. This clearly indicates the presence of potent bioactive principles in the crude extracts that might be useful as antiproliferative and antitumoragents. Thus, the active principle(s) need to be identified. Similar results were reported by our lab that methanolic and 70% ethanolic extract from Podophyllum hexandrum possess a potent cytotoxic effect on cultured human breast cancer cell lines (Ganie et al., Citation2011).
Conclusion
In conclusion, the present study reveals that the methanolic extract of C. songarica possesses profound in vivo antioxidant activity against CCl4-induced kidney and heart tissue damages. These findings indicate that the use of C. songarica might be considered as an adjunct therapeutic strategy to combat various disorders and other oxidative stress-related diseases. In addition, further studies to characterize the active principles of C. songarica and to elucidate the mechanism are in progress. The present study also shows that methanolic, ethanolic and ethyl acetate extracts of C. songarica possess in vitro cytotoxicity effect on different human cancer cell lines. However, further chemical work and investigations at molecular level are required to identify the active components from this plant.
Declaration of interest
The authors declare that there is no conflict of interest.
References
- Abraham P, Wilfred G, Cathrine SP. (1999). Oxidative damage to the lipids and proteins of the lungs, testis and kidney of rats during carbon tetrachloride intoxication. Clinic Chim Acta 289:177–9
- Ahmad FF, Cowan DL, Sun AY. (1987). Detection of free radical formation in various tissues after acute carbon tetrachloride administration in gerbil. Life Sci 41:2469–75
- Androutsopoulos V, Arroo RR, Hall JF, et al. (2008). Antiproliferative and cytostatic effects of the natural product eupatorin on MDA-MB-468 human breast cancer cells due to CYP1-mediated metabolism. Breast Cancer Res 10:1–12
- Bhattacharya H, Lun L, Gomez R. (2005). Biochemical effects to toxicity of CCl4 on rosy barbs (Puntius conchonius). Our Nature 3:20–5
- Cabre M, Comps J, Paternain JL, et al. (2000). Time course of changes in hepatic lipid peroxidation and glutathione metabolism in rats with carbon tetrachloride-induced cirrhosis. Clin Exp Pharmacol Physiol 27:694–9
- Castillo T, Koop DR, Kamimura S, et al. (1992). Role of cytochrome P-450 2E in ethanol, carbon tetrachloride and iron-dependent microsomal lipid peroxidation. Hepatology 1:992–6
- Charbonneau M, Brodeur J, Du Souich P, et al. (1986). Correlation between acetone potentiated CCl4-induced liver injury and blood concentrations after inhalation or oral administration. Toxicol Appl Pharmacol 84:286–94
- Churchill DN, Finn A, Gault M. (1983). Association between hydrocarbon exposure and glomerulonephritis, an appraisal of the evidence. Nephron 33:169–72
- Cook NC, Samman S. (1996). Flavonoids – Chemistry, metabolism, cardioprotective effects, and dietary sources. J Nut Bioch 7:66–76
- Ganie SA, Haq E, Hamid A, et al. (2011). Long dose exposure of hydrogen peroxide (H2O2) in albino rats and effect of Podophyllum hexandrum on oxidative stress. Eur Revr Med Pharmacol Sci 15:906–15
- Guven A, Guven A, Gulmez M. (2003). The effect of kefir on the activities of GSH-Px, GST, CAT, GSH and LPO levels in carbon tetrachloride-induced mice tissue. J Vet Med B Infect Dis Vet Public Health 50:412–16
- Halim AB, el-Ahmady A, Hassab-Allah S, et al. (1997). Biochemical effect of antioxidants on lipids and liver function in experimentally-induced liver damage. Ann Clin Biochem 34:656–63
- Halliwell B, Gutteridge JMC (eds.). (2007). Cellular responses to oxidative stress: Adaptation, damage, repair, senescence and death. In: Free Radicals in Biology and Medicine. Oxford: Oxford University Press Inc, 187–267
- Haque RH, Parvez B, Pandey S, et al. (2003). Aqueous extract of walnut protects mice against cyclophosphamide induced biochemical toxicity. Hum Exp Toxicol 22:473–80
- Hartley DP, Kolaja KL, Reinchord J, et al. (1999). 4-Hydroxynonenal and malondialdehyde hepatic protein adducts in rats treated with carbon tetrachloride: Immunochemical detection and lobular localization. Toxicol Appl Pharmacol 161:23–33
- Khan MR, Ahmed D. (2009). Protective effects of Digera muricata (L.) Mart. on testis against oxidative stress of carbon tetrachloride in rat. Food Chem Toxicol 47:1393–9
- Khan RA, Khan MR, Sahreen S, et al. (2010). Prevention of CCl4-induced nephrotoxicity with Sonchus asper in rat. Food Chem Toxicol 48:2469–76
- Ko KM, Ip SP, Poon MK, et al. (1995). Effect of a lignin enriched Fructus schisandrae extract on hepatic glutathione status in rats: Protection against carbon tetrachloride toxicity. Planta Med 61:134–7
- Lee KJ, Jeong HG. (2002). Protective effect of Platycodix radix on carbon tetrachloride-induced hepatotoxicity. Food Chem Toxicol 40:517–25
- Lequeux H, Hermans C, Lutts S, et al. (2010). Response to copper excess in Arabidopsis thaliana: Impact on the root system architecture, hormone distribution, lignin accumulation and mineral profile. Plant Physiol Biochem 48:673–82
- Limon-Pacheco JH, Hernandez NA, Fanjul-Moles ML, et al. (2007). Glutathione depletion activates mitogen activated protein kinase (MAPK) pathways that display organ-specific responses and brain protection in mice. Free Radic Biol Med 43:1335–47
- Lowry OH, Rosenbrough NJ, Farr AI, et al. (1951). Protein estimation with the Folin-phenol reagent. J Biol Chem 193:265–75
- Melin AM, Perromat A, Deleris G. (2000). Pharmacologic application of Fourier transform IR spectroscopy: In vivo toxicity of carbon tetrachloride on rat liver. Biopolymers 57:160–8
- Monks A, Scudiero D, Skehan P, et al. (1991). Feasibility of a high flux anticancer drug screen using a diverse panel of culture human tumor cell lines. J Natl Cancer Inst 83:757–66
- Moren MA, Depierre JW, Mannervick B. (1985). Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 582:67–78
- Murugesan GS, Sathishkumar M, Jayabalan R, et al. (2009). Hepatoprotective and curative properties of Kombucha tea against carbon tetrachloride-induced toxicity. J Microbiol Biotech 19:397–402
- Nichans WG, Samuelson D. (1968). Formation of malondialdehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem 6:126–30
- Ogeturk M, Kus I, Colakoglu N, et al. (2005a). Caffeic acid phenethyl ester protects kidneys against carbon tetrachloride toxicity in rats. J Ethnopharmacol 97:273–80
- Ogeturk M, Kus I, Kavaklý A, et al. (2005b). Reduction of carbon tetrachloride-induced nephropathy by melatonin administration. Cell Biochem Funct 23:85–92
- Ohkawa H, Ohishi N, Yagi K. (1979). Assay of lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal Biochem 95:351–8
- Ozturk F, Ucar M, Ozturk IC, et al. (2003). Carbon tetrachloride-induced nephrotoxicity and protective effect of betaine in Sprague-Dawley rats. Urology 62:353–6
- Perez AJ, Courel M, Sobrado J, et al. (1987). Acute renal failure after topical application of carbon tetrachloride. Lancet 1:515–16
- Raucy JL, Kraner JC, Lasker JM. (1993). Bioactivation of halogenated hydrocarbons by cytochrome P4502E1. Crit Rev Toxicol 23:1–20
- Sarkar K, Ghosh A, Kinter M, et al. (2006). Purification and characterization of a 43 kD hepatoproctective protein from the herb Cajanus indicus L. Protein J 25:411–21
- Sener G, Sehirli AO, Ayanoglu-Dülger G. (2003). Protective effects of melatonin, vitamin E, and N-acetylcysteine against acetaminophen toxicity in mice: A comparative study. J Pineal Res 35:61–8
- Shahjahan M, Sabitha KE, Jainu M, et al. (2004). Effect of Solanum trilobatum against carbon tetrachloride induced hepatic damage in albino rats. Ind J Med Res 120:194–8
- Sharma N, Trikha PA, Raisuddin SM. (2001). Inhibition of benzo[a]pyrene- and cyclophosphamide-induced mutagenicity by cinnamomum cassia. Mut Res 480–1:179–88
- Sheweita SA, El-Gabar MA, Bastawy M. (2001). Carbon tetrachloride changes the activity of cytochrome P450 system in the liver of male rats: Role of antioxidants. Toxicology 169:83–92
- Sies H. (1999). Glutathione and its role in cellular functions. Free Rad Biol Med 27:916–21
- Srinivasan M, Rukkumani R, Ram Sudheer A, et al. (2005). Ferulic acid, a natural protector against carbon tetrachloride-induced toxicity. Fund Clin Pharmacol 19:491–6
- Szymonik-Lesiuk S, Czechowska G, Stryjecka-Zimmer M, et al. (2003). Catalase, superoxide dismutase and glutathione peroxidase activities in various rat tissues after carbon tetrachloride intoxication. J Hepatobiliary Pancreat Surg 10:309–15
- Thieophile D, Emery TD, Paul Desire DD, et al. (2006). Effects of Alafia multiflora Stapf on lipid peroxidation and antioxidant enzyme status in carbon tetrachloride-treated rats. Pharmacologyonline 2:76–89
- Tirkey N, Pilkhwal S, Kuhad A, et al. (2005). Hesperidin, a citrous bioflavonoid, decreases the oxidative stress produced by carbon tetrachloride in rat liver and kidney. BMC Pharmacol 52:1–8
- Wu B, Ootani A, Iwakiri R, et al. (2005). T cell deficiency leads to liver carcinogenesis in Azoxymethane-treated rats. Exp Biol Med 231:91–8