Abstract
Context: Nepenthes mirabilis (Lour.) Rafarin (Nepenthaceae) is a carnivorous plant used as a folk medicine in the treatment of jaundice, hepatitis, gastric ulcers, ureteral stones, diarrhea, diabetes, and high blood pressure. Neither the phytochemical content nor biological activities of N. mirabilis have been reported.
Objective: The anti-inflammatory activity from the N. mirabilis methanolic extract led to the isolation of compounds (1–26).
Materials and methods: Chromatographic methods were used to isolate compounds from the methanol extract of N. mirabilis branches and leaves. The anti-inflammatory activity of these isolated compounds was investigated in lipopolysaccharide (LPS)-stimulated bone marrow-derived dendritic cells (BMDCs) using ELISA. Primary BMDCs were used to examine the production of pro-inflammatory cytokines (IL-12 p40, IL-6, and TNF-α, at concentrations of 0.1, 0.2, and 1.0 μM) as compared with a positive control, SB203580 (1.0 μM). MTT assays showed that isolated compounds (1–26) did not exhibit significant cytotoxicity at concentrations up to 20.0 μM.
Results: Compound 9 showed potent inhibition of IL-12 p40, IL-6, and TNF-α production (IC50 = 0.17 ± 0.02, 0.46 ± 0.01, and 8.28 ± 0.21 μM, respectively). Compound 4 showed potent inhibition of IL-12 p40 and IL-6 production (IC50 = 1.17 ± 0.01 and 2.15 ± 0.04 μM). In addition, IL-12 p40 inhibition by naphthalene derivatives (1–7, 9, and 10), phenolic compounds (11–15), lupeone (18), and flavonoids (22, 25, and 26) was more potent than with the positive control. The isolated compounds exhibited little and/or no inhibitory effects on TNF-α production in LPS-stimulated BMDCs.
Discussion and conclusion: Taken together, these data suggest that the isolated components have significant inhibitory effects on pro-inflammatory cytokine production and warrant further study concerning their potential medicinal use.
Introduction
Inflammation is an important defense mechanism in protecting human bodies from exogenous pathogens; it is defined as a part of the complex biological responses of vascular tissue toward exogenous harmful stimuli (Nathan, Citation2002). Inflammation, normally resulting from an excessive inflammatory response or failure of resolution (Gilroy et al., Citation2004), is recognized as a causative in various diseases, such as atherosclerosis, cancer, asthma, and some neuropathological disorders, such as Alzheimer’s disease or Parkinson’s disease (Serhan et al., Citation2008). Inflammation is a complex process, which is mediated by activated inflammatory cells of the immune system, including macrophages. The overproduction of pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6, and inflammatory mediators, including reactive oxygen species (ROS), nitric oxide (NO), and prostaglandin E2 (PGE2), generated by activated inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) by macrophages, plays an important role in inflammatory diseases (Walsh et al., Citation2005).
Numerous molecules are involved in the induction and maintenance of the inflammatory response. In addition to pivotal cytokines, such as IL-1, -6, -12, and TNF-α, prostaglandins and nitric oxide are important chemical mediators of inflammation. TNF-α is a well-characterized pro-inflammatory cytokine released primarily from monocytes and macrophages upon invasion of the host by a wide variety of pathogens. It plays a crucial role in host defense and in the inflammatory response. Although it has numerous beneficial roles in immune regulation, it has also been implicated in the pathogenesis of both acute and chronic inflammatory disease (Beutler & Cerami, Citation1986). IL-6 is a particularly interesting molecule because it has both pro- and anti-inflammatory effects. It has been implicated in many inflammatory diseases in both adults and neonates (Weinstein et al., Citation1997). IL-12 plays a central role in the initiation and regulation of cellular immunity. It is involved in type-1 helper T-cell-mediated inflammation as part of the normal immune response, as well as in inflammatory diseases, including rheumatoid arthritis, asthma, psoriasis, and Crohn’s disease (Gately et al., Citation1998; Philip, Citation2002; Trinchieri, Citation2003). Thus, inhibition of the production of these inflammatory mediators is an important target in the treatment of inflammatory diseases.
In recent years, many natural product resources, including medicinal plants, have been widely used for their various curative abilities. Plants synthesize many chemicals both as constitutive products and as secondary metabolites. Extracts from medicinal plants that are traditionally used include aromatic substances, mostly phenolic compounds, or their oxygen-substituted derivatives. They have attracted attention in the search for bioactive compounds as valuable functional foods and/or new drug candidates.
Nepenthes mirabilis (Lour.) Rafarin (Nepenthaceae) is a carnivorous plant used as a folk medicine in the treatment of jaundice, hepatitis, gastric ulcers, ureteral stones, diarrhea, diabetes, and high blood pressure (Chi, Citation2012). Naphthoquinones and naphthalene glycosides are characteristic of Nepenthes species and are chemotaxonomic markers within this genus (Aung et al., Citation2002; Cannon et al., Citation1980; Likhitwitayawuid et al., Citation1998; Rischer et al., Citation2002). However, neither the phytochemical content nor biological activities of N. mirabilis have been reported. In a previous study, we have screened the antioxidant and anti-osteoporosis components of the carnivorous plant N. mirabilis (Thanh et al., Citation2015a,Citationb). In this study, we continue to investigate the anti-inflammatory effects of the isolated constituents on the LPS-induced expression of the pro-inflammatory cytokines IL-12 p40, IL-6, and TNF-α in BMDCs using ELISA.
Materials and methods
Biological material
The samples of Nepenthes mirabilis were collected in May 2013 at Quang Tri province, Vietnam and were identified by Prof. Ninh Khac Ban (IMBC, VAST, Vietnam). A voucher specimen (NM.05.13-01) was deposited at the Herbarium of IMBC, VAST, Vietnam.
Compounds
From the methanol extract of N. mirabilis, 26 compounds (1–26) were isolated and structurally defined. Stock solutions of tested compounds in dimethyl sulfoxide (DMSO) were prepared, kept at −20 °C, and diluted to the final concentration in fresh media before each experiment. For not to affect cell growth, the final DMSO concentration did not exceed 0.5% in all experiments.
Cell viability assay
The cell viability was determined by standard procedure of MTT assays (Sigma, St. Louis, MO), which was based on the reduction of the dye MTT to formazan crystals, an insoluble intracellular blue product, by cellular dehydrogenases. Briefly, the cells at a concentration of 5 × 104 cells were seeded on a 96-well culture plate. After incubation for 1 h at 37 °C, cells were treated with compounds at various concentrations for 24 h. Cells were treated with 0.2 mg MTT (Sigma, St. Louis, MO) and then incubated at 37 °C for 4 h. The plate was centrifuged and the supernatants were aspirated. The formazan crystals in each well were dissolved in 250 μL DMSO (Amresco, Solon, OH). The plates were agitated to ensure complete dissolution of the purple formazan crystals, and the optical density was measured at a wavelength of 540 nm using an ELISA reader (Packard, Instrument Co., Downers Grove, IL).
Cell cultures
In this study, we used LPS-stimulated BMDCs as a model for testing the inhibitory effects of isolated compounds on the secretion of pro-inflammatory cytokines IL-12 p40, IL-6, and TNF-α. BMDCs (1 × 105) were seeded in 48-well plates at 37 °C, 5% CO2 for 1 h, and then treated for 1 h with the compounds at concentrations of 0.1, 0.2, and 1.0 μM, followed by stimulation with LPS (10.0 ng/mL, and ). The supernatants were harvested 16 h after stimulation and IL-12 p40 secretion was measured using ELISA. SB203580, an inhibitor of cytokine suppressive binding protein/p38 kinase, was used as a positive control (Lee et al., Citation1994). SB203580 inhibited IL-12 p40, IL-6, and TNF-α production with IC50 values of 5.00 ± 0.16, 3.50 ± 0.12, and 7.20 ± 0.13 μM, respectively.
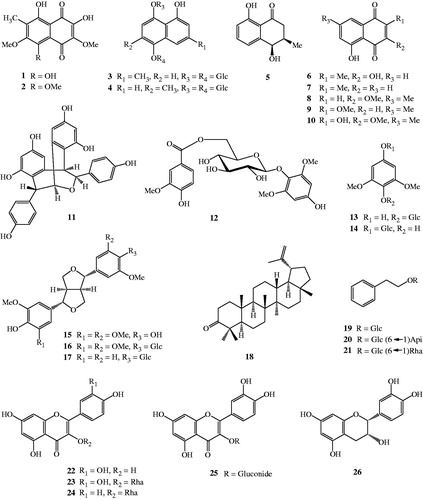
Figure 2. The effect of the selected compounds (0.1, 0.2, and 1.0 μM) on IL-12 p40 production by LPS-stimulated BMDCs. The data are presented as inhibition rate (%) compared with the value of vehicle-treated DCs. SB203580 was used as a positive control (Pos.).
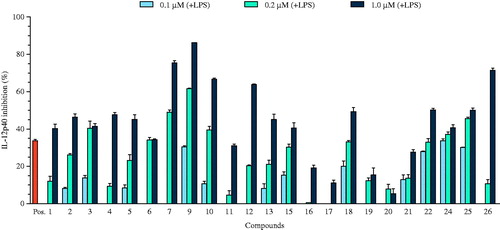
Figure 3. The effect of the selected compounds (0.1, 0.2, and 1.0 μM) on IL-6 production by LPS-stimulated BMDCs. The data are presented as inhibition rate (%) compared to the value of vehicle-treated DCs. SB203580 was used as a positive control (Pos.).
Bone marrow-derived dendritic cells were grown from wild-type C57BL/6 mice (Orient Bio Inc., Seongnam, South Korea) as previously described (Koo et al., Citation2012). All animal procedures were approved by and performed according to the guidelines of the Institutional Animal Care and Use Committee of Jeju National University (#2010-0028). Briefly, the mouse tibia and femur was obtained by flushing with Dulbecco’s modified Eagle medium to yield bone marrow cells. The cells were cultured in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum (FBS; Gibco, Grand Island, NY), 50.0 μM β-mercaptoethanol, and 2.0 mM glutamine supplemented with 3% J558L hybridoma cell culture supernatant containing granulocyte-macrophage colony-stimulating factor (GM-CSF). The culture medium was replaced with fresh medium every other day. At day 6 of culture, non-adherent cells and loosely adherent DC aggregates were harvested, washed, and resuspended in RPMI 1640 supplemented with 5% FBS.
Cytokine production measurements
The BMDCs were incubated in 48-well plates in 0.5 mL containing 1 × 105 cells per well, and then treated with the isolated compounds at different concentrations for 1 h before stimulation with 10.0 ng/mL LPS from Salmonella minnesota (Alexis, Troy, NY). Supernatants were harvested 18 h after stimulation. Concentrations of murine IL-12 p40, IL-6, and TNF-α in the culture supernatant were determined by ELISA (BD PharMingen, San Jose, CA) according to the instructions of the manufacturer.
IL-12 p40 level in unstimulated DC: not detectable. IL-12 p40 level in LPS-stimulated DC: 51.34 ± 0.66 (ng/mL). IL-6 level in unstimulated DC: not detectable. IL-6 level in LPS-stimulated DC: 41.12 ± 2.38 (ng/mL). TNF-α level in unstimulated DC: not detectable. TNF-α level in LPS-stimulated DC: 1.83 ± 0.02 (ng/mL).
The inhibitory activity (I) was expressed as the inhibition rate (%), which was calculated from the following formula:
Cdcv is the cytokine level (ng/mL) in vehicle-treated DC; Cdcc is the cytokine level (ng/mL) in compound-treated DC.
Statistical analysis
The results are expressed as means ± standard deviation (SD). All assays were performed in at least three independent experiments. Statistical analysis was performed using one-way ANOVA. p Value <0.05 was considered statistically significant.
Results and discussion
Phytochemical analysis
The methanolic extract of N. mirabilis (Lour.) Rafarin was subjected to various separation procedures and 26 compounds were isolated (1–26, ). Their structures were identified as nepenthone F (1), nepenthone G (2), nepenthoside A (3), nepenthoside B (4), cis-isoshinanolone (5), droserone (6), plumbagin (7), 3-methoxy-7-methyljuglone (8), 2-methoxy-7-methyljuglone (9), nepenthone C (10), (–)-heimiol A (11), 4-hydroxy-2,6-dimethoxyphenyl 6′-O-vanilloyl-β-d-glucopyranoside (12), leonuriside A (13), koaburaside (14), syringaresinol (15), syringaresinol-4′-β-d-glucopyranoside (16), pinoresinol-4-O-β-d-glucopyranoside (17), lupeone (18), phenylethyl-β-d-glucopyranoside (19), icariside D1 (20), phenethyl rutinoside (21), quercetin (22), quercitrin (23), kaempferol-3-O-α-l-rhamnoside (24), quercetin 3-O-β-d-glucuronide (25), and (–)-epicatechin (26) based on spectral and chemical evidence, consistent with previously published identifications (Thanh et al., Citation2015a,Citationb).
Biological activities
Inflammation helps to fight against diseases, to repair tissue damage, and ultimately, to restore homeostasis. It is a self-perpetuating feed-forward process that, once activated, produces more pro-inflammatory mediators, increasing the inflammatory response. Inflammation attracts immune cells and induces the local production of several cytokines and chemokines (Lawrence et al., Citation2002). LPS, which is derived from Gram-negative bacteria, stimulates macrophages to produce an array of pro-inflammatory mediators, including the potent vasodilator NO and cytokines (TNF-α, IL-1, IL-6, etc.).
IL-12 is a heterodimer formed by a 35-kDa light chain (known as p35 or IL-12α) and a 40-kDa heavy chain (known as p40 or IL-12β). p35 is homologous to other single-chain cytokines, while p40 is homologous to the extracellular domain of members of the hematopoietic cytokine-receptor family. IL-12 p40 is produced in great excess over the IL-12 and IL-23 heterodimers (Carra et al., Citation2000; Wysocka et al., Citation1995). IL-12 p40 secreted by human monocytes or DCs can be immunoprecipitated with p40-specific antibodies, indicating the p40 that is secreted in excess over the heterodimers and is present as free chains. IL-12 p40 is secreted by transfected cells both as a disulphide-bonded homodimer and as a monomer. In mice, production of p40 homodimers has been observed in vivo (Trinchieri et al., Citation2003).
In this study, the MeOH extract significantly inhibited the production of pro-inflammatory cytokines. Subsequently, all isolated compounds from the MeOH extract of N. mirabilis were tested for their inhibitory effects on the production of the pro-inflammatory cytokines IL-12 p40, IL-6, and TNF-α at various concentrations (0.1, 0.2, and 1.0 μM). SB203580, an inhibitor of cytokine suppressive binding protein/p38 mitogen-activated protein kinase, was used as a positive control, which inhibited IL-12 p40, IL-6, and TNF-α production (IC50 values of 5.00 ± 0.16, 3.50 ± 0.12, and 7.20 ± 0.13 μM, respectively). The results obtained from the 3 -(4,5-dimethyl-2,5 thiazolyl)-2,5 diphenyl tetrazolium bromide (MTT) assays showed that the isolated compounds (1–26) did not exhibit significant cytotoxicity at concentrations up to 20.0 μM at 24 h (data not shown).
Of the compounds tested, naphthalene derivatives (1–7, 9, and 10), phenolic compounds (11–15), lupeone (18), and flavonoids (22, 25, and 26) showed potent inhibition of IL-12 p40 production, with IC50 values ranging from 0.17 ± 0.02 to 4.19 ± 0.10 μM. In particular, IL-12 p40 inhibition by compounds 1–7, 9–15, 18, 22, 25, and 26 was the most effective and was greater than the positive control (). This variability in inflammatory response inhibition by compounds 1–7, 9–15, 18, 22, 25, and 26 may be explained by secretion of different levels of inflammatory factors upon LPS-stimulation. Among the tested compounds, nine naphthalene derivatives (1–7, 9, and 10) had inhibitory activities towards LPS-stimulated IL-12 p40 production that were comparable with the positive control, SB203580 (IC50 value of 5.00 ± 0.16 μM).
Table 1. Anti-inflammatory effects of isolated compounds on LPS-stimulated BMDCs.
Several naphthalene containing drugs are at present available, such as nafacillin, naftifine, tolnaftate, and terbinafine, which play vital roles in the control of microbial infection (Kotb et al., Citation2013). Several other synthetic derivatives were also reported to possess significant and satisfactory biological activities. Naphthalene has been identified as having a range of potent inhibitory effects against a variety of human pathogens. It occupies a central place among medicinally important compounds due to its diverse and interesting antibiotic properties with minimum toxicity (Rokade & Dongare, Citation2010). These data suggest that the isolated naphthalene derivatives 1–7, 9, and 10 have significant inhibitory effects on pro-inflammatory cytokine production and warrant further study concerning their potential for medicinal use. Compounds 16, 21, and 24 showed moderate suppressive effects on the production of IL-12 p40 of 35.00 ± 0.86, 29.23 ± 0.75, and 22.18 ± 0.78 μM, respectively ( and ), relative to the control group. The effects of these compounds on the production of the pro-inflammatory cytokines were evaluated at various concentrations in LPS-stimulated BMDCs.
IL-6 is an interleukin that acts as both a pro-inflammatory and an anti-inflammatory cytokine. It leads to inflammation when secreted by T-cells and macrophages in response to trauma, especially burns or other types of tissue damage (Ding et al., Citation2009). It is a cytokine that strongly activates the immune system and enhances inflammatory response, although considering some of its effects it may be classified as having anti-inflammatory interactions. IL-6 is a glycoprotein consisting of 184 amino acid residues, which during post-translational processing ultimately becomes an interconnected structure of four α-helices (Hammacher et al., Citation1994).
Naphthalene derivatives (4, 5, 7, 9, and 10), lignans (15 and 16), lupeone (18), and flavonoids (22, 25, and 26) considerably decreased the production of IL-6 in LPS-stimulated BMDCs with IC50 values ranging from 0.12 ± 0.01 to 12.68 ± 0.48 μM. Compounds 4, 9, 10, 16, 22, and 25 showed strong inhibitory effects on the LPS-stimulated production of IL-6 with IC50 values of 2.15 ± 0.04, 0.46 ± 0.01, 3.87 ± 0.09, 2.83 ± 0.08, 0.12 ± 0.01, and 0.14 ± 0.03 μM, respectively ( and ). The inhibitory effects of compounds 4, 9, 10, 16, 22, and 25 were more potent than the positive control (IC50 value of 3.50 ± 0.12 μM). The remaining compounds did not show significant activity (IC50 values > 50 μM) against IL-6 production ().
Phenolic compounds are a group of chemical substances, many of which are present in plants and are referred to as phytochemicals, and comprise a large variety of structures. Epidemiological studies indicate that consumption of phenol-rich foods reduces the incidence of chronic inflammatory diseases (Yoon & Baek, Citation2005). In fact, phenols are emergent for their therapeutic properties, such as anti-inflammatory, antioxidant, antiallergic, antiviral, antibacterial, and antitumor activities (Molina et al., Citation2003). For these reasons, the phenolic compounds are potential candidates for the development of new anti-inflammatory drugs.
Of the phenolic compounds, flavonoids 22 and 24–26 showed potent pro-inflammatory cytokine production in a dose-dependent manner at concentrations of 0.1, 0.2, and 1.0 μM. Some studies have shown an inverse relationship between consumption of flavonoid-rich foods and pathologies with inflammatory components (Mennen et al., Citation2004). Previous studies have indicated that the potency of anti-inflammatory activity of flavonoids in vivo depends on the patterns and number of hydroxy groups on the B-ring (a 3′,4′-dihydroxy substitution is more active than those with only a single hydroxy group) (Kim et al., Citation2004). Glycosylation also plays an important role in the biological action of flavonoids and flavonoid aglycones are more potent than their corresponding glycosides (Benavente-Garcia & Castillo, Citation2008; Gerritsen et al., Citation1995). The flavonoid glycosides may not penetrate the cell membrane due to their hydrophilicity, or there might be steric impediment due to their large glycosyl residues (Kim et al., Citation1999). However, glucoside acetylation may assist the ability of the compounds to suppress TNF-α expression. An important effect of flavonoids is the scavenging of oxygen-derived free radicals. In vitro experimental systems also showed that flavonoids possess anti-inflammatory, anti-allergic, anti-viral, and anti-carcinogenic properties (Middleton, Citation1998). The beneficial health effects of flavonoids can be attributed in part to their capacity to regulate oxidant production and pro-inflammatory signals. The flavanols are considered important candidates that may be responsible for the beneficial effects of flavanol-rich foods (Knaze et al., Citation2012).
TNF-α, a pro-inflammatory cytokine, is produced by many cell types, including macrophages, lymphocytes, fibroblasts, and keratinocytes, in response to inflammation, infection, and some environmental stresses. The binding of TNF-α to its two receptors (TNFR1 and TNFR2) results in the recruitment of signal transducers that activate at least three distinct effectors (Smith et al., Citation1994). Overexpression of the pro-inflammatory cytokines TNF-α and IL-6 is associated with the development of autoimmune, inflammatory, and immunopathological diseases. Therefore, blocking TNF-α, IL-6, and their respective signaling pathways can be effective in the treatment of inflammatory diseases.
The TNF-α assay results are shown in . 2-Methoxy-7-methyljuglone (9) showed potent inhibitory effects on the production of TNF-α, with an IC50 value of 8.28 ± 0.21 μM. The inhibitory effects of nepenthoside B (4) and 4-hydroxy-2,6-dimethoxyphenyl 6′-O-vanilloyl-β-d-glucopyranoside (12, IC50 values of 21.05 ± 0.92 and 41.99 ± 1.24 μM, respectively) were moderate compared with the positive control, SB203580 (IC50 value of 7.20 ± 0.13 μM), while the remaining compounds did not show significant inhibitory effects on TNF-α production (IC50 values > 50 μM, ).
Phenolic compounds present in fruits, vegetables, and beverages comprise a large array of molecules with biologic activities. Almost all phenolic compounds possess antioxidant properties contributing, in some extent, to the anti-inflammatory activity of plant-derived phenolics. In addition to their antioxidant properties, phenolic compounds could exert an anti-inflammatory action by modulating inflammatory signaling pathways. The identification and the characterization of phenolic compounds that modulate the signaling events involved in inflammation are important not only to validate the use of dietary phenolics in human health, but also to identify new molecules to serve as templates for further structural development of safe and effective anti-inflammatory drugs (Costa et al., Citation2012).
Conclusions
In conclusion, our study suggests that the isolated compounds have an anti-inflammatory effect mediated by suppression of the production of pro-inflammatory cytokines. However, further detailed testing is required to elucidate the exact mechanism of action and any potential impact on other pro-inflammatory mediators of these compounds. To our knowledge, this study is the first to report on the anti-inflammatory activity of chemical components isolated from N. mirabilis.
Declaration of interest
The authors report that they have no conflicts of interest. This work was financially supported by Vietnam National Foundation for Science & Technology Development (No: 104.01–2012.59) and the Priority Research Center Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2009–0093815), Republic of Korea.
References
- Aung HH, Chia LS, Goh NK, et al. (2002). Phenolic constituents from the leaves of the carnivorous plant Nepenthes gracilis. Fitoterapia 73:445–7
- Benavente-Garcia O, Castillo J. (2008). Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem 56:6185–205
- Beutler B, Cerami A. (1986). Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature 320:584–8
- Cannon JR, Lojanapiwatna V, Raston CL, et al. (1980). The quinones of Nepenthes rafflesiana. Aust J Chem 33:1075–93
- Carra G, Gerosa F, Trinchieri G. (2000). Biosynthesis and posttranslational regulation of human IL-12. J Immunol 164:4752–61
- Chi VV. Dictionary of Vietnamese Medicinal Plants. Vol. 2. Ha Noi: Publishing House Medicine; 2012
- Costa G, Francisco V, Lopes MC, et al. (2012). Intracellular signaling pathways modulated by phenolic compounds: Application for new anti-inflammatory drugs discovery. Curr Med Chem 19:2876–900
- Ding C, Cicuttini F, Li J, et al. (2009). Targeting IL-6 in the treatment of inflammatory and autoimmune diseases. Expert Opin Inv Drugs 18:1457–66
- Gately MK, Renzetti LM, Magram J, et al. (1998). The interleukin-12/interleukin-12-receptor system: Role in normal and pathologic immune responses. Immunology 16:495–521
- Gerritsen ME, Carley WW, Ranges GE, et al. (1995). Flavonoids inhibit cytokine-induced endothelial cell adhesion protein gene expression. Am J Pathol 147:278–92
- Gilroy DW, Lawrence T, Perretti M, et al. (2004). Inflammatory resolution: New opportunities for drug discovery. Nat Rev Drug Discov 3:401–16
- Hammacher A, Ward LD, Weinstock J, et al. (1994). Structure-function analysis of human IL-6: Identification of two distinct regions that are important for receptor binding. Protein Sci 3:2280–93
- Kim HK, Cheon BS, Kim YH, et al. (1999). Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure–activity relationships. Biochem Pharmacol 58:759–65
- Kim HP, Son KH, Chang HW, et al. (2004). Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci 96:229–45
- Knaze V, Ros RZ, Barroso LL, et al. (2012). Intake estimation of total and individual flavan-3-ols, proanthocyanidins and theaflavins, their food sources and determinants in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. British J Nutr 108:1095–108
- Koo JE, Hong HJ, Dearth A, et al. (2012). Intracellular invasion of Orientia tsutsugamushi activates inflammasome in ASC-dependent manner. PLoS One 7:e39042
- Kotb ER, Anwar MM, Abbas HAS, et al. (2013). A concise synthesis and antimicrobial activity of a novel series of naphthylpyridine-3-carbonitrile compounds. Acta Poloniae Pharm – Drug Res 70:667–79
- Lawrence T, Willoughby DA, Gilroy DW. (2002). Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Immunology 2:787–95
- Lee JC, Laydon JT, Mcdonnell PC, et al. (1994). A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372:739–46
- Likhitwitayawuid K, Kaewamatawong R, Ruangrungsi N, Krungkrai J. (1998). Antimalarial naphthoquinones from Nepenthes thorelii. Planta Med 64:237–41
- Mennen LI, Sapinho D, Bree AD, et al. (2004). Consumption of foods rich in flavonoids is related to a decreased cardiovascular risk in apparently healthy french women. J Nutr 134:923–26
- Middleton EJ. (1998). Effect of plan flavonoids on immune and inflammatory cell function. Adv Exp Med Biol 439:175–82
- Molina MF, Sanchez-Reus I, Iglesias I, et al. (2003). Quercetin, a flavonoid antioxidant, prevents and protects against ethanol-induced oxidative stress in mouse liver. Biol Pharm Bull 26:1398–402
- Nathan C. (2002). Points of control in inflammation. Nature 420:846–85
- Philip C. (2002). Protein kinases – The major drug targets of the twenty-first century. Nat Rev Drug Dis 1:309–15
- Rischer H, Hamm A, Bringmann G. (2002). Nepenthes insignis uses a C2-portion of the carbon skeleton of l-alanine acquired via carnivorous organs, to build up the allelochemical plumbagin. Phytochemistry 59:603–9
- Rokade Y, Dongare N. (2010). Synthesis and antimicrobial activity of some azetidinone derivatives with the β-naphthol. Rasayan J Chem 3:641–5
- Serhan CN, Chiang N, Dyke TEV. (2008). Resolving inflammation: Dual anti-inflammatory and proresolution lipid mediators. Nat Rev Immunol 8:349–61
- Smith CA, Farrah T, Goodwin RG. (1994). The TNF receptor superfamily of cellular and viral proteins: Activation, costimulation, and death. Cell 76:959–62
- Thanh NV, Thao NP, Dat LD, et al. (2015a). Two novel naphthalene glucosides and other bioactive compounds from the carnivorous plant Nepenthes mirabilis. Arch Pharm Res. [Epub ahead of print]. doi: 10.1007/s12272-015-0576-9
- Thanh NV, Thao NP, Huong PTT, et al. (2015b). Naphthoquinone and flavonoid constituents from the carnivorous plant Nepenthes mirabilis and their anti-osteoporotic and antioxidant activities. Phytochem Lett 11:254–59
- Trinchieri G. (2003). Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 3:133–46
- Trinchieri G, Pflanz S, Kastelein RA. (2003). The IL-12 family of heterodimeric cytokines: New players in the regulation of t cell responses. Immunity 19:641–4
- Walsh NC, Crotti TN, Goldring SR, et al. (2005). Rheumatic diseases: The effects of inflammation on bone. Immunol Rev 208:228–51
- Weinstein DL, O'Neill BL, Metcalf ES. (1997). Salmonella typhi stimulation of human intestinal epithelial cells induces secretion of epithelial cell-derived interleukin-6. Infect Immun 65:395–404
- Wysocka M, Kubin M, Vieira LQ, et al. (1995). Interleukin-12 is required for interferon-γ production and lethality in lipopolysaccharide-induced shock in mice. Eur J Immunol 25:672–6
- Yoon JH, Baek SJ. (2005). Molecular targets of dietary polyphenols with antiinflammatory properties. Yonsei Med J 46:585–96