Abstract
Context: Drug-induced liver injury (DILI) is associated with altering expression of hepatobiliary membrane transporters. Monoammonium glycyrrhizin (MAG) is commonly used for hepatic protection and may have a correlation with the inhibition effect of multidrug resistance-associated protein 2 (Mrp2).
Objective: This study evaluates the dynamic protective effect of MAG on rifampicin (RIF)- and isoniazid (INH)-induced hepatotoxicity in rats.
Materials and methods: Male Wistar rats were randomly divided into four groups of 15 rats. Liver injury was induced by co-treatment with RIF (60 mg/kg) and INH (60 mg/kg) by gavage administration; MAG was orally pretreated at the doses of 45 or 90 mg/kg 3 h before RIF and INH. Rats in each group were sacrificed at 7, 14, and 21 d time points after drug administration.
Results: Liver function, histopathological analysis, and oxidative stress factors were significantly altered in each group. The expression of Mrp2 was significantly increased 230, 760, and 990% at 7, 14, and 21 time points, respectively, in RIF- and INH-treated rats. Compared with the RIF and INH groups, Mrp2 was reduced and Ntcp was significantly elevated by 180, 140, and 160% in the MAG high-dose group at the three time points, respectively. The immunoreaction intensity of Oatp1a4 was increased 170, 190, and 370% in the MAG low-dose group and 160, 290, and 420% in the MAG high-dose group at the three time points, respectively, compared with the RIF and INH groups.
Discussion and conclusion: These results indicated that MAG has a protective effects against RIF- and INH-induced hepatotoxicity. The underlying mechanism may have correlation with its effect on regulating the expression of hepatobiliary membrane transporters.
Introduction
Tuberculosis (TB) remains a major global health problem, especially in developing countries. From the statistical data of the Global Tuberculosis Report of 2014 issued by World Health Organization (WHO), it is reported that an estimated 9 million people developed TB and about 64% of these who developed TB were identified as newly diagnosed cases in 2013. Rifampicin (RIF) and isoniazid (INH) are two effective front line drugs for anti-TB chemotherapy, which have been used for about 50 years. Although RIF and INH hold an irreplaceable status in TB treatment, the hepatotoxicity resulting from their long-term use remains a critical problem in anti-TB therapy (Du et al., Citation2013; Tostmann et al., Citation2008). Serum transaminase levels increased in 27% of patients taking INH/RIF and in 19% of patients taking INH alone. Currently, toxic metabolites of INH and oxidative stress are reported to play a crucial role in RIF- and INH-induced hepatotoxicity, but the exact pathogenic mechanism remains unclear.
In recent years, it is found that drug-induced liver injury (DILI) may be correlated with the alteration of hepatobiliary transporters which expresses at the two polar surface domains of hepatocytes (Schuetz et al., Citation2014; Zhou et al., Citation2013). The expression and/or function of hepatobiliary transporters can be up-/down-regulated by drugs or metabolites (Aleksunes et al., Citation2006; Corsini & Bortolini, Citation2013; Van Staden et al., Citation2012). It is reported that RIF could inhibit bile salt export pump (Bsep), organic anion transporting polypeptides (Oatps), and sodium taurocholate cotransporting plypeptide (Ntcp)-mediated Na+-independent uptake of taurocholate in vitro, this may interfere with normal bile formation and excretion in mammalian liver (Wolf et al., Citation2010). Recent studies have shown that RIF- and INH-induced hepatic injury occurs through the induction of oxidative stress, and hepatic glutathione (GSH), the important marker of oxidative stress, is known be transported from hepatocytes into bile mainly via liver transporter Mrp2 (Paulusma et al., Citation1999). Based on the above findings, it is possible that RIF- and INH-induced liver injury may have a correlation with altered expression or function of certain membrane transporters. Therefore, it is proposed the strategies that focus on regulating the expression of certain transporters, i.e., Oatps, Ntcp, Mrp2, and Bsep, might be useful in RIF- and INH-induced hepatotoxicity.
Chinese herbal formulas, such as compound glycyrrhizin tablets, Potenline®, have been used for hepatic protection, and glycyrrhizin acid is frequently presented in these formulas (Koga et al., Citation2007; Statti et al., Citation2004). Monoammonium glycyrrhizin (MAG), the monoammonium salt of glycyrrhizin acid (q.v.) (Statti et al., Citation2004), is characterized by anti-inflammatory and antiviral activity, and commonly used for the prevention and treatment of viral hepatitis (Fu et al., Citation2004). Recently, Xu et al. (Citation2012) revealed that glycyrrhizin has a protective effect in liver injury, the underlying mechanism may be correlated with its inhibitory effect on the function of Mrp2. It is well known that MAG is the main component of glycyrrhizin, whether MAG can induce alteration of hepatobiliary membrane transporters and be responsible for the liver protective activity of glycyrrhizin is of great interest.
In this study, we hypothesized that the expression of liver transporters might be altered in RIF- and INH-induced hepatotoxicity, while MAG may exert protective activity in RIF- and INH-induced liver injury, and the mechanism may be correlated with its effect on regulating hepatobiliary transporter expression. Liver injury was induced by RIF and INH administration, biochemical and pathological analyses were carried out to evaluate the hepatotoxicity induced by RIF and INH and the hepatoprotective effect of MAG in it. Effect of MAG on the alterations of GSH and malondialdehyde (MDA) levels was also estimated. In addition, the expression of Mrp2, Ntcp, and Oatp1a4 was evaluated by Western blot and immunohistochemstry techniques in order to determine whether liver transporters were altered in RIF- and INH-induced liver injury and whether MAG has regulatory effect.
Materials and methods
Reagents
RIF and INH were purchased from Shanghai Tongyong Pharmaceutical Co., Ltd (Shanghai, China). MAG (purity > 90%, MW = 839.97) was provided by Xinjiang TIANSHAN Pharmaceutical Co., Ltd (Xinjiang, China). Rabbit polyclonal to ABCB11 (lot number: ab112494), mouse monoclonal [M2 III-6] to MRP2 (lot number: ab3373), rabbit polyclonal to SLC10A1 (lot number: ab85611), goat polyclonal secondary antibody to mouse IgG-H&l (HRP) (lot number: ab6789), and goat polyclonal secondary antibody to rabbit IgG-H&l (HRP) (lot number: ab6721) were obtained from Abcam (Hong Kong, China), and rabbit anti-OATP1a4 affinity-purified polyclonal antibody (lot number: JC1690755) was provided by Millipore (Billerica, MA). Other chemicals and reagents were of analytical grade and readily available from commercial sources.
Animals
Male Wistar rats (180–220 g) were obtained from the Laboratory Animal Center of Lanzhou University and maintained at 25 °C for 12 h alternating light–dark cycle with free access to food and water. Rats were maintained in laboratory conditions for 1 week to adapt to the environment before experiments. All studies were carried out in accordance with animal experimental protocols approved by the Institutional Animal Experimentation Committee of Lanzhou University.
Experimental design
Rats were randomly divided into four groups, i.e., control group, RIF and INH group, MAG low-dose group, and MAG high-dose group, each group had 15 rats. Rats in the RIF and INH group received RIF (60 mg/kg) and INH (60 mg/kg) by gavage administration once daily; rats in MAG groups were pretreated with MAG at the doses of 45 or 90 mg/kg, RIF (60 mg/kg) and INH (60 mg/kg) were given 3 h after MAG administration; rats in the control group were treated with saline. To evaluate the dynamic effect of drugs, rats in each group were sacrificed on 7, 14, and 21 d after drug administration. At each time point, five rats were randomly selected and anesthetized with ether, blood was collected by abdominal aortic puncture, and serum was obtained for biochemical analysis. The livers were harvested immediately, a portion of liver was fixed in 10% formaldehyde for histological analysis, the remaiders were frozen with liquid nitrogen and stored at −80 °C for GSH and MDA measurements as well as western blot analysis.
Biochemistry parameters
Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBIL), direct bilirubin (DBIL), indirect bilirubin (IBIL), and total bile acids (TBA) were determined using kits (Thermo Fisher Scientific, Cincinnati, OH) according to the protocols of the manufacturer. The determination was performed using the standard clinical method by Automatic Biochemistry Analyzer (OLYMPUS, AU2700, Tokyo, Japan).
Histopathology examination
The liver tissue was fixed in 10% formaldehyde for 24–48 h and embedded in paraffin. Liver sections (5 μm in thickness) were stained with hematoxylin and eosin. The light microscopy digital images were acquired with a scanscope digital slide scanner. A researcher blinded to treatment counted five random fields at a 200 magnification for each section. Histological assessments were graded using the histological activity index (HAI) according to the criteria of Knodell et al. (Citation1981).
Measurement of GSH and MDA levels
The level of GSH was measured by the colorimetric microplate assay kit (Beyotime Institute of Biotechnology, Beijing, China), the extent of lipid peroxidation in the livers was assessed by measuring the thiobarbituric acid-reactive substances with a lipid peroxidation MDA assay kit (Beyotime Institute of Biotechnology, Beijing, China). According to the protocols of the manufacturer, both GSH and MDA levels were measured in the liver tissue homogenate and calculated in µmol/mg liver. Briefly, 40 μL metaphosphoric acid were added into 10 μL liver homogenates and then centrifuged at 10 000 g for 10 min at 4 °C. The supernatant was used for GSH and MDA assay (Chen et al., Citation2012; Zhu et al., Citation2008).
Western blot analysis
Liver tissue was homogenized in RIPA buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA, 1.0% Nonidet P-40, 0.1% sodium dodecyl sulfate (SDS), 0.5% sodium deoxycholate, and PMSF) and centrifuged at 14 000 g for 10 min at 4 °C, the supernatants were harvested. After determination of protein content, the samples were denatured at 100 °C for 5 min. Protein samples were separated by 8% SDS-PAGE gel and transferred to PVDF membrane (Bio-Rad, Hercules, CA). Non-specific binding sites were blocked with 5% non-fat milk for 1 h at room temperature, and then the membranes were incubated overnight at 4 °C with rabbit anti-MRP2 (1:1500, Abcam, Shatin, Hong Kong), rabbit anti-SLC10A1 (1:3000, Abcam, Shatin, Hong Kong). The membrane was then incubated with secondary antibodies for 1 h at room temperature. The immunoreaction was detected with an ECL western blotting kit (GE Healthcare, Amersham, UK). Bands were visualized on X-ray film (Kodak, Shanghai, China) and captured by a scanner. The scanned digital images were quantified with ImageJ 1.37c software (NIH, Bethesda, MD). In order to adjust for any variations in loading, all detected protein bands were normalized to β-actin levels.
Immunohistochemistry
The sections (5 μm thick) were deparaffinized and rehydrated for immunohistochemistry, the DAB method was performed as described (Morimoto et al., Citation2003). Primary antibody Oatp1a4 (1:100, Calbiochem, National Harbor, MD) was incubated overnight at 4 °C, followed by the labeled streptavidin biotin. The primary antibody was omitted on one section of each reaction as a negative control. All the sections were counterstained with hematoxylin for nuclei labeling. The immunoreaction products were observed under a light LEICA microscope equipped with a LEIKA color digital camera system (LEIKA, San Francisco, CA), images were captured with 40× and analyzed using ImageJ 1.37c software (NIH, Bethesda, MD) by an investigator blinded to the sample groups.
Statistical analysis
Data are presented as mean ± standard derivation (SD). Differences between two groups were analyzed by Student’s t-test (St). When multiple groups were compared, data were analyzed by analysis of variance followed by the Student–Newman–Keuls (SNK) test. p < 0.05 was considered to be statistically significant.
Results
MAG reversed RIF- and INH-induced alteration in liver function
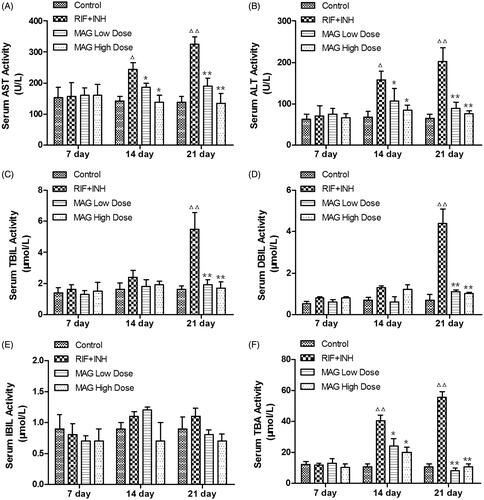
As shown in , the levels of serum ALT, AST, TBIL, and TBA were markedly elevated at 14 and 21 d time points after RIF and INH administration when compared with that of the control, suggesting the occurrence of liver injury induced by RIF and INH. Low- and high-dose MAG treatment significantly reduced the AST, ALT, TBIL, and TBA levels at 14 and 21 d time points when compared with that of the RIF and INH group, suggesting the protective effect of MAG on RIF- and INH-induced liver injury. The level of DBIL in the RIF and INH group was significantly elevated at 21 d time point when compared with that of the control group, while MAG administration significantly and dose dependently reversed the elevated DBIL level at this time point. No significant differences were found in the IBIL level among the control group, the RIF and INH group, as well as low- and high-dose MAG-treated groups.
Figure 1. Effect of MAG on serum AST, ALT, TBIL, DBIL, IBIL, and TBA activities in control, RIF + INH, MAG low-dose, and MAG high-dose groups. RIF + INH group: co-administered with RIF (60 mg/kg) and INH (60 mg/kg). MAG low-dose group: co-administered with MAG at 45 mg/kg and RIF/INH at 60/60 mg/kg. MAG high-dose group: co-administered with MAG at 90 mg/kg and RIF/INH at 60/60 mg/kg. Data are shown as mean ± SD. Each group at each time point contained at least five rats. Δp < 0.05 versus the control group; ΔΔp < 0.01 versus the control group; *p < 0.05 versus the RIF + INH group; **p < 0.01 versus the RIF + INH group.
MAG reversed RIF- and INH-induced histopathology changes in liver
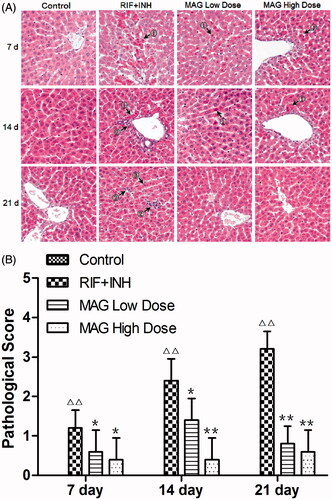
As shown in , the control group showed a normal histological architecture, while histopathological feature of inflammation and necrosis was found in RIF- and INH-treated rats. Histological sections in high-dose MAG-treated rats showed patterns similar to those of the control group after 21 d treatment, that is, with normal architecture and without acidophilic, pyknosis, steatosis, inflammation, or necrosis. As shown in , results from histopathological evaluation showed that HAI scores in the RIF and INH group was significantly and time dependently higher at 7, 14, and 21 d time point when compared with that of the control, indicating the liver damage caused by RIF and INH administration. The HAI scores in low- and high-dose MAG-treated rats were significantly and dose dependently decreased at each time point.
Figure 2. Histopathology changes of control, RIF + INH, MAG low-dose, and MAG high-dose groups after treated for 7, 14, and 21 d. (A) Photomicrography of liver sections; (B) statistical analysis of HAI (histological activity index) scores. ① acidophilic and pyknosis ② inflammation and necrosis ③ fatty degeneration. Images of all groups were taken at ×200 magnification. Data are shown as mean ± SD. Each group at each time point contained five rats. Δp < 0.05 versus the control group; ΔΔp < 0.01 versus the control group; *p < 0.05 versus the RIF + INH group; **p < 0.01 versus the RIF + INH group.
Effect of MAG on GSH and MDA levels in liver
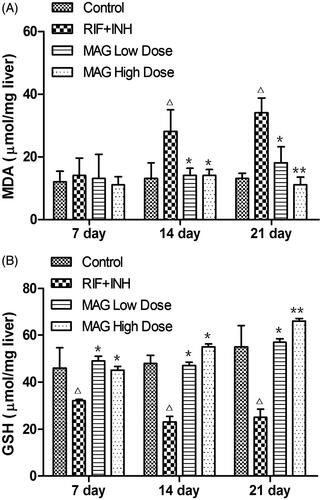
As shown in , compared with the control group, RIF and INH treatment significantly increased the hepatic MDA level at 14 and 21 d time points, while the GSH level was gradually decreased at 7, 14, and 21 d time points, suggesting the liver damage caused by the administration of RIF and INH. MAG treatment groups elevated the hepatic GSH level at 7, 14, and 21 d time points and markedly reduced the MDA level at 14 and 21 d time points in RIF- and INH-treated rats, suggesting the protective effect of MAG in RIF- and INH-induced liver injuries.
Figure 3. Effect of MAG on GSH and MDA levels in control, RIF + INH, MAG low-dose, and MAG high-dose treated groups. RIF + INH group: co-administered with RIF (60 mg/kg) and INH (60 mg/kg). MAG low-dose group: co-administered with MAG (45 mg/kg) and RIF/INH at 60/60 mg/kg. MAG high-dose group: co-administered with MAG (90 mg/kg) and RIF/INH at 60/60 mg/kg. Data are shown as mean ± SD. Each group at each time point contained five rats. Δp < 0.05 versus the control group; ΔΔp < 0.01 versus the control group; *p < 0.05 versus the RIF + INH group; **p < 0.01 versus the RIF + INH group.
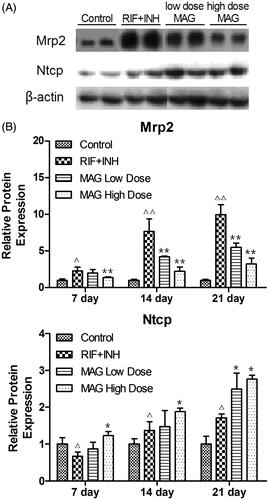
Effect of MAG on transporter Mrp2 and Ntcp expression
As shown in , compared with the control group, the expression of sinusoidal efflux transporter Mrp2 was significantly increased by 230, 760, and 990%, respectively, at 7, 14, and 21 d time points in RIF- and INH-treated rats. Conversely, compared with RIF- and INH-treated rats, Mrp2 expression in rats treated with low-dose MAG was reduced 87, 54, and 55% at 7, 14, and 21 d time points, respectively (); while in rats treated with high-dose MAG was decreased by 61, 29, and 32% at 7, 14, and 21 d time points, respectively ().
Figure 4. Western blot analysis of Mrp2 and Ntcp from the rats of the control, RIF + INH, MAG low-dose, and MAG high-dose groups. (A) The representative western immunoblots of Mrp2 and Ntcp at 21-d time point; (B) the statistical analysis of mean relative protein expression (normalized to control rats). Each group at each time point contained at least five rats. Δp < 0.05 versus the control group; ΔΔp < 0.01 versus the control group; *p < 0.05 versus the RIF + INH group; **p < 0.01 versus the RIF + INH group.
Compared with the control group, the expression of Ntcp in the RIF- and INH-treated group was reduced to 66% at 7 d time point, and then increased by 140 and 170% at 14 and 21 d time points, respectively (). Compared with RIF- and INH-treated rats, the expression of Ntcp was significantly elevated by 180, 140, and 160% in the MAG high-dose group at 7, 14, and 21-d time points, respectively (); while in the MAG low-dose group, the Ntcp expression was significantly increased by 140% at 21 d time point, no significant differences were found at 7 and 1 d time points.
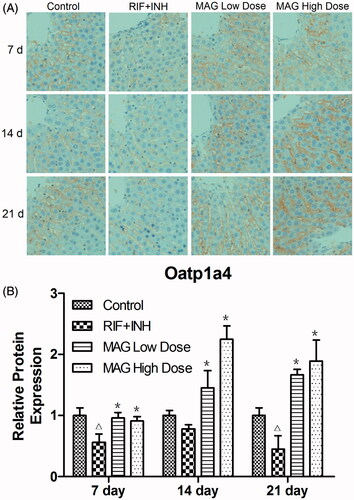
Effect of MAG on expression of Oatp1a4
As shown in , the immunoreaction of Oatp1a4 was strongly evident in hepatic sinusoid and mainly localized around central vein of liver tissue. Compared with the control group, the immunoreaction of Oatp1a4 was decreased by 56, 78, and 45% in the RIF- and INH-treated group at 7, 14, and 21 d time points, respectively; the alterations in each time point showed significant differences except 14 d time point, which may come from the adaptive change of body to liver injury caused by RIF and INH (). MAG treatment significantly increased Oatp1a4 staining intensity when compared with that in RIF- and INH-treated rats, the Oatp1a4 immunoreaction intensity was increased by 170, 190, and 370% in the MAG low-dose group and 160, 290, and 420% in the MAG high-dose group at 7, 14, and 21 d time points, respectively.
Figure 5. Immunohistochemical images and statistical results of Oatp1a4 in liver tissue from rats of the control, RIF + INH, MAG low-dose, and MAG high-dose groups. The data were presented as representative pictures (A) and as mean relative Oatp1a4 protein expression ± SD (B). Each group at each time point contained at least 15 immunohistochemistry images. Δp < 0.05 versus the control group; ΔΔp < 0.01 versus the control group; *p < 0.05 versus the RIF + INH group; **p < 0.01 versus the RIF + INH group.
Discussion
RIF and INH are two important anti-TB medicines, but their hepatotoxicity remains a difficult problem in anti-TB therapy, and the exact pathogenic mechanism about RIF- and INH-induced hepatotoxicity remains unclear. In this present work, we first demonstrated that the expression of transporter Mrp2, Ntcp, and Oatp1a4 was altered in the liver of RIF- and INH-treated rats, and this alteration was consistent with RIF- and INH-induced liver injuries in a time-dependent manner. We also found that RIF- and INH-induced liver injuries and transporter alteration can be partly reversed by MAG pretreatment, and the potential underlying mechanism may be related to the regulatory effect on oxidative stress.
Results from the present work show that liver function, histopathological features, and oxidative stress factors were significantly altered in RIF- and INH-treated rats, and the alteration was presented in a time-dependent manner, suggesting that the severity of liver injury induced by RIF and INH had close correlation with long-term drug usages (Pal et al., Citation2006). Coincided with our findings, the hepatotoxicity induced by RIF and INH administration was also observed and reported by other investigators (Jatav et al., Citation2014; Santhosh et al., Citation2006, Citation2007). Our results also showed that MAG treatment reversed the RIF- and INH-induced liver injuries, and the effect of high-dose MAG-treated group was superior to that of the low-dose-treated group, suggesting the protective effect of MAG on RIF- and INH-induced liver injuries.
It is reported that the enhanced lipid peroxidation plays an important role in the development of RIF- and INH-induced hepatotoxicity (Dong et al., Citation2014; Saad et al., Citation2010). In this study, a significant increase in the level of MDA together with a significant decrease in GSH activity was found in RIF- and INH-treated rats, suggesting that oxidative stress indeed was involved in the pathogenesis of RIF- and INH-induced liver injuries. Moreover, our results showed that MAG treatment significantly decreased the MDA level and markedly increased the GSH level. These results indicated that the protective effect of MAG on RIF- and INH-induced hepatotoxicity might be associated with its strong anti-oxidative effect. Recently, it was reported that the alteration of hepatobiliary transporters may be responsible for drug-induced liver injury, and RIF has been discovered to inhibit transporter Oatps- and Ntcp-mediated uptake of taurocholate in vitro (Vavricka et al., Citation2002). In this study, we found that the expression of Mrp2 was elevated time dependently after RIF and INH treatment, this finding was consistent with previous report, in which the up-regulation of Mrp2 expression has be considered to have correlation with RIF-mediated pregnane X receptor (PXR) activation (Tirona et al., Citation2003, Citation2004). It is well known that GSH is transported from hepatocytes into bile mainly through Mrp2 (Paulusma et al., Citation1999; Saad et al., Citation2010), which may explain why the level of GSH was decreased in RIF- and INH-treated rats. Moreover, our results showed that MAG treatment significantly and dose dependently reversed the elevated expression of Mrp2 in RIF- and INH-treated rats in a time-dependent manner, which may resulted in reduction of GSH transportation from hepatocytes into bile, and thus the GSH level was elevated in liver tissue. It is possible that the elevated GSH level in MAG-treated rats may exert antioxidative activity and thus play a protective role in RIF- and INH-induced hepatotoxicity.
Bilirubin is an endogenous molecule that is a byproduct of heme catabolism produced predominantly from red blood cells. It has been reported that transporter Oatp1a4 transports unconjugated or conjugated bilirubin from blood to hepatocytes (Reichel et al., Citation1999), while Mrp2 is responsible for bilirubin glucuronides excretion from the hepatocytes into the bile (Wolf et al., Citation2010). In this present study, reduced Oatp1a4 and elevated Mrp2 expression have been found in RIF- and INH-treated rats, this alteration is consistent with the increase of serum bilirubin and unconjugated bilirubin level, suggesting that Oatp1a4 and Mrp2 both play an important role in keeping the homeostasis of bilirubin. As we expected, MAG treatment markedly reversed RIF- and INH-induced alteration in Oatp1a4 and Mrp2 expression, both contributed to converting the elevated bilirubin level to a normal level.
Nowadays, rats hepatobiliary transporter Ntcp and Mrp2 have been viewed as two major possible mechanisms by which bile acids (BAs) are transported (Gonzalez, Citation2012; Ridlon et al., Citation2006). Our results showed that serum TBA was significantly increased after treatment of RIF and INH for 14 and 21 d, this alteration was consistent with elevated Ntcp and Mrp2 protein expression. It is possible that, in RIF- and INH-treated rats, the increased expression of Ntcp leads to more conjugated BAs uptake to hepatocytes and the elevated Mrp2 leads to the bile efflux to bile duct, which resulted in the increased re-absorption of BAs in the intestinal, thus serum TBA was finally elevated. MAG treatment dose dependently down-regulated transporter Ntcp and Mrp2 protein expression in RIF- and INH-treated rats, which may contribute to its effect on reversing the elevated TBA level in RIF and INH induced liver injury, and thus maintain the homeostasis of BAs.
Conclusion
This study comprehensively characterized the notable alterations in hepatobiliary transporters Mrp2, Ntcp, and Oatp1a4 expressions, in the protective effect of MAG on RIF- and INH-induced hepatotoxicity for the first time. This study indicated that the protective effects of MAG on RIF- and INH-induced liver injuries have close correlation with its effect on regulating the expression of hepatobiliary membrane transporters. The coordinated reduction of efflux transporter expression (e.g., Mrp2) together with a corresponding increase of uptake carriers expression (e.g., Oatp1a4) suggest a protective mechanism of MAG for maintaining bilirubin and BAs homeostasis in RIF- and INH-induced hepatotoxicity. A better understanding on the altered expression of Ntcp (decreased at 7 d time point and increased at 14 and 21 d time points, respectively) in the RIF and INH group is necessary to address the functional contribution of transport mechanisms to the changed hepatic of xenobiotics during injury as well as conferring resistance to subsequent toxicant exposure.
Declaration of interest
The authors report no conflict of interest. This present study was financially supported by a grant from the National Natural Science Foundation of China (Nos. 81373494 and 81041086).
Acknowledgements
The authors would like to thank the department of pathology in the first hospital of Lanzhou University for technical assistance.
References
- Aleksunes LM, Scheffer GL, Jakowski AB, et al. (2006). Coordinated expression of multidrug resistance-associated proteins (Mrps) in mouse liver during toxicant-induced injury. Toxicol Sci 89:370–9
- Chen T, Zhang L, Yue JQ, et al. (2012). Prenatal PFOS exposure induces oxidative stress and apoptosis in the lung of rat off-spring. Reprod Toxicol 33:538–45
- Corsini A, Bortolini M. (2013). Drug-induced liver injury: The role of drug metabolism and transport. J Clin Pharmacol 53:463–74
- Dong Y, Huang J, Lin X, et al. (2014). Hepatoprotective effects of yulangsan polysaccharide against isoniazid and rifampicin-induced liver injury in mice. J Ethnopharmacol 152:201–6
- Du H, Chen X, Fang Y, et al. (2013). Slow N-acetyltransferase 2 genotype contributes to anti-tuberculosis drug-induced hepatotoxicity: A meta-analysis. Mol Biol Rep 40:3591–6
- Fu Y, Hsieh TC, Guo J, et al. (2004). Licochalcone-A, a novel flavonoid isolated from licorice root (Glycyrrhiza glabra), causes G2 and late-G1 arrests in androgen-independent PC-3 prostate cancer cells. Biochem Biophys Res Commun 322:263–70
- Gonzalez FJ. (2012). Nuclear receptor control of enterohepatic circulation. Compr Physiol 2:2811–28
- Jatav SK, Kulshrestha A, Zacharia A, et al. (2014). Spirulina maxima protects liver from isoniazid and rifampicin drug toxicity. J Evid Based Complementary Altern Med 19:189–94
- Knodell RG, Ishak KG, Black WC, et al. (1981). Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1:431–5
- Koga K, Kawashima S, Shibata N, Takada K. (2007). Novel formulations of a liver protection drug glycyrrhizin. Yakugaku Zasshi 127:1103–14
- Morimoto R, Hayashi M, Yatsushiro S, et al. (2003). Co-expression of vesicular glutamate transporters (VGLUT1 and VGLUT2) and their association with synaptic-like microvesicles in rat pinealocytes. J Neurochem 84:382–91
- Pal R, Vaiphei K, Sikander A, et al. (2006). Effect of garlic on isoniazid and rifampicin-induced hepatic injury in rats. World J Gastroenterol 12:636–9
- Paulusma CC, Van Geer MA, Evers R, et al. (1999). Canalicular multispecific organic anion transporter/multidrug resistance protein 2 mediates low-affinity transport of reduced glutathione. Biochem J 338:393–401
- Reichel C, Gao B, Van Montfoort J, et al. (1999). Localization and function of the organic anion-transporting polypeptide Oatp2 in rat liver. Gastroenterology 117:688–95
- Ridlon JM, Kang DJ, Hylemon PB. (2006). Bile salt biotransformations by human intestinal bacteria. J Lipid Res 47:241–59
- Saad EI, El-Gowilly SM, Sherhaa MO, Bistawroos AE. (2010). Role of oxidative stress and nitric oxide in the protective effects of alpha-lipoic acid and aminoguanidine against isoniazid-rifampicin-induced hepatotoxicity in rats. Food Chem Toxicol 48:1869–75
- Santhosh S, Sini TK, Anandan R, Mathew PT. (2006). Effect of chitosan supplementation on antitubercular drugs-induced hepatotoxicity in rats. Toxicology 219:53–9
- Santhosh S, Sini TK, Anandan R, Mathew PT. (2007). Hepatoprotective activity of chitosan against isoniazid and rifampicin-induced toxicity in experimental rats. Eur J Pharmacol 572:69–73
- Schuetz JD, Swaan PW, Tweedie DJ. (2014). The role of transporters in toxicity and disease. Drug Metab Dispos 42:541–5
- Statti GA, Tundis R, Sacchetti G, et al. (2004). Variability in the content of active constituents and biological activity of Glycyrrhiza glabra. Fitoterapia 75:371–4
- Tirona RG, Leake BF, Podust LM, Kim RB. (2004). Identification of amino acids in rat pregnane X receptor that determine species-specific activation. Mol Pharmacol 65:36–44
- Tirona RG, Leake BF, Wolkoff AW, Kim RB. (2003). Human organic anion transporting polypeptide-C (SLC21A6) is a major determinant of rifampin-mediated pregnane X receptor activation. J Pharmacol Exp Ther 304:223–8
- Tostmann A, Boeree MJ, Aarnoutse RE, et al. (2008). Antituberculosis drug-induced hepatotoxicity: Concise up-to-date review. J Gastroenterol Hepatol 23:192–202
- Van Staden CJ, Morgang RE, Ramachandran B, et al. (2012). Membrane vesicle ABC transporter assays for drug safety assessment. Curr Protoc Toxicol doi: 10.1002/0471140856.tx2305s54. Chapter 23, Unit 23 5
- Vavricka SR, Van Montfoort J, Ha HR, Meier PJ, Fattinger K. (2002). Interactions of rifampicin SV and rifampicin with organic anion uptake systems of human liver. Hepatology 36:164–72
- Wolf KK, Vora S, Webster LO, et al. (2010). Use of cassette dosing in sandwich-cultured rat and human hepatocytes to identify drugs that inhibit bile acid transport. Toxicol In Vitro 24:297–309
- Xu R, Zhang X, Yang J, et al. (2012). Effects of glycyrrhizin on biliary transport and hepatic levels of glutathione in rats. Biopharm Drug Dispos 33:235–45
- Zhou Y, Zhang GQ, Wei YH, et al. (2013). The impact of drug transporters on adverse drug reaction. Eur J Drug Metab Pharmacokinet 38:77–85
- Zhu MT, Feng WY, Wang B, et al. (2008). Comparative study of pulmonary responses to nano- and submicron-sized ferric oxide in rats. Toxicology 247:102–11
