Abstract
Context: Previous studies demonstrated that sodium tanshinone IIA sulfonate (STS) could inhibit MDV replication in vitro. The mechanism about how STS inhibits MDV replication is still not well understood.
Objective: In this study, we evaluated the effect of STS on gB gene/protein of Marek’s disease virus (MDV).
Materials and methods: The concentration of 0.25 mg/ml of STS was used in this study. Meanwhile, 0.25 mg/ml of acyclovir (ACV) was used as a positive control. About 9–11-d-old embryonated specific-pathogen-free (SPF) chicken eggs were used to prepare CEF cells. CEF cells were infected with MDV 2 h, followed by treatment with STS. Real-time PCR and western blot assay were used to measure the gB (UL27) gene/protein expression in STS treatment group at 24, 48, 72, and 96 h post-infection.
Results: Compared with MDV control, the gB gene copies were significantly decreased in STS and ACV treatment groups at 72 h and 96 h (p < 0.05), both in the DNA and in the mRNA level. Furthermore, the expression of gB protein was also inhibited by STS at 24, 72, and 96 h.
Discussion and conclusion: Our study demonstrated that STS could effectively inhibit the MDV replication by suppressing gB gene/protein expression in cell culture.
Introduction
Tanshinone IIA (Tan IIA), a major active component extracted from the root of Salvia miltiorrhiza Bunge (Labiatae), is known to have various functions including inducing differentiation (Wang et al., Citation2007), anti-inflammatory (Bai et al., Citation2008), and apoptosis activities (Tang et al., Citation2010). In addition, tanshinone IIA was also reported to have inhibitory effects on tumor cells, such as human colon adenocarcinoma cells (Su et al., Citation2008), human hepatocellular carcinoma cells (Chien et al., Citation2012), and prostate cancer cells (Chiu et al., Citation2013).
Sodium tanshinone IIA sulfonate (STS), a water-soluble derivative of tanshinone IIA (Tan IIA), is widely used in treating cardiovascular diseases for a long time (Zhou et al., Citation2003). Our previous studies suggested that STS was capable of inhibiting PRRSV replication in vitro (Sun et al., Citation2012, Citation2014b) and it also inhibited the replication and proliferation of Marek’s disease virus (MDV) demonstrated by cytopathic effect reduction (CPE), plaque forming reduction, and MTT method in vitro. Moreover, it has been confirmed that STS had an inhibitory effect on the expression of Meq and UL49 genes of MDV (Sun et al., Citation2014a).
Similar to most alphaherpes viruses, MDV is considered to be highly cell-associated in vitro, and virions could spread from cells to cells by direct contacts. Glycoprotein B (gB), a viral envelope glycoprotein, is coded by UL27 gene. It is a homolog of α-herpesvirus gB, which plays an essential role in the transmission of MDV. It has been long known that virions with structure integrity can be infectious. gB and other glycoproteins, the key components of MDV envelope, are all required for virion entry into cells. Evidence indicates that gB is the core member of glycoprotein complex in mediating the membrane fusion (Connolly et al., Citation2011; Heldwein & Krummenacher, Citation2008). The analysis of crystal structure of gB and gH/gL suggested that gB is the major protein in initiating membrane fusion process (Chowdary et al., Citation2010; Heldwein et al., Citation2006).
Vaccination is the main strategy to control MD. However, vaccination could not prevent MDV from spreading in vaccinated birds (Subramaniam et al., Citation2013). Thus, new approach is needed to eliminate MD. Our previous study demonstrated that STS could inhibit MDV replication in vitro. The mechanisms about how STS protected host from MDV virions and involvement of gB on virus replication are still not well understood. In this study, we investigated the anti-MDV mechanisms of STS, targeting on UL27 (gB) gene/protein of MDV.
Materials and methods
Cell cultures
Chick embryo fibroblasts (CEF) cultures were prepared from specific-pathogen-free (SPF) eggs following the standard methods (Xing & Schat, Citation2000). CEF cells were maintained in DMEM supplemented 10% fetal bovine serum (FBS) and penicillin-streptomycin at 37°C with a humidified, 5% CO2 atmosphere.
Viruses
MDV vaccine strain (Merial Select, Inc., Monett, MO, Vet. license no. 279) was propagated in CEF which was infected with 100 PFU/0.25 ml virus suspension.
Materials and reagents
STS (catalog number: 111605) and acyclovir (ACV) (catalog number: 140630) were purchased from National Institutes for Food and Drug Control (Beijing, PR China). Specific-pathogen-free (SPF) embryonated eggs were purchased from Longkeer Company (Shanxi, PR China). 2 × Es Taq MasterMix (CW0690) was obtained from Cowin Bio-Technology Limited (Seattle, WA). RNAiso Plus, SYBR® Premix Ex Taq™ II kit, PrimeScriptTM RT reagent Kit with gDNA Eraser and gel extraction kit were obtained from Takara Bio Inc (Dalian, PR China). Total protein extraction Kit was purchased from Applygen (Beijing, PR China). Anti-gB monoclonal antibody is a gift from Prof. Qing Ai-jian of Yangzhou University.
DNA extraction
CEF was prepared by using 9–11-d-old embryonated specific pathogen-free (SPF) chicken eggs. CEF cells (2 × 106/ml) in a 6-well plate were infected with MDV for 2 h. After washed twice with PBS, cells were treated with STS at the concentration of 0.25 mg/ml. ACV was used as a positive control. Total DNA was extracted from the infected and uninfected cells using the phenol/chloroform/isopentanol extraction method, a conventional method. The concentration of total DNA was determined using the NanoDrop 1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE).
Construction of recombinant plasmid
Primer sequences were as follows: forward 5′-GTCAAGCGTAACTTTCTCA-3′ and reverse 5′-CCATAAATCTGTATCTCCC-3′ and the PCR production length was 145 bp. PCR for DNA was performed by using 2 × Es Taq MasterMix (CWbio Inc., Beijing, China) for 35 cycles of 94 °C for 30 s; 51.5 °C for 30 s and 72 °C for 30 s. Products were separated by electrophoresis on 2% agarose gels and visualized under UV light. The amplified PCR fragments of gB gene of MDV were purified by gel extraction kit, respectively, and ligated to T-vector. Plasmids containing gB gene were confirmed by sequencing and used to generate the standard curve for real time PCR.
RNA extraction
Total RNA from CEF or MDV-infected CEF were extracted using RNAiso Plus (Takara Bio Inc., Dalian, PR China, Code no. 9108), and transcribed reversely to cDNA with PrimeScriptTM RT reagent Kit with gDNA Eraser (Takara Bio Inc., Dalian, PR China).
Real-time PCR
To examine UL27 gene expression, real-time PCR were performed using IQ™ SYBR Green Supermix (Bio-Rad, Hercules, CA) for 40 cycles of 95 °C for 5 s and 51.5 °C for 30 s, according to instruction providing in SYBR® Premix Ex Taq™ II kit (Takara Bio Inc., Dalian, PR China).
Western blot analysis
Total proteins were extracted according to the instruction of a total protein extraction kit (Applygen, Beijin, PR China). Western blot analysis was performed to detect virus glycoprotein gB at different time points. Proteins were separated on SDS-PAGE (15%) followed by transferring onto a PVDF membrane and blocked with 5% nonfat dry milk. Subsequently, the membrane was incubated with monoclonal antibodies against gB (1:100 dilution) and β-actin (1:1000 dilution) antibodies against β-actin overnight at 4°C. After washed thrice with PBST (PBS plus 0.1% Tween-20) for 10 min each wash. The membrane was then incubated with goat anti-mouse secondary antibody IgG (1:1000 dilution) for 1 h at room temperature. Following three 10 min washes with PBST, the protein bands were detected with eECL Western Blot Kit (CWbio Inc., Beijin, China).
Statistical analysis
Data were analyzed by one-way ANOVA implemented in GraphPad Prism version 5.01 (full) software (GraphPad Software, Inc., San Diego, CA). p < 0.05 was considered to be significant.
Results
Effect of STS on MDV gB (UL27) gene expression
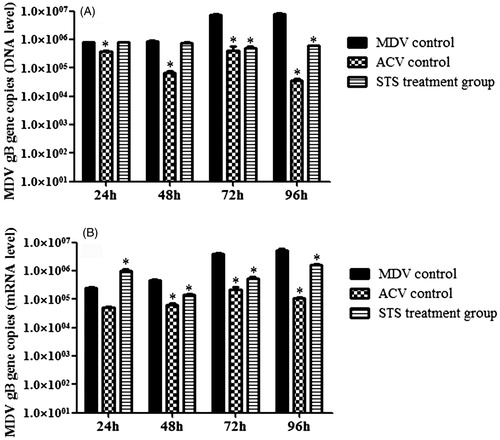
At the level of DNA (), the gB copies in the STS-treated group was much lower than the MDV-infected group at time points of 72 and 96 h, and there was significant difference between the ACV treatment group and the MDV group at the time points of 24, 48, 72, and 96 h (p < 0.05). At the level of mRNA (), compared with the MDV group, the gB (UL27) gene copies in the STS treatment group was higher at 24 h but lower at 48, 72, and 96 h. The gB (UL27) gene copies in the STS group had significant decrease at 48, 72, and 96 h (p < 0.05) as compared with that of the MDV group. Additionally, treatment of ACV significantly decreased the amount of MDV gB (UL27) gene copies at 48, 72, and 96 h (p < 0.05). Taken all these results together, STS could inhibit MDV replication by suppressing gB gene expression in cell culture.
Figure 1. The effect of STS on UL27 (gB) gene expression at different time points. Real-time PCR was used to quantify MDV titers based on gB gene. (A) The effect of STS on UL27 (gB) gene expression at different time points on DNA level. At the level of DNA, addition of STS in MDV infected cells resulted in a decrease in gB (UL27) gene load compared with the virus group at time point of 72 h and 96 h, and there was significant difference (p < 0.05). (B) The effect of STS on UL27 (gB) gene mRNA level at different time points. In the level of mRNA, STS showed a little enhance gB (UL27) gene expression at 24 h, and STS decreased gB (UL27) gene expression compared with the MDV group from 48, 72, and 96 h, and also there was significant difference (p < 0.05). *Significant difference compared with MDV control.
Effect of STS on gB protein
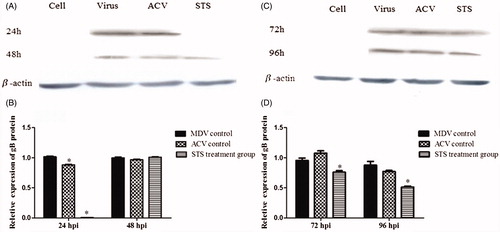
Western blot assay were used to analyze the expression of gB protein in all groups (). A 98-kD protein band was detected in the MDV group, and it could not be detected in the STS treatment group at 24 h. With the longer incubation time, the protein band at 72 and 96 h were weaker in the STS group compared with the MDV group (p < 0.05), which demonstrated that STS had an inhibitory effect on gB protein expression.
Figure 2. STS inhibited protein expression of gB gene in the MDV-infected CEF cells and β-actin was used as an internal control. STS significantly reduced the expression of gB protein (p < 0.05) in the MDV-infected cells after 24 h treatment with STS (A and B). Effect of STS on gB expression for 72 h and 96 h treatment is shown in (C) and (D). The protein band in the STS treatment group was much weaker than the virus group at time points of 72 h and 96 h (p < 0.05). *Significant difference compared with MDV control.
Discussion
Sodium tanshinone IIA sulfonate (STS) is a water-soluble derivative of tanshinone IIA isolated as the main pharmacologically active natural compound from a traditional Chinese herbal medicine, the dried root of Salvia miltiorrhiza known as Danshen. It has been widely used in China to treat cerebrovascular diseases and coronary artery diseases (Zhou et al., Citation2005).
Our previous results demonstrated that the maximum safe concentration of STS for CEF cells is 0.25 mg/ml (Sun et al., Citation2014c) and 0.25 mg/ml STS showed the significant antivirus effect in MDV-infected CEF cells. MDV UL27 gene, one of the MDV late genes, encodes virus glycoprotein gB, a virion important component. It is well known that virus structural integrity is indispensable for virus infection. Exploring the interactions and functions of MDV envelope glycoproteins will provide information for a better understanding of virus entry and assembly, which may lead to potential antiviral targets drug.
In this experiment, both DNA and mRNA of MDV UL27 gene were detected by real-time PCR to study whether STS could inhibit MDV replication. Results demonstrated that UL27 expression on both DNA and mRNA levels were diminished by STS treatment and the analysis by western blotting further indicated that STS interferes gB protein expression. gB, one of the major MDV envelope glycoproteins, plays an essential role in viral replication (Schumacher et al., Citation2000). Herpesvirus gB glycoprotein is the most highly conserved of all surface glycoproteins and primarily involved in the virus entry (Chowdary et al., Citation2010). Moreover, MDV gB glycoprotein has been significantly inhibited in viral replication by RNAi (Chen et al., Citation2008). Additionally, treating with STS could also affect the MDV Meq gene copies and VP22 gene/protein, which play an important role in MDV replication (Sun et al., Citation2014a). Considering all these results together, STS inhibited MDV replication by suppressing multi-genes/proteins related with virus replication.
Conclusion
Inhibition of MDV replication by STS was demonstrated by measuring the expression of gB. Treatment with STS reduced the expression of MDV UL27 gene and STS also decreased the expression of gB protein.
Declaration of interest
The authors report that they have no conflicts of interest. This project was supported by the key scientific and technological grant from Shanxi Province (Grant nos. 2010311047 and 2011021007-4). All experiments comply with the current laws of PR China.
References
- Bai A, Lu N, Guo Y, Fan X. (2008). Tanshinone IIA ameliorates trinitrobenzene sulfonic acid (TNBS)-induced murine colitis. Digest Dis Sci 53:421–8
- Chen M, Payne WS, Hunt H, et al. (2008). Inhibition of Marek's disease virus replication by retroviral vector-based RNA interference. Virology 377:265–72
- Chien SY, Kuo SJ, Chen YL, et al. (2012). Tanshinone IIA inhibits human hepatocellular carcinoma J5 cell growth by increasing Bax and caspase 3 and decreasing CD31 expression in vivo. Mol Med Rep 5:282–6
- Chiu SC, Huang SY, Chen SP, et al. (2013). Tanshinone IIA inhibits human prostate cancer cells growth by induction of endoplasmic reticulum stress in vitro and in vivo. Prostate Cancer Prostatic Dis 16:315–22
- Chowdary TK, Cairns TM, Atanasiu D, et al. (2010). Crystal structure of the conserved herpesvirus fusion regulator complex gH-gL. Nat Struct Mol Biol 17:882–8
- Connolly SA, Jackson JO, Jardetzky TS, et al. (2011). Fusing structure and function: A structural view of the herpesvirus entry machinery. Nat Rev Microbiol 9:369–81
- Heldwein EE, Krummenacher C. (2008). Entry of herpesviruses into mammalian cells. Cell Mol Life Sci 65:1653–68
- Heldwein EE, Lou H, Bender FC, et al. (2006). Crystal structure of glycoprotein B from herpes simplex virus 1. Science 313:217–20
- Schumacher D, Tischer BK, Fuchs W, et al. (2000). Reconstitution of Marek's disease virus serotype 1 (MDV-1) from DNA cloned as a bacterial artificial chromosome and characterization of a glycoprotein B-negative MDV-1 mutant. J Virol 74:11088–98
- Su CC, Chen GW, Kang JC, et al. (2008). Growth inhibition and apoptosis induction by tanshinone IIA in human colon adenocarcinoma cells. Planta Med 74:1357–62
- Subramaniam S, Preeyanon L, Cheng HH. (2013). Transcriptional profiling of Meq-dependent genes in Marek’s disease resistant and susceptible inbred chicken lines. PLoS One 8:e78171
- Sun N, Cong XM, Jiang JB, et al. (2014a). Sodium tanshinone IIA sulfonate inhibits the meq, ul49 and VP22 expression of Marek's disease virus. Antivir Ther 19:793–8
- Sun N, Li E, Wang Z, et al. (2014b). Sodium tanshinone IIA sulfonate inhibits porcine reproductive and respiratory syndrome virus via suppressing N gene expression and blocking virus induced apoptosis. Antivir Ther 19:89–95
- Sun N, Zhao X, Bai XY, et al. (2012). Anti-PRRSV effect and mechanism of sodium tanshinone IIA sulfonate in vitro. J Asian Nat Prod Res 14:721–8
- Sun Y, Niu L, Song MQ, et al. (2014c). Screening compounds of Chinese medicinal herbs anti-Marek's disease virus. Pharm Biol 52:841–7
- Tang C, Xue HL, Huang HB, et al. (2010). Tanshinone IIA inhibits constitutive STAT3 activation, suppresses proliferation, and induces apoptosis in rat C6 glioma cells. Neurosci Lett 470:126–9
- Wang J, Wang X, Jiang S, et al. (2007). Growth inhibition and induction of apoptosis and differentiation of tanshinone IIA in human glioma cells. J Neurooncol 82:11–21
- Xing Z, Schat KA. (2000). Expression of cytokine genes in Marek's disease virus infected chickens and chicken embryo fibroblast cultures. Immunology 100:70–6
- Zhou L, Zuo Z, Chow MSS. (2005). Danshen: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol 45:1345–59
- Zhou G, Jiang W, Zhao Y, et al. (2003). Sodium tanshinone IIA sulfonate mediates electron transfer reaction in rat heart mitochondria. Biochem Pharmacol 65:51–7
