Abstract
Context: Argemone mexicana Linn. (Papaveraceae) has been used as traditional medicine in India and Taiwan for the treatment of skin diseases, inflammations, bilious, fever, etc. Some alkaloids of A. mexicana have been screened for their cytotoxicity on different cancer cell lines.
Objective: The study investigates potential cytotoxic effects of alkaloids isolated from aerial part of A. mexicana on SW480 human colon cancer cell line.
Materials and methods: Six alkaloids, 13-oxoprotopine, protomexicine, 8-methoxydihydrosanguinarine, dehydrocorydalmine, jatrorrhizine, and 8-oxyberberine were isolated from the methanol extract of A. mexicana. Cytotoxicity of these alkaloids was studied on SW480 human colon cancer cell line at 1, 25, 50, 75, 100, 125, 150, and 200 µg/mL for 24 and 48 h. Cells were seeded in a 96-well micro-plate at a concentration of 2 × 104 cells per well and MTS assay was performed to assess cytotoxicity in terms of cell viability.
Results: At 200 µg/mL, protomexicine and 13-oxoprotopine showed mild cytotoxicity (∼24–28%) whereas dehydrocorydalmine exhibited moderate cytotoxicity (∼48%). 8-Oxyberberine was mildly cytotoxic (∼27%) at 24 h but was more potent (∼76%) at 48 h. Jatrorrhizine and 8-methoxydihydrosanguinarine were most potent (∼95–100%) in inhibiting the human colon cancer cell proliferation showing complete reduction in cell viability.
Discussion and conclusion: This is the first study on the effect of these alkaloids on SW480 human colon cancer cell line. This study indicates that some alkaloids of A. mexicana strongly inhibit the cell proliferation in human colon cancer cells, and it might be a basis for future development of a potent chemotherapeutic drug.
Introduction
Argemone mexicana Linn. (Papaveraceae) is a spiny herbaceous annual plant which grows mainly in subtropical countries. In India, it generally grows over agricultural waste lands from January to June. It is widely used in the Indian System of Medicine in curing skin diseases, fever, diarrhea, and dysentery (Anonymous, Citation1985). Toxicity of seed oil of A. mexicana was observed due to the presence of physiologically active benzophenanthridine alkaloids, sanguinarine, and dihydrosanguinarine (Das & Khanna, Citation1997). Earlier chemical investigations of this plant had revealed the presence of many alkaloids (Deopke et al., Citation1976; Haisova & Slavik, Citation1975; Mishra et al., Citation1961; Singh et al., Citation2009, Citation2010, Citation2012), phenolics (Bharadwaj et al., Citation1982; Harborne & Williams, Citation1983; Singh et al., Citation2009), and amino acids (Dinda & Bandopadhyay, Citation1986). Some of the isolated alkaloids had shown anti-HIV (Chang et al., Citation2003b), anticancer (Chang et al., Citation2003a), and hepatotoxic activities (Dalvi, Citation1985).
The isoquinoline alkaloids are one of the largest groups of alkaloids including benzylisoquinolines, protopines, benzophenanthridines, and protoberberines. Protoberberine had been shown to cause cytotoxicity in most of the cell lines such as ovarian, breast, lung, gastric, and cervical cancer cell lines (Jing & Zhengwei, Citation2008). Some other alkaloids isolated from this plant had been screened for their cytotoxicity on human gastric cancers (HONE) and nasopharyngeal carcinoma (NUGC) (Chang et al., Citation2003a).
Colon cancer is third most common cancer in the world and its treatment includes surgery, radiation therapy, and chemotherapy (Enker et al., Citation1995). All these ways of treatments have major complications as well as many limitations. In the present study, three tertiary and three quaternary alkaloids were isolated from aerial part of A. mexicana. Cytotoxicity of these alkaloids was studied on SW480 human colon cancer cell line at 24 and 48 h of post-treatment.
Materials and methods
General experimental procedure
Melting points were determined using a Tempo Innovative® melting point apparatus (Merck Millipore, Darmstadt, Germany) and are uncorrected. Absorbance was recorded using Cary 14 UV/Vis/NIR spectrophotometer (Merck Millipore, Darmstadt, Germany). 1H and 13C NMR, DEPT, and HMBC were performed at Bruker HX-90 (Bruker Inc., Billerica, MA) with TMS as an internal reference standard. Mass spectrometry (MS) was performed on Kratos MS-50 mass spectrometer (Kratos Analytical Inc., Arvada, CO) operating at 70 eV with evaporation of sample in the ion source at 200 °C. Thin-layer chromatography (TLC) was monitored on silica gel G (Merck, Darmstadt, Germany)-coated glass plates. Alkaloid spots on TLC were visualized by spraying Dragendorff’s reagent. Cell viability was observed on microtitre plate reader Perkin Elmer R 1420 Multilable Counter Victor 3 TMV (PerkinElmer Health Sciences, Inc., Shelton, CT).
Plant material, extraction, and isolation of alkaloids
The aerial part of A. mexicana (3 kg) was collected in May 2007 from District Varanasi, UP, India, and identified by Prof N. K. Dubey, Department of Botany, BHU, Varanasi (voucher specimen no. 221). Plant material was extracted with methanol as described previously (Singh et al., Citation2009, Citation2010, Citation2012). Briefly, air-dried plant material was coarsely powdered and extracted with methanol in a Soxhlet apparatus. Tertiary and quaternary alkaloid fractions were separated from the methanol extract. Both fractions were subjected to column chromatography. Six compounds (A–F) were obtained and identified with the help of various methods such as mp, UV, IR, 1H and 13C NMR, DEPT, HMBC, and MS spectrometry.
Cell culture
The SW480 human colon cancer cell line was purchased from American Tissue Culture Collection (ATCC, Ontario, Canada). SW480 cells were grown in Leibovitz L-15 medium (Gibco, Omaha, NE) supplemented with 10% fetal calf serum (Gibco, Omaha, NE) and 1% antibiotics penicillin (100 µg/mL) and streptomycin (100 µg/mL). Incubation was carried out at 37 °C in a humidified atmosphere of 100% air without CO2. Cytotoxicity analysis was performed using MTS assay kit purchased from Promega (Madison, WI).
Cytotoxicity assay
For cell culture purpose, all compounds (A–F) were dissolved in DMSO and diluted in growth medium to make the concentration of DMSO to 1%. The same amount of DMSO and media were used for control experiments. All the drug solutions were stored at −10 °C. The cytotoxic activities of isolated alkaloids were evaluated at different concentrations in terms of cell viability. Colon cells SW480 were seeded in a 96-well micro-plate at a concentration of 2 × 104 cells per well. Cells were grown in Leibovitz complete growth media with 10% FBS for 24 h at 37 °C without CO2. Monolayer was washed after 24 h and covered with fresh media having different concentrations (1, 25, 50, 75, 100, 125, 150, and 200 μg/mL) of isolated compounds. Cells grown in non-modified medium served as control.
After the incubation period of 24 and 48 h, culture media were altered by adding 20 µL/well of [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] (MTS) assay (Cell Titer 96®AQueous One Solution Cell Proliferation assay, Promega Corporation, Madison, WI). The cells were then allowed to incubate for 4 h. After 4 h of incubation with MTS, the absorbance in each well was recorded at 490 nm using microtitre plate reader (Perkin Elmer R 1420 Multilable Counter Victor 3 TMV, PerkinElmer Health Sciences, Inc., Shelton, CT). Cell viability (%) was calculated from the following equation:
Cytotoxicity curves were obtained by plotting percentage of viability against different concentrations of different compounds.
DAPI staining
Differences in cell morphological characteristics of apoptotic cells were examined in DAPI (4,6-diamidino-2-phenylindole)-stained cells by fluorescent microscope. Monolayer of cells was fixed with 4% paraformaldehyde for 30 min at ambient temperature. Cells were permeabilized with 0.2% Triton X-100 in PBS for three times and incubated with 1 μg/mL of DAPI for 30 min. Apoptotic nuclei were detected under 200× magnification using a fluorescent microscope.
Statistical analysis
Results are expressed as mean ± standard deviation in triplicates and evaluated by Student’s t-test for significance.
Results and discussion
Chromatographic resolution of the crude tertiary and quaternary base fractions of the aerial part of A. mexicana (3 kg) resulted in the isolation of compounds A–F (). Two protopine type alkaloids: 13-oxoprotopine A (19 mg) and protomexicine B (18 mg); a phenanthridine type alkaloid: 8-methoxydihydrosanguinarine C (21 mg) and three protoberberine type alkaloids: dehydrocorydalmine D (23 mg), jatrorrhizine E (17 mg), and 8-oxyberberine F (24 mg) were isolated and characterized with the help of various spectroscopic data (Singh et al., Citation2009, Citation2010, Citation2012). Homogeneity of isolated compounds was confirmed by single spot on TLC plate. In order to screen their biological activity, the isolated alkaloids were tested for their cytotoxic activity on SW480 colon cancer cell line for 24 and 48 h at different concentrations.
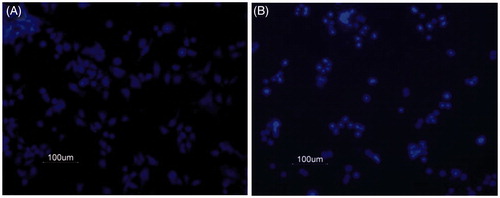
The MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay was preferred over MTT [(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay for cell viability as it requires fast incubation period of only 4 h, with no further solubilization of formazan crystals with DMSO. It is non-radioactive and flexible to use. DAPI staining was performed to examine the morphological characteristics of apoptotic cells. DAPI (4,6-diamidino-2-phenylindole dihydrochloride) is a water soluble, blue fluorescent nucleic acid stain which prefers to stain dsDNA (although it also binds to RNA). In control wells (media plus 1% DMSO, 24 h), cells were more in number and fully stretched indicating alive cells (), whereas in treatment wells, supplied with drug, e.g., 8-methoxydihydrosanguinarine (5 µg/mL, 24 h), cells were less in number and more condensed () indicating the cells that had undergone apoptosis.
Figure 2. DAPI stained control and drug-treated cells. SW480 human colon cancer cells were treated with an alkaloid and stained with DAPI as described in methods. (A) Cells treated with 0.1% DMSO in media alone was taken as control. (B) Cells treated with 5 μg/mL 8-methoxydihydrosanguinarine.
Cytotoxicity of three alkaloids from tertiary alkaloid fraction was studied in terms of % cell viability at 24 and 48 h cultures, as shown in and . 13-Oxoprotopine exhibited mild cytotoxic activity at higher dose of 200 μg/mL. Cell viability was reduced by 24% at 24 h and 28% at 48 h post-treatment. Lesser reduction in cell viability was observed at lower concentrations. Protomexicine is a new protopine type alkaloid reported for the first time from this laboratory (Singh et al., Citation2012). This alkaloid also showed mild cytotoxicity even at higher doses and the reduction in cell viability was observed up to 24% both at 24 and 48 h post-treatment. 8-Methoxydihydrosanguinarine showed strong cytotoxic activity. This drug reduced 34% and 47% cell viability even at a small concentration of 1 μg/mL at 24 and 48 h, respectively. The cell viability was considerably reduced with increase in time and drug concentration. The drug concentration of 100 μg/mL was enough to reduce the cell viability by 98% at 48 h ( and ).
Figure 3. Dose–response curve of 13-oxoprotopine, 8-methoxydihydrosanguinarine, and protomexicine on SW480 colon cancer cell line at 24 h and 48 h post-treatment.
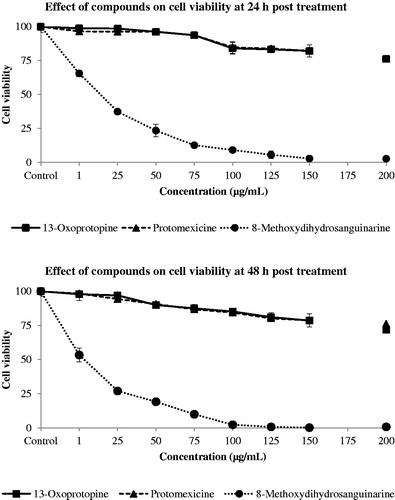
Table 1. Cytotoxicity of 13-oxoprotopine, 8-methoxydihydrosanguinarine, and protomexicine on SW480 colon cancer cell line in terms of % cell viability ± SD at 24 h and 48 h post–treatment.
The above results revealed that protopine-type alkaloids, protomexicine and 13-oxoprotopine, are mild cytotoxic whereas 8-methoxydihydrosanguinarine, a benzophenanthridine alkaloid, is a very potent cytotoxic agent. These results are in conformity with earlier reports that showed protopine type alkaloids possess both mild and moderate cytotoxic activity whereas benzophenanthridine-type alkaloids exhibit strong cytotoxic effect (Alexandrova Citation2000; Chang et al., Citation2003a).
Three protoberberine type alkaloids, dehydrocorydalmine, jatrorrhizine, and 8-oxyberberine isolated from quaternary fraction, were also studied for their cytotoxicity on SW480 colon cancer cell line in vitro. Cytotoxicity of these three compounds in terms of % cell viability at 24 and 48 h is shown in and .
Figure 4. Dose–response curve of dehydrocorydalmine, jatrorrhizine, and 8-oxyberberine on SW480 colon cancer cell line at 24 h and 48 h post-treatment.
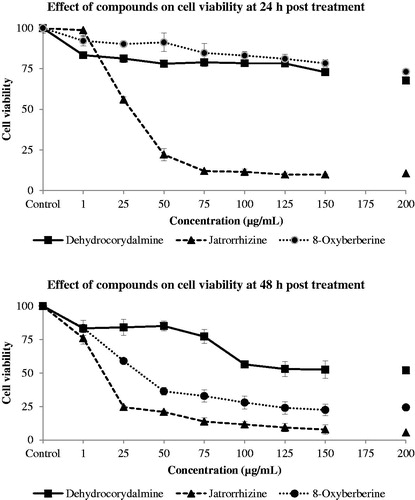
Table 2. Cytotoxicity of dehydrocorydalmine, jatrorrhizine, and 8-oxyberberine on SW480 colon cancer cell line in terms of % cell viability ± SD at 24 h and 48 h culture.
Jatrorrhizine at 25 μg/mL reduced the cell viability by 44 and 76% at 24 and 48 h, respectively. Cell viability was decreased by 90% at 24 and 48 h with 100 μg/mL concentration. Dehydrocorydalmine reduced the cell viability by 32% at 24 h and 48% at 48 h at a concentration of 200 μg/mL. This drug showed stronger cytotoxic activity at 48 h in comparison with 24 h post-treatment. 8-Oxyberberine, an oxo-derivative of berberine, exhibited mild to potent cytotoxicity depending on the time of exposure and concentration used. It reduced cell viability by 27% at a concentration of 200 μg/mL at 24 h. However, cell viability was reduced by more than 76% at a concentration of 125 μg/mL at 48 h post-treatment.
Protoberberine type of alkaloids, dehydrocorydalmine and 8-oxyberberine, showed a time-dependent cytotoxicity effect. They both exhibited more potent cytotoxic effect at 48 h post-treatment. However, jatrorrhizine had a similar cytotoxic effect both at 24 and 48 h post-treatment at higher concentrations. These results showed that jatrorrhizine is a very potent cytotoxic alkaloid but dehydrocorydalmine and 8-oxyberberine are cytotoxic only at higher incubation time periods. This result may be due to the lower cell permeability (thus higher exposure times) of dehydrocorydalmine and 8-oxyberberine alkaloids. The structure of dehydrocorydalmine and jatrorrhizine is quite similar (), but there was a large difference in their cytotoxicity. Dehydrocorydalmine was shown to be moderately cytotoxic only at 48 h but jatrorrhizine has strong cytotoxic effect both at 24 and 48 h. This may be due to the difference in their structures at positions 3 and 10. Thus, probably the presence of methoxy (–OCH3) and hydroxyl (–OH) groups at positions 3 and 10 in dehydrocorydalmine and hydroxyl and methoxy groups at positions 3 and 10 in jatrorrhizine (), respectively, are responsible for greater observed cytotoxicity effects.
Conclusion
Six alkaloids, namely 13-oxoprotopine, protomexicine, dehydrocorydalmine, 8-oxyberberine, 8-methoxydihydrosanguinarine, and jatrorrhizine, were isolated from aerial part of A. mexicana. This study demonstrated the inhibitory effect of these alkaloids on proliferation of SW480 human colon cancer cell line. 13-Oxoprotopine and protomexicine were found to be mild cytotoxic even at higher doses. Dehydrocorydalmine was mild to moderately cytotoxic depending on the period of incubation. 8-Methoxydihydrosanguinarine, jatrorrhizine, and 8-oxyberberine were most potent in inhibiting the proliferation of colon cancer cells in vitro as they exhibited significant reduction in cell viability up to 100%. The inhibitory effect of these three alkaloids on SW480 human colon cancer cells might be a basis for future development of a potent chemotherapeutic drug against human colon cancer.
Acknowledgements
Authors are thankful to Dr. Fred Morin, Department of Chemistry, McGill University, Montreal, Canada, for providing place for performing cytotoxic activities.
Declaration of interest
The authors report that they have no conflicts of interests.
References
- Alexandrova R, Varadinova T, Velcheva M, et al. (2000). Cytotoxic effect of isoquinoline alkaloids on tumor cell lines. Exp Pathol Parasitol 4:8–14
- Anonymous. (1985). The Wealth of India, Raw Materials, I. New Delhi (India): CSIR
- Bharadwaj DK, Bist MS, Jain RK, Munjal A. (1982). Phenolics from the seeds of Argemone mexicana. Phytochemistry 21:2154–56
- Chang YC, Chang FR, Khalil AT, et al. (2003a). Cytotoxic benzophenanthridine and benzylisoquinoline alkaloids from Argemone mexicana. Naturforsch 58:521–26
- Chang YC, Hsieh PW, Chang FR, et al. (2003b). Two new protopine Argemexicaines A and B and the anti-HIV alkaloid 6-acetonyldihydrochelerythrine from Formosan Argemone mexicana. Plant Med 69:148–52
- Dalvi RR. (1985). Sanguinarine: Its potential as a liver toxic alkaloid present in the seeds of Argemone mexicana. Experientia 41:77–8
- Das M, Khanna SK. (1997). Clinicoepidemiological, toxicological, and safety evaluation studies on Argemone Oil. Crit Rev Toxicol 27:273–97
- Deopke W, Hess U, Jimenez V. (1976). Structure of new alkaloid from Argemone mexicana. Z Chem 16:54–55
- Dinda B, Bandopadhyay J. (1986). Free amino acids of Argemone mexicana. J Indian Chem Soc 63:934–36
- Enker WE, Thaler HT, Cranor ML, Polyak T. (1995). Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg 181:335–46
- Haisova K, Slavik J. (1975). On the minor alkaloids from Argemone mexicana L. Collect Czech Chem Commun 40:1576–78
- Harborne JB, Williams CA. (1983). Flavonoids in the seeds of Argemone mexicana: A reappraisal. Phytochemistry 22:1520–21
- Jing L, Zhengwei H. (2008). Rationale and problems for use of coptis and berberine in cancer chemoprevention. NAJ Med Sci 1:38–43
- Mishra PS, Bhakuni DS, Sharma VN, Kaul KN. (1961). Chemical constituents of Argemone mexicana. J Sci Ind Res 20:186
- Singh S, Pandey VB, Singh TD. (2012). Alkaloids and flavonoids of Argemone mexicana. Nat Prod Res 26:16–21
- Singh S, Singh TD, Singh VP, Pandey VB. (2009). Alkaloids of Argemone mexicana. J Indian Chem Soc 86:756–57
- Singh S, Singh TD, Singh VP, Pandey VB. (2010). Quaternary alkaloids of Argemone mexicana. Pharm Biol 48:158–60