Abstract
Context: Curcumol has recently attracted special attention due to its potential activities in many chronic disorders. Moreover, the traditional role of turmeric [Curcuma longa L. (Zingiberaceae)] in suppression of hyperglycemia is of great interest.
Objectives: The present work explores the potential acute and subchronic antihyperglycemic, antinociceptive, and in vivo antioxidant effects of curcumol in alloxan-diabetic mice.
Materials and methods: Bio-guided fractionation, column-chromatography, and GC–MS were utilized to identify the most active compound of turmeric (curcumol). Turmeric (25, 50, and 100 mg/kg), the curcumol rich fraction (CRF) (7 mg/kg), and curcumol (20, 30, and 40 mg/kg) were assessed for their acute (6 h) and subchronic (8 d) antihyperglycemic potentials and antinociceptive effects (8 weeks) were measured, using hot-plate and tail-flick latencies and von-Frey filaments method and in vivo antioxidant effects in alloxan-diabetic mice.
Results: The most-active turmeric fraction was found to be rich in curcumol (45.5%) using GC–MS analysis method. The results proved that the highest dose levels of turmeric extract and curcumol exerted remarkable hypoglycemic activity with 41.4 and 39.3% drop in the mice glucose levels after 6 h, respectively. Curcumol (40 mg/kg) was found to be 9.4% more potent than turmeric extract (100 mg/kg) in subchronic management of diabetes. Curcumol also showed a significant improvement of peripheral nerve function as observed from the latency and tactile tests.
Discussion: The antioxidant potential of curcumol may cause its ability to ameliorate diabetes and diabetes-related complications.
Conclusions: Curcumol, a natural metabolite with a good safety-profile, showed results comparable with tramadol in reversing diabetes-induced tactile allodynia and hyperalgesia.
Introduction
The world prevalence of type 2 diabetes mellitus (DM) as reported in 2013 was 8.3% (Anonymous, Citation2014). This figure is also similar to that found in Lebanon 2014 which is about 8.5% (Guariguata et al., Citation2014). Type 2 diabetes mellitus is an important cause of morbidity and mortality in the Middle East, which currently has the highest global prevalence of DM, ranging from 8 to 24% (Anonymous, Citation2014; Guariguata et al., Citation2014). Patients with diabetes frequently develop acute or subacute painful allodynia and hyperalgesia (Weinbroum et al., Citation2001).
In view of the lack of novel and safer drugs for effective amelioration of DN, therapeutic plant extracts or herbal phytopharmaceuticals can be a complementary source for modern medicine in the development of new drugs (Newman & Cragg, Citation2007).
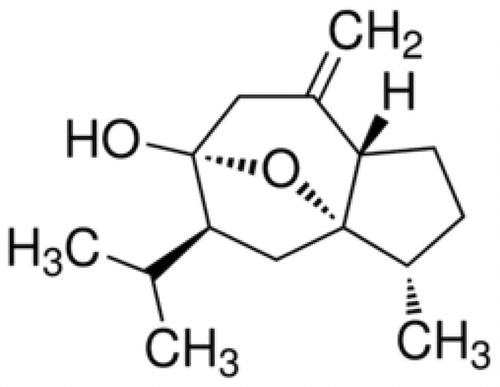
Curcumol is a guaiane-type sesquiterpenoid hemiketal () and one of the major components of the essential oil of Curcuma longa L. (Zingiberaceae) (turmeric). Turmeric is an important commercial grown crop for its aromatic rhizomes and is used as a condiment, preservative, and traditional medicine for treating certain diseases since 4000 BC (Liu et al., Citation2013). Turmeric ethanolic extract was previously reported to suppress hyperglycemia in genetically diabetic mice (Kuroda et al., Citation2005). To date, only a few phyto-pharmacological investigations have been reported on curcumol, possibly due to its hydrophobicity (Wang et al., Citation2014). Recent studies have indicated that curcumol has potential anti-inflammatory, antihepatic fibrosis (Jiang et al., Citation2005), and antimicrobial (Lou et al., Citation2010) activities. Curcumol has also attracted the attention of researchers due to its excellent antitumor effect both in vitro and in vivo (Wang et al., Citation2014). However, a literature review indicated that there are no investigations that deeply study curcumol antidiabetic and antinociceptive potentials.
Presently, oxidative stress is recognized to be related to more than 200 diseases (Nelson et al., Citation2006). Consequently, high oxidative stress has been found to be associated with persistent and chronic hyperglycemia caused by decreased utilization of glucose by the tissue (Kamalakkannan & Prince, Citation2006). Supplementation with certain nutraceutical antioxidants such as vitamin E, C, and flavonoids is the recent approach for alleviating the oxidative damage in DM (Raafat et al., Citation2014; Rahimi et al., Citation2005).
Direct scavenging of reactive oxygen species (ROS) has been primary done using the enzymatic antioxidant, catalase (CAT) (Arulselvan & Subramanian, Citation2007). CAT is an indigenous hemoprotein, which catalyzes the lessening of hydrogen peroxides (Punitha et al., Citation2005) and known to be involved in the detoxification of H2O2 concentrations (Manonmani et al., Citation2005). As a consequence of non-enzymatic glycosylation and oxidation, CAT has been found to be inhibited in DM (Al-Azzawie & Alhamdani, Citation2006). In order to totally or partly ease CAT inhibition, antioxidant compounds might be used, and thus could be used in DM management (Sepici-Dincel et al., Citation2007).
Therefore, the aim of the present work involves the study of the potential in vivo acute and subchronic antihyperglycemic, in vivo antioxidant, and tactile allodynia- and hyperalgesia-management effects of curcumol in the diabetic mouse model, compared with that of turmeric ethanolic extract.
Materials and methods
Materials
Dried Curcuma longa rhizomes were purchased commercially (Summer, 2013) from Ibn-Al-Nafess herbalist, Beirut, Lebanon. Plant species was identified by Prof. J. Habib (LU, Lebanon) and a specimen was placed in the faculty herbarium (specimen voucher no. 2014-P-029). Curcumol, alloxan, extraction solvents, and standards were purchased from Sigma-Aldrich (St. Louis, MO). Glibenclamide (GB) (Lansa Pharm., Hebei, China) and tramadol hydrochloride (TRA) (Deutsche Labs., Darmstadt, Germany) were obtained commercially. All additional chemicals were of analytical grade chemicals.
Ultrasound plant extraction
The dried rhizomes were extracted using 80% ethanol and ultrasound extraction (ROHS, Zhongshan, China). The ethanolic extract was dried in a rotary evaporator (Buchi, Essen, Germany) at temperature 40°C under vacuum.
Bio-guided chromatographic fractionation and identification of the effective compounds
The turmeric ethanolic extract was fractionated using column chromatography. Preparative chromatography column, 50 mm diameter and 100 cm height, was used.
Stepwise elution was done using, diethyl ether/n-pentane (25/75, v/v), then diethyl ether/n-pentane (50/50, v/v), then diethyl ether/n-pentane (75/25, v/v), and finally with 100% diethyl ether, as a gradient mobile phase, and utilizing silica gel as a stationary phase. During the whole chromatography process, the eluent was collected in a series of over 200 fractions by time. Each fraction was tested, for its antidiabetic activity, the same way as the tested solutions using in vivo alloxan-diabetic mice. The most active fraction was analyzed using GC–MS.
GC–MS analysis
The most active fraction was identified by gas chromatography (GC) coupled with a quadrupolar mass spectrometer selective detector (MSD) using an Agilent 6890 N Network for GC (Agilent Technologies, Santa Clara, CA) and an Agilent 5975 Network detector for MSD (Agilent Technologies, Santa Clara, CA). The GC/MS system also equipped with NIST (NIST 11.0, National Institute of Standards and Technology, Gaithersburg, MD) and Wiley (Wiley, Chichester, West Sussex, England) library search database.
The most active fraction was separated on a (30 mm × 0.25 mm × 0.25 mm) HP-5 MS capillary column. The carrier gas was helium and it was used at a flow rate of 1 mL/min. The temperature of the split injector was adjusted at 250°C, the split ratio was 1:5, and the injection volume was 1 mL. The column temperature has been adjusted to 50°C and then programmed at 20°C/min to 150°C, followed by 2°C/min to 180°C, then at 20°C/min to 200°C, and this temperature was then maintained for 3 min. The ion source and GC–MS interface temperatures were 200 and 260°C, respectively. The selected ion monitoring (SIM) method was used for quantification of the three identified sesquiterpenes. Fragmentations were used for quantification, m/z 105 for curcumol, m/z 107 for germacrone, and m/z 180 for curdione and the electron energy was 70 eV (Cheng et al., Citation2009).
Preparation of solutions
The most active fraction was dissolved in n-hexane. The standard identified curcumol stock solution was prepared using the same method and diluted to appropriate concentrations for the construction of calibration curve. Five concentrations of standard curcumol were injected in triplicate.
Animals
One week before the experimentation, male Swiss–Webster mice (Faculty of Pharmacy, Beirut Arab University) were housed in standard cages utilizing a 12 h light/dark cycle. The temperature was set to 22 ± 1°C, animals had free access to water and laboratory standard pellets (proteins 20%, fats 5%, and multivitamins 1%) (Raafat et al., Citation2010, Citation2013b). Mice were held in those conditions for a 7-d period before the beginning of the experiment. Sixteen hours before the experiment, mice were voided from food overnight, but allowed free access to water. All animal care and experiments were accomplished abiding by Lebanese Ministry of Higher Education and with the approval of Beirut Arab University Institutional Review Board (Approval no. 2014A-012-P-R-0023).
Diabetes induction
Intraperitoneal (IP) injection of freshly prepared alloxan dissolved in sterile saline every 48 h three times at a dose of 180 mg/kg has been utilized for experimental induction of DM. After 72 h from the last alloxan injection, blood samples were collected from the tail of each mouse and fasting glucose levels were measured with Accu-chek Active TM glucose strips in Accu-chek Active TM Test Meter (Roche, Indianapolis, IN). After induction of diabetes, glucose (5%) has been added to mice drinking water. The mice were considered diabetic if their fasting blood glucose levels were above 200 mg/dL, and thus used in the experiments.
Acute antidiabetic effect of the turmeric extract, the most active fraction (CRF), and the most active compound (curcumol) in alloxan-induced diabetic mice
The most active fraction was found to contain ca. 45.5% curcumol using GC–MS analysis, hence named curcumol-rich fraction (CRF).
Diabetic mice were divided into nine groups (n = 7 animals/group). Animals of each group had a single IP injection of the following:
Control group I, received only the vehicle (sterile saline). | |||||
Group II, received GB (5 mg/kg) dissolved in DMSO as a reference drug. | |||||
Groups III, IV, and V, received the turmeric extract dissolved in vehicle at doses of 25, 50, and 100 mg/kg, respectively. | |||||
Group VI, received CRF (7 mg/kg) dissolved in vehicle. | |||||
Groups VII, VIII and IX received curcumol dissolved in the vehicle at doses 20, 30, and 40 mg/kg, respectively. | |||||
Blood samples were collected from the tail just prior to dosing and 0.5, 2, and 6 h after dosing, and blood glucose was measured.
Subchronic antidiabetic effect of the turmeric extract, CRF, and curcumol in alloxan-induced diabetic mice
The action of curcumol was tested during a longer duration of treatment. The mice were divided into groups comprising non-diabetic and diabetic animals. Group I (non-diabetic mice, n = 7) served as normal control and only received the vehicle, IP for 7 d (Bakirel et al., Citation2008). Diabetic mice were divided into nine groups (II–IX) seven animals each. The animals of each group were treated for 7 d with a daily IP injection of the following:
Group II, received only vehicle (sterile saline). | |||||
GB group III, received GB dissolved in DMSO as reference drug (5 mg/kg, IP) and was considered as diabetic control. | |||||
Groups IV, V, and VI received turmeric extract dissolved in the vehicle at doses of 25, 50, and 100 mg/kg, respectively. | |||||
Group VII received CRF dissolved in the vehicle at 7 mg/kg. | |||||
Groups VIII, IX, and X received curcumol dissolved in the vehicle at doses 20, 30, and 40 mg/kg, respectively. Each animal was monitored every other day for its blood glucose levels on the 1st, 3rd, 5th and 8th days after 6 h of each injection. | |||||
The level of the antioxidant enzyme (catalase) was measured, and the body weights of animals were recorded at the same day.
Management of diabetic neuropathy
After 6 weeks of induction of DM in animals, DN success rate (i.e. loss of sensory of thermal sensitivity significantly below 10S (Sullivan et al., Citation2007) was ca. 90%, and their neurological function was measured at 1 week intervals for 8 weeks, with TRA 10 mg/kg as a positive control; using the following tests.
Hot plate latency test
Tested animals were placed one at a time on the hot plate (hot plate analgesia meter, Ugo Basile, Monvalle, Italy) adjusted to a temperature of 55 ± 0.1°C. Response latency either to jump or a hindpaw lick was measured by means of an electronic timer. A cut-off time of 30 s was attempted to avoid tissue damage (Ulugol et al., Citation2012).
Tail flick latency test
Briefly, a beam of light was focused on the dorsal surface of the mouse tail using a tail-flick apparatus with suitable tail-flick mice restrainer (Hugo Sachs Elektronik, March, Germany) and the time until the tail flicked was monitored. The tail-withdrawal latency (time from onset of the radiant heat to the withdrawal of the tail) was measured with a timer. The light intensity in the apparatus was adjusted so that the baseline tail-withdrawal latency was about 5.6 s in all mice. A cut-off time of 10.00 ± 0.50 s was set for tissue damage prevention (Ulugol et al., Citation2012).
Von Frey filaments test
Paw withdrawal thresholds were used to access tactile allodynia in mice, by measuring an increasing pressure stimulus placed onto the dorsal surface of the paw using calibrated von Frey filaments (OptiHair TM, Marstock Nervtest TM, Marburg, Germany) with intensities ranging from 0.5 to 45.3 g. Filaments having a force above 32 g were not used, as they produced lifting of the paws. On the test day, animals were placed in a chamber with mesh metal floor and were allowed to adapt for 30 min. The lowest filament force initially has been applied perpendicular to the hind paw, with sufficient force to cause slight bending against the paw and has been held for a few seconds. This was repeated four times with an interval of 1–2 s. If the paw was sharply withdrawn or there was flinching upon removal of the filament that was considered a positive response, then the next higher force filament was applied, if no response was noted. The trial was repeated, with a 5 min interval between consecutive trials, and the filament which produced a positive response was denoted as the threshold. Withdrawal thresholds were monitored on the mouse hind limb prior to (predose) and then up to 8 weeks following drug or vehicle administration (Fox et al., Citation2003).
Estimation of antioxidant activity
Catalase (CAT) activity was measured in serum using the modified method previously described (Yasmineh et al., Citation1995). The CAT activity was expressed as kU/L.
Statistical analysis
All values were presented as means ± SEM. Statistical differences between the test and the control were measured by one-way analysis of variance (ANOVA) followed by the Student–Newman–Keuls test using the “OriginPro” statistic computer program (OriginLab Corporation, Northampton, MA). A difference in the mean values of p ≤ 0.05 was considered statistically significant.
Results
Bio-guided fractionation and GC–MS identification of the effective compound
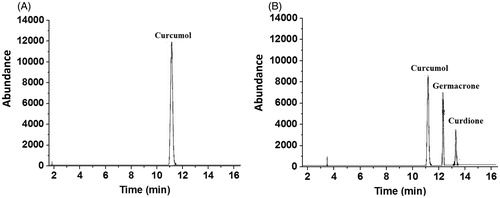
Following bio-guided fractionation, the most active fraction of turmeric extract was injected into the GC–MS instrument to study its pattern. The most active fraction major peak was identified as curcumol, utilizing standard curcumol calibration curves, and hence this fraction was named “curcumol rich fraction” (CRF).
CRF major peaks were curcumol (45.5%), germacrone (24.2%), and curdione (12.1%) (
). Nevertheless, curcumol was the most active compound in CRF when injected in alloxan-diabetic mice.Acute antidiabetic effect of turmeric extract, CRF, and curcumol in alloxan-induced diabetic mice
The acute antidiabetic effect of various doses of turmeric extract, CRF, and curcumol, in diabetic animals, is summarized in . The turmeric extract at all doses (25, 50, and 100 mg/kg) showed a significant effect compared with the control, with blood glucose levels dropping after 6 h of administration to 34.9, 36.6, and 41.4%, respectively. GB given to alloxan-induced diabetic mice prevented the drastic elevation of blood glucose after 1 h of glucose loading. Nevertheless, GB significantly reduced the glucose level, 2 and 6 h after the glucose loading. CRF (7 mg/kg) showed a significant glucose lowering effect compared with the control, with blood glucose levels dropping after 6 h of administration by 47.0%.
Table 1. Acute effect of C. longa extract (turmeric), CRF, and curcumol on blood glucose level in diabetic mice.
Curcumol showed significant decrease in blood glucose level after 6 h at all doses (20, 30, and 40 mg/kg) with the glucose level dropping by 33.9, 39.1, and 39.3%, respectively.
Subchronic effect of turmeric extract, CRF, and curcumol in alloxan-induced diabetic mice
Blood glucose levels of diabetic control mice were significantly higher than those of the control mice during the experiment period as shown in .
Table 2. Subchronic effect of C. longa extract (turmeric), CRF and curcumol on blood glucose level.
The highest reduction in blood glucose using turmeric extract was observed in group VI, showing 40.6% reduction in blood glucose levels on the 8th day compared with 33.9 and 38.1% reduction in case of 25 and 50 mg/kg doses, respectively.
CRF (7 mg/kg) showed a significant hypoglycemic effect on the 8th day compared with that of the diabetic control, with a blood glucose level dropping to 49.0%. Curcumol at all doses (20, 30, and 40 mg/kg) showed significant decrease in blood glucose level on the 8th day compared with that of the diabetic control, with a blood glucose level dropping by 39.6, 42.8, and 50.0%, respectively.
During subchronic study, mice treated with turmeric extract, CRF, and curcumol were also monitored for changes in body weight (). The turmeric extract showed 3.4, 6.7, and 15.3% increase in body weight at doses of 25, 50, and 100 mg/kg, respectively, on the 8th day. CRF (7 mg/kg) showed 9.4% increase in body weight on the 8th day. Similarly, curcumol at doses level of 20, 30, and 40 mg/kg showed 3.3, 6.7, and 30.0% increase in body weight on the 8th day, respectively.
Table 3. Subchronic effect of C. longa extract (turmeric), CRF, and curcumol on body weights in alloxan-induced diabetic mice.
In order to evaluate the in vivo antioxidant effect of the tested extract, CRF, and curcumol, the level of serum CAT of each mouse was measured on the 1st, 3rd, 5th and 8th days following administration ().
Table 4. In vivo assessment of the antioxidant activity of C. longa extract (turmeric), CRF, and curcumol using CAT levels in serum of alloxan-induced diabetic mice.
Treatment of mice with turmeric extract at doses of 25, 50, and 100 mg/kg resulted in a gradual rise in serum CAT activity to reach a significant difference on the 8th day (44.6, 60.2, and 70.7%, respectively) as compared with diabetic control mice. Treatment of mice with CRF (7 mg/kg) had a gradual rise in serum CAT activity to reach significant differences on the 8th day (48.1%) as compared with diabetic control mice. Similarly, curcumol had a gradual rise in serum CAT activity to reach a significant difference on the 8th day with CAT activities with increases of 39.4, 61.1, and 79.8% for doses 20, 30, and 40 mg/kg, respectively.
Management of diabetic neuropathy
An important indicator for diabetic patients having peripheral neuropathy is the decline of peripheral nerve conduction (Said, Citation2007; Tesfaye et al., Citation2010; Widenfalk et al., Citation2009). Therefore, the effect of turmeric extract and curcumol treatment on sensory function was studied by measuring the thermal latency using tail flick and hot plate tests and tactile allodynia using von Frey filaments on the 8th week after alloxan injection.
Treatment of the alloxan-induced diabetic mice with turmeric extract markedly has improved the thermal latency compared with TRA 10 mg/kg-treated mice (). Diabetic mice exhibited temporary hyperalgesic response in thermal tests. On the 8th week after alloxan injection, treatment of mice with turmeric extract in doses of 25, 50, and 100 mg/kg showed a marked improvement in hot-plate latency compared with the vehicle-treated group by 65.3, 73.5, and 91.8%, respectively ().
Figure 3. Effect of C. longa ethanolic extract (Turmeric) on the hot plate and tail withdrawal latencies in alloxan-treated mice. (A) Hot plate latency: (crossed-triangles, straight line) NORM, normal control mice. (Closed-squares, straight-line) DIA + VEH, diabetic animals treated with vehicle as control. (Open-circles, straight-line) DIA + turmeric 25 mg/kg, diabetic animals treated with turmeric 25 mg/kg. (Up-triangles, dashed-line) DIA + turmeric 50 mg/kg, diabetic animals treated with turmeric 50 mg/kg. (Right-triangles, dashed-dotted-line) DIA + turmeric 100 mg/kg, diabetic animals treated with turmeric 100 mg/kg. (B) Tail withdrawal latency: (Crossed-triangles, straight line) NORM, normal control mice. (Closed-squares, straight-line) DIA + VEH, diabetic animals treated with vehicle as control. (Open-circles, straight-line) DIA + turmeric 25 mg/kg, diabetic animals treated with turmeric 25 mg/kg. (Up-triangles, dashed-line) DIA + turmeric 50 mg/kg, diabetic animals treated with turmeric 50 mg/kg. (Right-triangles, dashed-dotted-line) DIA + turmeric 100 mg/kg, diabetic animals treated with turmeric 100 mg/kg. Data (n=7) are expressed as mean ± SEM. “*” p < 0.05 compared with control.
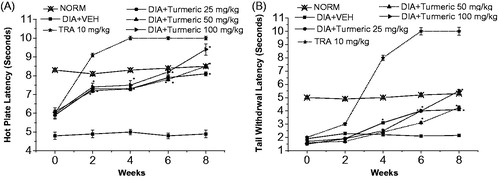
Nevertheless, treatment with all doses of turmeric extract on the 8th week after alloxan injection demonstrated a marked improvement in tail-flick latency by ca. 0.6-, 0.7-, and 1.2-fold for doses of 25, 50, and 100 mg/kg, respectively, compared with the vehicle-treated group ().
Furthermore, on the 8th week, treatment with all doses (25, 50, and 100 mg/kg) of turmeric extract markedly improved tactile allodynia utilizing von Frey filaments by 2.8-, 4.8-, and 6.0-fold, respectively, compared with the vehicle-treated group ().
Additionally, treatment of mice at all dose levels (20, 30, and 40 mg/kg), on the 8th week following alloxan, curcumol markedly improved hot-plate latency by 63.3, 85.7, and 91.8%, respectively, compared with the vehicle-treated group ().
Figure 4. Effect of curcumol on the hot plate and tail withdrawal latencies in alloxan-treated mice. (A) Hot plate latency: (Crossed-triangles, straight line) NORM: normal control mice. (Closed-squares, straight-line) DIA + VEH, diabetic animals treated with vehicle as control. (Open-circles, straight-line) DIA + curcumol 20 mg/kg: diabetic animals treated with curcumol 20 mg/kg. (Up-triangles, dashed-line) DIA + curcumol 30 mg/kg: diabetic animals treated with curcumol 30 mg/kg. (Right-triangles, dashed-dotted-line) DIA + curcumol 40 mg/kg, diabetic animals treated with curcumol 40 mg/kg. (B) Tail withdrawal latency: (Crossed-triangles, straight line) NORM, normal control mice. (Closed-squares, straight-line) DIA + VEH, diabetic animals treated with vehicle as control. (Open-circles, straight-line) DIA + curcumol 20 mg/kg, diabetic animals treated with curcumol 20 mg/kg. (Up-triangles, dashed-line) DIA + curcumol 30 mg/kg, diabetic animals treated with curcumol 30 mg/kg. (Right-triangles, dashed-dotted-line) DIA + curcumol 400 mg/kg, diabetic animals treated with curcumol 40 mg/kg. Data (n=7) are expressed in mean ± SEM. “*”p < 0.05 compared with control.
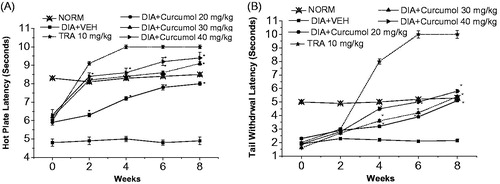
Figure 5. The effect of C. Longa EtOH extract (turmeric), curcumol, and tramadol (TRA) 10 mg/kg on tactile allodynia in the neuropathic model in alloxan-induced diabetic mice. (A) Turmeric group: paw withdrawal thresholds to von Frey filaments were determined on hind paw prior to (Predose) and up to 8 weeks following i.p. injection of 25, 50, and 100 mg/kg turmeric. (B) Curcumol group: paw withdrawal thresholds to von Frey filaments were determined on hind paw prior to (predose) and up to 8 weeks following i.p. injection of (20, 30, and 40 mg/kg) curcumol. (NORM) normal non-diabetic untreated mice. *p ≤0.05 and **p ≤0.01 compared with vehicle (VEH) (n=7 animals/group).
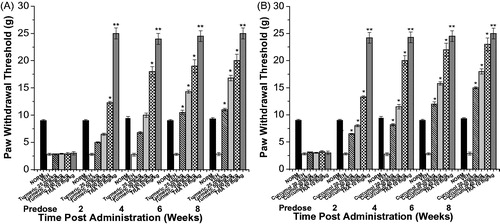
Nonetheless, treatment of mice with at all dose levels of curcumol (20, 30, and 40 mg/kg) has markedly improved the tail-flick latency by ca. 1.3-, 1.5-, and 1.7-fold, respectively ().
On the 8th week, treatment of mice with curcumol at all dose levels (20, 30, and 40 mg/kg) significantly improved tactile allodynia utilizing von Frey filaments by 4.3-, 5.3-, and 7.1-fold, respectively, compared with the vehicle-treated group ().
Discussion
DM is one of the most common metabolic disorders, clinically characterized by hyperglycemia and associated with a number of complications such as DN (Mathis et al., Citation2001). The current therapy for DM and DN is restricted to stimulating Beta-cells in the pancreas and managing painful neuropathy, but their side effects restrict their long-term use (Singh et al., Citation2013). Consequently, wider investigation of potent natural antidiabetics with fewer side effects was the aim of many scientists worldwide. Moreover, the elevated oxidative stress in diabetics significantly contributes to the complications of this disease (Baynes & Thorpe, Citation1999) and free radicals excessive production is a discovered phenomenon associated with diabetic complications (Young et al., Citation1995). Therefore, the aim of the present work was to study the potential in vivo antioxidant, acute and subchronic antihyperglycemic, and management of its related complications by curcumol compared with that of the turmeric ethanol extract.
In the current study, turmeric extract was selected based on its folkloric use in the treatment of many chronic diseases in Lebanon.
Bio-guided fractionation utilizing column chromatography and GC–MS indicated that CRF is the most effective fraction, and curcumol is the most effective compound in CRF. However, turmeric extract showed a faster onset of decreasing blood glucose level than curcumol in diabetic mice. Nevertheless, CRF has shown a greater potency in decreasing blood glucose level than curcumol in diabetic mice. This may be attributed to the synergistic pattern of augmentation between the most active compound and other compounds in the active fraction (Raafat et al., Citation2014). On the contrary, in longer duration of treatment, curcumol showed a more powerful pattern of amelioration of DM and DN than the original extract.
Turmeric extract and curcumol had a dose-dependent effect in diabetic mice, with significant decrease of blood glucose levels at the highest dose levels.
It has been found that the highest dose levels of turmeric extract (100 mg/kg), CRF (7 mg/kg), and curcumol (40 mg/kg) were the most effective doses in the acute and subchronic studies. Compared with the GB group, curcumol showed significantly higher effect on blood glucose level. During studying the acute antidiabetic effect, an initial increase in blood glucose levels was detected during the first 2 h after IP administration of turmeric extract. This temporary hyperglycemic effect could be attributed to glucose pre-loading, and the tested compounds which did not start to give their effect directly after administration, as previously observed (Raafat & Samy, Citation2014).
Concerning the acute antidiabetic effect, turmeric extract showed a significantly higher reduction in glucose level compared with curcumol. This could be advantageous in fast reduction of blood sugar level. However, in the subchronic experiments, curcumol showed a significantly higher reduction in glucose level compared with turmeric extract, an effect that could be beneficial for designing a long acting therapy for controlling DM.
Turmeric extract, CRF, and curcumol showed a significant elevation in body weight, as a consequence of amelioration of hyperglycemia, as previously demonstrated with medications used in the management of DM (Raafat et al., Citation2013a; Russell-Jones & Khan, Citation2007).
Currently, much attention has been given on the role of oxidative stress as the main and common event in the pathogenesis of different diabetic complications (Sepici-Dincel et al., Citation2007).
In this study, the activity of CAT was reduced in the untreated diabetic mice, as reported earlier (Al-Azzawie & Alhamdani, Citation2006; Sepici-Dincel et al., Citation2007). This could be the result of CAT deactivation caused by alloxan-generated reactive oxygen species (ROS). Subchronic treatment of diabetic mice, especially with the highest dose of turmeric extract, CRF and curcumol, lessened the oxidative stress, as evidenced by the elevation in CAT activity.
In the subchronic study, curcumol showed a comparatively similar pattern of decreasing blood glucose level and oxidative stress to that of turmeric extract, which might indicate that curcumol is the most effective bioactive component in turmeric extract.
The present study describes for the first time the antihyperalgesic activity of curcumol in an alloxan-induced diabetic mouse model, where the administration of either turmeric extract or curcumol alleviated hyperalgesia in pain conditions compared with that of TRA.
The current data show that curcumol is highly effective against thermal hyperalgesia and tactile allodynia in animal models of neuropathic pain. It was shown that curcumol possesses more potent antihyperalgesic activity in the alloxan-induced diabetic mice compared with the parent extract. The difference in potencies has been observed before among other natural compounds, like rutin, and synthetic ones, like carbamazepine (Fox et al., Citation2003; Raafat et al., Citation2014).
Curcumol was the most active compound against tactile allodynia showing about a 7.1-fold improvement.
These findings provide a useful information for a promising natural remedy intended for symptomatic amelioration of diabetic neuropathy, a safe (Wang et al., Citation2014) antidiabetic agent as well as management of micro- and macrovascular complications of DM with lower side effects. Relatively rapid onset medication (turmeric extract) may be better for acute management, while slower ones (curcumol) may be more beneficial in long-term management.
In conclusion, the present study indicated that curcumol exerted a remarkable antihyperglycemic activity and reduced the oxidative stress that improved peripheral nerve function in diabetic animals. Therefore, the observed in vivo antioxidant potential of curcumol might possibly be one of the mechanisms of action responsible for its antinociceptive effect.
The safety profile and the current results signify curcumol benefit in the treatment of neuropathic pain conditions, without the long-term side effects of the existing therapies. Therefore, curcumol might prove to be a better therapy for diabetic neuropathy, which may require further clinical investigation.
Acknowledgements
Thanks extends to for Mrs. G. Onsy for proof reading the manuscript. Authors are also grateful to the Junior Research Team Department of Pharmaceutical Sciences, Faculty of Pharmacy, BAU for technical support in methodology preparation.
Declaration of interest
The authors report that they have no conflict of interest. Authors would like to thank Dr. A. Yacout (IPS Holding, Lebanon) for partially funding this research.
References
- Al-Azzawie HF, Alhamdani MS. (2006). Hypoglycemic and antioxidant effect of oleuropein in alloxan-diabetic rabbits. Life Sci 78:1371–7.
- Anonymous. (2014). Global guideline for type 2 diabetes. Diabetes Res Clin Pract 104:1–52.
- Arulselvan P, Subramanian SP. (2007). Beneficial effects of Murraya koenigii leaves on antioxidant defense system and ultra structural changes of pancreatic beta-cells in experimental diabetes in rats. Chem Biol Interact 165:155–64.
- Bakirel T, Bakirel U, Keles OU, et al. (2008). In vivo assessment of antidiabetic and antioxidant activities of rosemary (Rosmarinus officinalis) in alloxan-diabetic rabbits. J Ethnopharmacol 116:64–73.
- Baynes JW, Thorpe SR. (1999). Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes 48:1–9.
- Cheng XJ, Liu HG, Liao YK, et al. (2009). Comparisons of volatile components in different parts of three species of Rhizoma Curcumae. Zhong Yao Cai 32:1551–3.
- Fox A, Gentry C, Patel S, et al. (2003). Comparative activity of the anti-convulsants oxcarbazepine, carbamazepine, lamotrigine and gabapentin in a model of neuropathic pain in the rat and guinea-pig. Pain 105:355–62.
- Guariguata L, Whiting DR, Hambleton I, et al. (2014). Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 103:137–49.
- Jiang J, Tan SZ, Tan CY, et al. (2005). Effect of FZHY recipe on elastase expression in liver fibrosis in rats. Zhonghua Gan Zang Bing Za Zhi 13:307–8.
- Kamalakkannan N, Prince PS. (2006). Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic wistar rats. Basic Clin Pharmacol Toxicol 98:97–103.
- Kuroda M, Mimaki Y, Nishiyama T, et al. (2005). Hypoglycemic effects of turmeric (Curcuma longa L. rhizomes) on genetically diabetic KK-Ay mice. Biol Pharm Bull 28:937–9.
- Liu Y, Roy SS, Nebie RH, et al. (2013). Functional food quality of Curcuma caesia, Curcuma zedoaria and Curcuma aeruginosa endemic to Northeastern India. Plant Foods Hum Nutr 68:72–7.
- Lou Y, Zhang H, He H, et al. (2010). Isolation and identification of phase 1 metabolites of curcumol in rats. Drug Metab Dispos 38:2014–22.
- Manonmani G, Bhavapriya V, Kalpana S, et al. (2005). Antioxidant activity of Cassia fistula (Linn.) flowers in alloxan induced diabetic rats. J Ethnopharmacol 97:39–42.
- Mathis D, Vence L, Benoist C. (2001). Beta-cell death during progression to diabetes. Nature 414:792–8.
- Nelson SK, Bose SK, Grunwald GK, et al. (2006). The induction of human superoxide dismutase and catalase in vivo: A fundamentally new approach to antioxidant therapy. Free Radic Biol Med 40:341–7.
- Newman DJ, Cragg GM. (2007). Natural products as sources of new drugs over the last 25 years. J Nat Prod 70:461–77.
- Punitha IS, Rajendran K, Shirwaikar A. (2005). Alcoholic stem extract of Coscinium fenestratum regulates carbohydrate metabolism and improves antioxidant status in streptozotocin-nicotinamide induced diabetic rats. Evid Based Complement Alternat Med 2:375–81.
- Raafat K, Aboul-Ela M, El-Lakany A. (2014). Alloxan-induced diabetic thermal hyperalgesia, prophylaxis and phytotherapeutic effects of Rheum ribes L. in mouse model. Arch Pharm Res. [Epub ahead of print]. doi:10.1007/s12272-014-0372-y.
- Raafat K, Boukhary R, Aboul-Ela M, et al. (2013a). Endogenous Lebanese plants treating diabetes and related complications. Nat Prod Chem Res 1:112–20.
- Raafat K, Breitinger U, Mahran L, et al. (2010). Synergistic inhibition of glycinergic transmission in vitro and in vivo by flavonoids and ftrychnine. Toxicol Sci 118:171–82.
- Raafat K, Samy W. (2014). Amelioration of diabetes and painful diabetic neuropathy by Punica granatum L. extract and its spray dried biopolymeric dispersions. Evid Based Complement Alternat Med 2014:180495.
- Raafat KM, Jassar H, Aboul-Ela M, et al. (2013b). Protective effects of Origanum majorana L. against neurodegeneration: Fingerprinting, isolation and in vivo glycine receptors behavioral model. Int J Phytomed 5:46–57.
- Rahimi R, Nikfar S, Larijani B, et al. (2005). A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother 59:365–73.
- Russell-Jones D, Khan R. (2007). Insulin-associated weight gain in diabetes – causes, effects and coping strategies. Diabetes Obes Metab 9:799–812.
- Said G. (2007). Diabetic neuropathy – a review. Nat Clin Pract Neurol 3:331–40.
- Sepici-Dincel A, Acikgoz S, Cevik C, et al. (2007). Effects of in vivo antioxidant enzyme activities of myrtle oil in normoglycaemic and alloxan diabetic rabbits. J Ethnopharmacol 110:498–503.
- Singh R, Kaur N, Kishore L, Gupta GK. (2013). Management of diabetic complications: A chemical constituents based approach. J Ethnopharmacol 150:51–70.
- Sullivan KA, Hayes JM, Wiggin TD, et al. (2007). Mouse models of diabetic neuropathy. Neurobiol Dis 28:276–85.
- Tesfaye S, Boulton AJ, Dyck PJ, et al. (2010). Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 33:2285–93.
- Ulugol A, Oltulu C, Gunduz O, et al. (2012). 5-HT7 receptor activation attenuates thermal hyperalgesia in streptozocin-induced diabetic mice. Pharmacol Biochem Behav 102:344–8.
- Wang H, Wang Y, Jiang X, et al. (2014). The molecular mechanism of curcumol on inducing cell growth arrest and apoptosis in Jurkat cells, a model of CD4(+) T cells. Int Immunopharmacol 21:375–82.
- Weinbroum AA, Gorodezky A, Niv D, et al. (2001). Dextromethorphan attenuation of postoperative pain and primary and secondary thermal hyperalgesia. Can J Anaesth 48:167–74.
- Widenfalk J, Wu W, Hao J, et al. (2009). Treatment of transected peripheral nerves with artemin improved motor neuron regeneration, but did not reduce nerve injury-induced pain behaviour. Scand J Plast Reconstr Surg Hand Surg 43:245–50.
- Yasmineh WG, Kaur TP, Blazar BR, et al. (1995). Serum catalase as marker of graft-vs-host disease in allogeneic bone marrow transplant recipients: Pilot study. Clin Chem 41:1574–80.
- Young IS, Tate S, Lightbody JH, et al. (1995). The effects of desferrioxamine and ascorbate on oxidative stress in the streptozotocin diabetic rat. Free Radic Biol Med 18:833–40.