Abstract
Context The root of Helicteres angustifolia L. (Sterculiaceae) has been used as folk herbal drug to treat cancer, bacterial infections, inflammatory, and flu in China. However, there is no report on its antidiabetic activity.
Objective This study evaluates the antidiabetic activity of ethanol extract from H. angustifolia root.
Materials and methods The promoting effect of H. angustifolia root ethanol extract (25, 50, and 100 μg/mL) on glucose uptake was evaluated using HepG2 cell, differentiated C2C12 myotubes, and differentiated 3T3-L1 adipocytes. The antidiabetic activity of the extract was assessed in vivo using STZ-induced diabetic rats by orally administration of the extract (200 and 400 mg/kg b.w.) once per day for 28 d. Blood glucose, TG, TC, TP, HDL-C, UA, BUN, AST, ALT, insulin, and HOMA-IR were analyzed.
Results The results showed that the extract increased glucose uptake in C2C12 myotubes and 3T3-L1 adipocytes with an IC50 value of 79.95 and 135.96 μg/mL, respectively. And about 12%, 19%, and 10% (p < 0.05) in HepG2 cells when compared with the control at the concentration of 25, 50, and 100 μg/mL, respectively. After 28 days’ treatment with the extract, significant reduction was observed in blood glucose, HOMA-IR, TC, TG, UA, BUN, AST, and ALT levels, while the levels of TP and HDL cholesterol increased.
Discussion and conclusion These results suggest that H. angustifolia root ethanol extract possess potent antidiabetic activity, which is the first report on antidiabetic activity of this plant.
Introduction
Diabetes mellitus is a chronic metabolic disease characterized by high blood glucose level. It is estimated that the number of diabetics will increase up to 439 million in 2030 (Shaw et al. Citation2010), and more than 90% of the diabetics are type 2 diabetics (Attele et al. Citation2002). Type 2 diabetes is characterized by insulin resistance and a progressive decline in β-cell function (Laakso Citation2001). It has been reported that insulin resistance is an important factor in the pathogenesis of type 2 diabetes (Meier & Bonadonna Citation2013), which is characterized not only by decreased responsiveness of the peripheral target tissues (such as liver, adipose tissue, and skeletal muscle) to insulin but also by remarkable decrease in glucose uptake and utilization (Yang et al. Citation2014). Thus, increasing glucose uptake of the above three key tissues is one of the effective therapeutic approaches for treating diabetes.
Helicteres angustifolia L. (Sterculiaceae), namely Shan-Zhi-Ma in Chinese, is one of the traditional medicinal plants which are wildly distributed in South China and Southeast Asia. Helicteres isora L. has shown significant antidiabetic activity (Chakrabarti et al. Citation2002). Helicteres angustifolia root has been used as a folk herbal drug to treat cancer, bacterial infections, inflammatory and flu in China (Jiangsu New Medical College Citation1986). It has also been reported to possess antihepatic fibrosis activity (Huang et al. Citation2012) and antiviral activity (Huang et al. Citation2013). Different classes of phytochemical constituents have been isolated from the root of H. angustifolia (Chen et al. Citation1994 Citation2006), e.g. triterpenoids (betulinic acid, oleanolic acid, helicteric acid, and methyl heliceate), flavonoids (kaempferol 3-O-β-d-glucopyranoside, 5,8-dihydroxy-7,4′-dimethoxyflavone, takakin 8-O-β-d-glucuronide 6″-methyl ester and takakin 8-O-β-d-glucuronide 2″-sodium sulfate), phenolic acids (rosmarinic acid), quinines (mansonone E, F, H, and H methyl ester), lignans (lariciresinol, lirioresinol-B and (+)-pinoresinol), and cucurbitacins (cucurbitacin D and J). Among these phytochemical constituents, betulinic acid (De Melo et al. Citation2009), oleanolic acid (Gao et al. Citation2007), and rosmarinic acid (Jayanthy & Subramanian Citation2014) have been shown to exhibit antidiabetic activity. Up to now, however, there is no report on the antidiabetic activity of H. angustifolia root. Therefore, this study was to investigate whether the root of H. angustifolia possessed antidiabetic activity or not.
This study evaluates the glucose uptake activity of H. angustifolia root ethanol extract in HepG2 cell, differentiated C2C12 myotubes and differentiated 3T3-L1 adipocytes in vitro, and antidiabetic activity in STZ-induced diabetic rats. The total flavonoid content, cytotoxicity, and acute oral toxicity of the extract were also discussed.
Materials and methods
Chemicals, reagents, and culture medium
CMC-Na, STZ, glibenclamide, and glucose CII-Test were purchased from Wako Pure Chemical (Osaka, Japan). Insulin, 3-isobutylmethylxanthine (IBMX), dexamethasone (DEX), DMSO, and DMEM essential medium were purchased from Sigma Aldrich, Inc. (St. Louis, MO). Horse serum (HS) was from Cosmo-bio (Tokyo, Japan). Fetal bovine serum (FBS) was from Giboco (Grand Island, NY), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Life Technologies (Grand Island, NY). All other chemicals were of analytical grade.
Plant material
The roots of H. angustifolia were collected in the rural area near the city of Vientiane, Laos in June, 2013. The collected plant was identified by Dr. Ende Liu from Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, China, of which a voucher specimen (KUN-2014-141) was deposited at the herbarium of the same institute.
The dried H. angustifolia root (500 g) was powdered and extracted with 70% ethanol (5 L × 3 times) at room temperature (25 ± 2°C) for 3 d. Then the extract was concentrated and lyophilized to get powdered product (yielding 10.8%, w/w). The obtained 70% ethanol extract from H. angustifolia root was stored at −20°C until further use.
Cell culture and differentiation
Murine C2C12 myoblasts and human hepatocarcinoma cell line HepG2 were purchased from RIKEN Bioresource Center (Tsukuba, Japan). Mouse 3T3-L1 adipocyte was purchased from JCRB Cell Bank (Osaka, Japan). All cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37°C and 5% CO2 incubator. The medium was changed every 2–3 d.
Differentiation of C2C12 myoblasts to myotubes was carried out according to Kang et al. (Citation2012) with slight modifications. Briefly, cells were reseeded in 12-well plates at a density of 2 × 104 cells/mL. When the cells were over 80% confluent, the medium was replaced with DMEM containing 2% horse serum and was changed every 2 d. Cells were used for glucose uptake assay until myotubes being observed under microscope.
Differentiation of 3T3-L1 cells to adipocytes was performed according to Zhang et al. (Citation2014) with some modifications. In brief, cells were reseeded in 96-well plates at a density of 5 × 103 cells/well. When the cells were over 80% confluent, the medium was replaced with DMEM containing 1 μM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, 10 μg/mL insulin for 3 d. Then the cells were incubated in DMEM containing 10% FBS, 1% penicillin–streptomycin, and 10 μg/mL insulin for 2 d. Finally, the cells were incubated in DMEM containing 10% FBS and 1% penicillin–streptomycin for 5–9 d. The cells were used for glucose uptake assay until 80–90% of them changed into lipid droplets under microscope.
Cytotoxicity assay
The cytotoxic effect of H. angustifolia root extract on HepG2, C2C12, and 3T3-L1 cells was determined by using MTT assay (Lu et al. Citation2011). Briefly, HepG2, C2C12, and 3T3-L1 cells were seeded at a density of 5 × 103 cells/well in 96-well plate and incubated for 24 h, and then incubated with various concentrations of H. angustifolia extract for another 24 h. After incubation, 10 μL MTT (5 mg/mL) was added into each well, incubated for 4 h, then the culture medium was removed before the addition of 100 μL DMSO. After shaking for 10 min, the optical density (OD) was measured at 570 nm with a microplate reader (Bio-Rad, Tokyo, Japan).
Glucose uptake assay
Glucose uptake in HepG2 cells was determined according to Lv et al. (Citation2014) with slight modifications. In brief, HepG2 cells were seeded at a density of 1 × 104 cells/well in 96-well plate with some wells left blank. After the cells reached confluence, the medium was replaced by DMEM containing 0.2% BSA. After 12 h incubation, the cells were treated with DMEM containing 0.2% BSA with or without the designated concentrations of test samples for another 24 h. Then 10 μL of medium was removed from each well (being placed into a new 96-well plate) into which 200 μL of glucose oxidase reagent (Glucose CII-Test, Wako, Japan) was added. The plates were further incubated at 37°C for 15 min and the optical density (OD) was measured at 490 nm with a microplate reader (Bio-Rad, Tokyo, Japan). The amount of glucose uptake was calculated by subtracting the glucose concentrations of the blank wells from the remaining glucose in the cell plated wells. Insulin (10 μg/mL) was utilized as positive while sterilized water as negative control.
Glucose uptake in differentiated C2C12 myotubes and differentiated 3T3-L1 adipocytes was determined according to the method used by Shimokawa et al. (Citation2000) with some modifications. Briefly, the differentiated C2C12 myotubes and differentiated 3T3-L1 adipocytes were first added into 96-well plates. After incubation with DMEM containing 0.2% BSA for 12 h, the cells were treated with various concentrations of test samples for 24 h. The amount of glucose uptake was calculated as that used for HepG2 cells. Insulin (10 μg/mL) and sterilized water were also utilized as positive and negative controls.
Animals
Sprague–Dawley rats, 6-week-old, were obtained from Japan SLC, Inc. (Shizuoka, Japan) and maintained in Laboratory Animal Resource Center, University of Tsukuba, Japan. The rats were kept in cages with three rats in each cage under controlled conditions of temperature (23 ± 1°C), humidity (55 ± 5%), and 12/12-h light/dark cycle. The rats had free access to tap water and food before the experiments. All the animal experiments were based on the guideline of the maintenance and use of laboratory animals at the Laboratory Animal Resource Center of University of Tsukuba and were approved by the Animal Experiments Committee, University of Tsukuba (Approval number 14-344).
Acute oral toxicity study
Acute oral toxicity assay of ethanol extract from H. angustifolia root was performed according to the OECD Guideline 423. Overnight fasted Sprague–Dawley rats were randomly divided into two groups with three rats in each group. The control group was given distilled water, and the experimental group was given the extract (5 g/kg). The rats were observed for 24 h. After 14 d, the rats were dissected and their major organs were used for determination.
Induction of experimental diabetic rats
Induction of experimental diabetic rats was performed according to the literature (Masiello et al. Citation1998). Briefly, after 1 week of preliminary breeding, the rats were fasted overnight and then were injected intraperitoneally with streptozotocin (dissolved in 0.1 M cold citrate buffer, pH 4.5) at a dose of 50 mg/kg. After 3 d, the fasting blood glucose of rats was measured by one touch select glucometer (Sanwa Kagaku Kenkyusho, Nagoya, Japan). The rats with the fasting blood glucose greater than 11 mmol/L were regarded as diabetic and used for the following antidiabetic study.
Animal experimental protocol
The STZ-induced diabetic rats were randomly divided into five groups with six in each group. The groups were as follows:
Group I (NC): Normal control rats treated with 0.5% CMC-Na solution (p.o.).
Group II (DC): Diabetic control rats treated with 0.5% CMC-Na solution (p.o.).
Group III (DE200): Diabetic rats treated with extract (200 mg/kg b.w. p.o.).
Group IV (DE400): Diabetic rats treated with extract (400 mg/kg b.w. p.o.).
Group V (DG): Diabetic rats treated with glibenclamide (1 mg/kg b.w. p.o.).
The ethanol extract from H. angustifolia root and an antidiabetic drug, glibenclamide were suspended in 0.5% CMC-Na solution, respectively, which were given by oral route (p.o.) to the rats once per day for 28 d according to the above experimental design.
Plasma collection and biochemical analysis
On day 28, all the rats were fasted overnight and anesthetized with isoflurane, and then blood was collected and centrifuged at 3000 rpm and 4°C for 15 min to obtain serum. The serum sample was then measured with an automated biochemical analyzer (DRI-CHEM 7000V, Fujifilm, Tokyo, Japan) for the determination of some biochemical parameters including blood glucose, total glycerides (TG), total cholesterol (TC), total proteins (TP), HDL cholesterol (HDL-C), uric acid (UA), urea nitrogen (UN), aspartate transaminase (AST), and alanine transaminase (ALT). Serum insulin levels were determined using a commercial kit (Mercodia, Uppsala, Sweden) according to the instructions of the manufacturer. Insulin resistance was assessed by Homeosasis Model Assessment of Insulin Resistance (HOMA-IR) which was calculated from fasting glucose (mmol/L × fasting insulin (μU/mL)/22.5 (Matthews et al. Citation1985).
Determination of total flavonoid content
The total flavonoid content was measured by using aluminum chloride colorimetric assay (Yao et al. Citation2013). In brief, 1 mL extract or rutin at various concentration was mixed with 4 mL 70% aqueous ethanol, and then 0.5 mL NaNO2 (5%, w/v) was added. After 6 min, 0.5 mL AlCl3 (10%, w/v) and 3 mL NaOH (1 M) were added, followed by the addition of distilled water to reach 10 mL. After the solution was mixed and incubated for 15 min at 25°C, the absorbance was read at 510 nm using a UV-1800 spectrophotometer (Shimadzu, Tokyo, Japan). The total flavonoid content of in the ethanol extract was calculated based on the standard curve obtained with rutin and expressed as rutin equivalent (RE) in mg/g of dry sample.
Statistical analysis
All the data were expressed as mean ± SD, and ANOVA followed by Duncan’s multiple range test (DMRT) was used for statistical analysis by using SPSS software (version 17.0, SPSS Inc., Chicago, IL). Significance was assumed if p < 0.05.
Results
Cytotoxicity assay
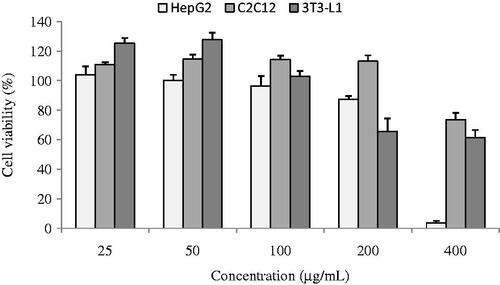
The cytotoxicity of the ethanol extract from H. angustifolia root on HepG2, C2C12, and 3T3-L1 cells was assessed by MTT assay (). At the concentration of 100 μg/mL, the cell viability of the extract was about 96% for HepG2, 114% for C2C12, and 103% for 3T3-L1 cells. Therefore, different doses of the ethanol extract from H. angustifolia root, i.e. 25, 50, and 100 μg/mL were employed in the following glucose uptake experiments.
Glucose uptake assay
The effect of the ethanol extract on glucose uptake in HepG2 cells, C2C12 myotubes, and 3T3-L1 adipocytes is illustrated in . The extract increased glucose uptake in C2C12 myotubes and 3T3-L1 adipocytes with an IC50 value of 79.95 and 135.96 μg/mL, respectively. And about 12, 19, and 10% (p < 0.05) in HepG2 cells when compared with the control at the concentration of 25, 50, and 100 μg/mL, respectively. As a contrast, insulin, as a positive control at 10 μg/mL, increased glucose uptake in HepG2 cells, C2C12 myotubes, and 3T3-L1 adipocytes with a value of about 80, 91, and 75%, respectively.
Figure 2. Effects of ethanol extract of H. angustifolia root at different concentrations on glucose uptake in HepG2 cells, C2C12 myotubes, and 3T3-L1 adipocytes. Insulin and sterilized water were utilized as positive and negative controls. *p < 0.05, **p < 0.01 compared to negative control.
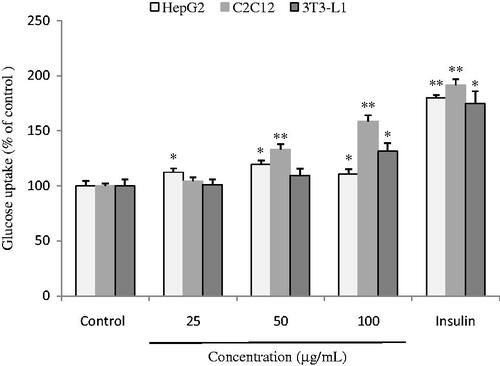
The above results indicate that the extract has promoting effect on HepG2 cells, differentiated C2C12 myotubes and differentiated 3T3-L1 adipocytes which represent the three key tissues involving in glucose homeostasis, such as liver, muscle, and adipose tissue.
Acute oral toxicity study
During the acute oral toxicity experiments, oral administration of the extract at a dose of 5 g/kg did not produce any signs of toxicity, and no death occurred. This observation aroused us to seek more in depth facts through the following long-term antidiabetic experiments.
Effect on blood glucose level, insulin level, and HOMA-IR
As shown in , the diabetic rats were found to have significantly higher fasting blood glucose concentrations, insulin concentrations, and HOMA-IR than the normal control rats. After 28 days’ administration of the extract (200 and 400 mg/kg), the fasting blood glucose concentrations were observed to decrease when compared with the diabetic rats (p < 0.05). This observation suggests that the extract has hypoglycemic effect on STZ-induced diabetic rats, resulting in decrease in the insulin resistance.
Table 1. Effect of ethanol extract of Helicteres angustifolia root on blood glucose, insulin, and HOMA-IR in the control and diabetic rats (n = 6) after 28 days’ experiments.
Effect on serum lipid profile, renal, and hepatic parameters
As seen from , the TG, TC, UA, BUN, AST, and ALT levels of the diabetic rats were higher while TP and HDL-C were lower than those of the normal rats. After 28 days’ administration of the extract (200 and 400 mg/kg) and glibenclamide (1 mg/kg), however, the TG, TC, UA, BUN, AST, and ALT levels were significantly decreased; in contrast, significant increases in TP and HDL-C levels were detected in the tested rats when compared with diabetic rats (p < 0.01).
Table 2. Effect of ethanol extract of H. angustifolia root on serum biochemical parameters in the control and diabetic rats (n = 6) after 28 days’ experiments.
Determination of total flavonoid content
The total flavonoid content in the ethanol extract of H. angustifolia root was determined according to the calibration curve equation (y = 0.0012x – 0.0115, R2 = 0.9991) and expressed in rutin equivalents (RE). Result showed that the extracts contained 430.70 ± 0.92 mg/g of total flavonoids.
Discussion
The results from this study indicate that the ethanol extract of H. angustifolia root has promoting effect on HepG2 cells, differentiated C2C12 myotubes, and differentiated 3T3-L1 adipocytes that represent the three key tissues involving in glucose homeostasis liver, muscle, and adipose tissue, respectively. The extract at a dose of 100 μg/mL could significantly promote the glucose uptake in C2C12 myotubes (p < 0.01), HepG2 cells (p < 0.05), and differentiated 3T3-L1 adipocytes (p < 0.05). This phenomenon is similar to the positive control of insulin. This study for the first time confirms that the ethanol extract of H. angustifolia root has antidiabetic effects by stimulating glucose uptake in HepG2 cells, C2C12 myotubes, and 3T3-L1 adipocytes.
Hypertriglyceridaemia and hypercholesterolaemia have been reported to be the primary factors of diabetic state involving in the development of atherosclerosis and coronary heart disease which are the secondary complications of diabetes (Ananthan et al. Citation2003). Results from this study indicate that the extract administration could significantly reduce TG and TC levels while significantly increase HDL-C level in the STZ-induced diabetic rats. Therefore, it can be inferred that the extract has a potential for improving serum lipid abnormalities in diabetic conditions and thus decreasing the risk of atherosclerosis and coronary heart disease.
Liver is the center of glucose metabolism, which not only maintains normal blood glucose level but also provides glucose as energy to organs. ALT and AST are enzymatic markers of liver function and they are usually used to signal liver damage in clinics. Compared with the normal rats, the diabetic rats had significantly higher levels of ALT and AST while lower level of TP (). The results showed that the diabetic rats were in a liver injury state. After administration of the extract, the ALT and AST levels were found to be significantly decreased in the STZ-induced diabetic rats while TP was significantly increased, implying that the extract could potentially protect liver against diabetic damage.
Kidney is an important organ to remove the metabolic wastes such as blood urea nitrogen (BUN) and creatinine from body, thereby helping to maintain body homeostasis of above mentioned substances (Ramachandran et al. Citation2012). The results from show that the extract administration could significantly decrease BUN, an indicator of renal function. It is well known that diabetic renal disease is one of the diabetic complications. Therefore, results from this study imply that the ethanol extract has potential for treating diabetic complications.
UA is the last metabolite of the purine catabolic pathway in humans. High UA content in blood is a characteristic of hyperuricemia (Haidari et al. Citation2008). Reducing UA level is one of the effective ways for preventing and treating gout. The results () show that administration of the extract could significantly decrease UA, implying that the extract has a potential for treating gout.
HOMA-IR is a reliable and useful parameter for assessing insulin resistance in patients with type 2 diabetes (Okita et al. Citation2013). Results from this study demonstrate that administration of the extract did not lead to increased insulin levels, but could significantly lower the blood glucose level and HOMA-IR. These results are in consistence with the results from glucose uptake assay, proving that the extract could increase glucose uptake in insulin-target tissues and improve insulin resistance. So it can be concluded that the mechanism involving in the promoting effect of the extract on antidiabetic activity is probably realized by reducing insulin resistance and also by increasing glucose uptake in insulin-target tissues.
The results from acute oral toxicity experiments indicate that the ethanol extract of H. angustifolia root is non-toxic even at a high oral dose of 5 g/kg. In the long-term antidiabetic test, doses of 200 and 400 mg/kg were applied in the experiments.
From the results of animal experiments, the extract exhibited antidiabetic effect on the STZ-induced diabetic rats and could decrease the insulin resistance. In this study, no significant change in body weight was observed between the treatment group and the control group (data not shown). This is also the first study to disclose the antidiabetic activity of the ethanol extract from H. angustifolia root in STZ-induced diabetic rats.
It is commonly recognized that certain flavonoids and triterpenoids from plant exhibit antidiabetic activity (Perez et al. Citation1998). In previous studies, several flavonoids and triterpenoids have been isolated from H. angustifolia root (Chen et al. Citation1994 Citation2006), and some of them have been proven to have antidiabetic activity. In this study, the total flavonoid content in the ethanol extract of H. angustifolia root was also determined which is much higher than those of mulberry leaves exhibiting antidiabetic activity (Wanyo et al. Citation2011). Further studies are necessary for the characterization and quantification of the active ingredients in the ethanol extract of H. angustifolia root.
Conclusion
Based on the results from cell-based bioassays and animal-based assays, it can be concluded that the ethanol extract from H. angustifolia root possesses potent antidiabetic activity.
Funding information
This study was funded by Taisei Kogyo Co., Ltd (No. CDE26014).
Disclosure statement
The authors confirmed that there is no conflict of interests in the present study.
References
- Ananthan R, Latha M, Ramkumar KM, Pari L, Baskar C, Narmatha Bai V. 2003. Effect of Gymnema montanum leaves on serum and tissue lipids in alloxan diabetic rats. Exp Diabetes Res. 4:183–189.
- Attele AS, Zhou YP, Xie JT, Wu JA, Zhang L, Dey L, Pugh W, Rue PA, Polonsky KS, Yuan CS. 2002. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 51:1851–1858.
- Chakrabarti R, Vikramadithyan RK, Mullangi R, Sharma VM, Jagadheshan H, Rao YN, Sairam P, Rajagopalan R. 2002. Antidiabetic and hypolipidemic activity of Helicteres isora in animal models. J Ethnopharmacol. 81:343–349.
- Chen WL, Tang WD, Lou LG, Zhao WM. 2006. Pregnane, coumarin and lupane derivatives and cytotoxic constituents from Helicteres angustifolia. Phytochemistry. 67:1041–1047.
- Chen ZT, Lee SW, Chen CM. 1994. New flavoid glycosides of Helicteres angustifolia. Heterocycles. 38:1399–1406.
- De Melo CL, Queiroz MG, Arruda Filho AC, Rodrigues AM, de Sousa DF, Almeida JG, Pessoa OD, Silveira ER, Menezes DB, Melo TS, et al. 2009. Betulinic acid, a natural pentacyclic triterpenoid, prevents abdominal fat accumulation in mice fed a high-fat diet. J Agric Food Chem. 57:8776–8781.
- Gao D, Li Q, Li Y, Liu Z, Liu Z, Fan Y, Han Z, Li J, Li K. 2007. Antidiabetic potential of oleanolic acid from Ligustrum lucidum Ait. Can J Physiol Pharmacol. 85:1076–1083.
- Haidari F, Rashidi MR, Keshavarz SA, Mahboob SA, Eshraghian MR, Shahi MM. 2008. Effects of onion on serum uric acid levels and hepatic xanthine dehydrogenase/xanthine oxidase activities in hyperuricemic rats. Pak J Biol Sci. 11:1779–1784.
- Huang Q, Huang R, Wei L, Chen Y, Lv S, Liang C, Zhang X, Yin F, Li H, Zhuo L, et al. 2013. Antiviral activity of methyl helicterate isolated from Helicteres angustifolia (Sterculiaceae) against hepatitis B virus. Antiviral Res. 100:373–381.
- Huang Q, Li Y, Zhang S, Huang R, Zheng L, Wei L, He M, Liao M, Li L, Zhuo L, et al. 2012. Effect and mechanism of methyl helicterate isolated from Helicteres angustifolia (Sterculiaceae) on hepatic fibrosis induced by carbon tetrachloride in rats. J Ethnopharmacol. 143:889–895.
- Jayanthy G, Subramanian S. 2014. Rosmarinic acid, a polyphenol, ameliorates hyperglycemia by regulating the key enzymes of carbohydrate metabolism in high fat diet-STZ induced experimental diabetes mellitus. Biomed Prev Nutr. 3:431–437.
- Jiangsu New Medical College. 1986). Dictionary of Chinese herb medicines. Shanghai: Shanghai Scientific and Technologic Press.
- Kang C, Lee H, Jung ES, Seyedian R, Jo M, Kim J, Kim JS, Kim E. 2012. Saffron (Crocus sativus L.) increases glucose uptake and insulin sensitivity in muscle cells via multipathway mechanisms. Food Chem. 135:2350–2358.
- Laakso M. 2001. Insulin resistance and its impact on the approach to therapy of type 2 diabetes. Int J Clin Pract. 121:8–12.
- Lu Z, Jia Q, Wang R, Wu X, Wu Y, Huang C, Li Y. 2011. Hypoglycemic activities of A-and B-type procyanidin oligomer-rich extracts from different Cinnamon barks. Phytomedicine. 18:298–302.
- Lv HW, Zhu MD, Luo JG, Kong LY. 2014. Antihyperglycemic glucosylated coumaroyltyramine derivatives from Teucrium viscidum. J Nat Prod. 77:200–205.
- Masiello P, Broca C, Gross R, Roye M, Manteghetti M, Hillaire-Buys D, Novelli M, Ribes G. 1998. Experimental NIDDM: Development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes. 47:224–229.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. 1985. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 28:412–419.
- Meier JJ, Bonadonna RC. 2013. Role of reduced β-cell mass versus impaired β-cell function in the pathogenesis of type 2 diabetes. Diabetes Care. 36:S113–S119.
- Okita K, Iwahashi H, Kozawa J, Okauchi Y, Funahashi T, Imagawa A, Shimomura I. 2013. Homeostasis model assessment of insulin resistance for evaluating insulin sensitivity in patients with type 2 diabetes on insulin therapy. Endocr J. 60:283–290.
- Perez GRM, Zavala SMA, Perez GS, Perez GC. 1998. Antidiabetic effect of compounds isolated from plants. Phytomedicine. 5:55–75.
- Ramachandran S, Rajasekaran A, Manisenthilkumar K. 2012. Investigation of hypoglycemic, hypolipidemic and antioxidant activities of aqueous extract of Terminalia paniculata bark in diabetic rats. Asian Pac J Trop Biomed. 2:262–268.
- Shaw JE, Sicree RA, Zimmet PZ. 2010. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 87:4–14.
- Shimokawa T, Kagami M, Kato M, Kurosaki E, Shibasaki M, Katoh M. 2000. Effect of YM-126414 on glucose uptake and redistribution of glucose transporter isotype 4 in muscle cells. Eur J Pharmacol. 410:1–5.
- Wanyo P, Siriamornpun S, Meeso N. 2011. Improvement of quality and antioxidant properties of dried mulberry leaves with combined far-infrared radiation and air convection in Thai tea process. Food Bioprod Process. 89:22–30.
- Yang TC, Chao HF, Shi LS. 2014. Alkaloids from Coptis chinensis root promote glucose uptake in C2C12 myotubes. Fitoterapia. 93:239–244.
- Yao X, Zhu L, Chen Y, Tian J, Wang Y. 2013. In vivo and in vitro antioxidant activity and α-glucosidase, α-amylase inhibitory effects of flavonoids from Cichorium glandulosum seeds. Food Chem. 139:59–66.
- Zhang CH, Yu RY, Liu YH, Tu XY, Tu J, Wang YS, Xu GL. 2014. Interaction of baicalin with berberine for glucose uptake in 3T3-L1 adipocytes and HepG2 hepatocytes. J Ethnopharmacol. 151:864–872.