Abstract
Context: Citrus limon (L.) Burm.f. (Rutaceace) is a commonly available fruit variety with high medicinal and industrial values.
Objective: Lemon peel (LP) extract was studied as a potent preventive and curative agent for experimentally induced hyperoxaluric rats.
Materials and methods: Gas chromatography–mass spectrometry (GC–MS) analyses and toxicity study were performed for aqueous methanol LP extract. Twenty-four Wistar rats were segregated into four groups. Group 1: Control; Group 2: Urolithic (ethylene glycol (EG) – 0.75%); Group 3: Preventive study (EG + LP extract administration from 0th to 7th week); Group 4: Curative study (EG + LP extract administration from 4th to 7th week). Animals received LP extract daily by oral administration (100 mg/kg body weight) for 7 weeks.
Results and discussion: GC–MS analyses revealed that compound 6 was abundant in the LP extract (32%) followed by compound 1 (∼21%). The LD50 value of LP extract was found to be >5000 mg/kg of body weight. Urolithic rats showed significantly higher urinary calcium and oxalate (4.47 ± 0.44 and 18.86 ± 0.55 mg/24 h, respectively) excretion compared with control and experimental rats. Renal function parameters like urea (84 ± 8.5 and 96.1 ± 3.6 mg/dL), creatinine (1.92 ± 0.27 and 1.52 ± 0.22 mg/dL), and urinary protein (2.03 ± 0.02 and 2.13 ± 0.16 mg/24 h) were also reduced by LP extract (p < 0.001) and corroborated with tissue analyses (SOD, catalase, and MDA levels) and histological studies in normal and experimental animals. Immunohistochemical staining of THP and NF-κB in urolithic animals showed elevated expression than the control, while LP extract suppressed the expression of these proteins.
Conclusion: In conclusion, lemon peel is effective in curing kidney stone disease and also can be used to prevent the disease and its recurrence.
Introduction
Calcium oxalate (CaOx) stone formation in kidney is well known for its long history and a wide range of occurrence. A person becomes urolithic due to various factors such as genetic, socio-economical, environmental, and metabolic factors. A study reported by Coe et al. (Citation2005) states that about 12% of the global population are affected by urolithiasis and its recurrence rate is more than 50% within 10 years after first incidence (Kalaiselvi & Selvam, Citation2001). The process of stone formation (lithogenesis) is a complex phenomenon which starts with nucleation caused by supersaturation of urine. Aggregation occurs when the nucleated crystals bind to each other and enhanced aggregation can be seen in the presence of metabolically active urinary macromolecules. Eventually, the aggregated crystals damage the renal epithelium and get deposited (Li et al., Citation2009). Researchers have found that the multifactorial etiology and intricate pathophysiology of the stone formation is a challenging factor for designing a drug (Rathod et al., Citation2012).
Among the various stone forming causatives, reduced citrate excretion or hypocitraturia is a common phenomenon in CaOx stone formation. Alkalizing medications like citrate and bicarbonate salts are found to increase the protective urinary components (e.g., citrate, potassium, and pH) and reduce calcium oxalate supersaturation (Boruczkowska, Citation1994). The studies indicated that supplementation of citrate salts increase the expression of CaOx inhibitory protein, especially Tamm–Horsfall protein (THP) in the hyperoxaluric conditions (Pourmand et al., Citation2005). Increased expression of certain inflammatory markers like iNOS, p65- NF-κB, p38-MAPK, and oxidative stress markers were also observed in EG supplemented animals (Ilbey et al., Citation2009). This can be a possible evidence for CaOx crystal-induced lipid peroxidation in the renal epithelium. Hence, a potential antiurolithic drug must protect the membrane from CaOx crystals and avoid supersaturation by improving the urinary pH.
Citrus limon (L.) Burm.f. was classified under the family Rutaceace and consumption of its fruits aid in treating various disorders. The polyphenols and vitamin C contents in these fruits make them highly valuable in various medical and industrial purposes. Bioflavonoids are a group of polyphenols in Citrus that are the major contributors in antimicrobial, anti-inflammatory, and antioxidant properties (Sood et al., Citation2009). Apart from a bunch of biologically active polyphenolics, high content of citrate present in these fruits may also help in reduction of supersaturation. The study reported by Touhami et al. (Citation2007) proved that lemon juice reduced CaOx crystal deposition in the kidney of the experimental animals. Further, diets rich in citrate contents are commonly prescribed for stone prevention (Agarwal et al., Citation2011). In addition to that, Citrus fruits and their peels are found to help in improving various metabolic and inflammatory disorders attributing to the abundance in its bioactive compounds (Kundusen et al., Citation2011; Sood et al., Citation2010). Hence, this study was designed to investigate the efficacy of LP extract in experimentally induced hyperoxaluric animals.
Materials and methods
Chemicals and reagents
For the present study, solvents and other standard chemicals were purchased from Sisco Research Laboratories. Pvt. Ltd., Mumbai, India. The kits used for the estimation of calcium, urea, creatinine and protein were purchased from Span Diagnostics Ltd., Gujarat, India.
Preparation of LP extract
Lemon fruits were purchased from the local market and the specimens were authenticated and verified (Voucher no. VITRRL008/2013) as Citrus limon by Dr. B. Angeline Vijayakumari, Head and Department of Botany, Voorhees College, Vellore. The peels were removed, shade dried, and powdered. Extraction procedure was targeted to obtain flavonoids as followed by Nogata et al. (Citation2007). Lemon peels (100 g) were first extracted with n-hexane and the residue was further extracted with chloroform followed by hot ethanol. The extract was dried and partitioned with 80% aqueous methanol and n-hexane. The aqueous methanol fraction was air dried and dissolved in water for animal supplementation.
Gas chromatography–mass spectrometry (GC–MS) analysis
The phytochemical composition of the LP extract was analyzed using a GC–MS (GCD-HP1800A, Hewlett-Packard, Palo Alto, CA) equipped with a split/splitless capillary injection port. For GC–MS detection, an electron ionization system (Quadruples Analyzer – mass range: 10–425 amu) with an ionization energy of 70 eV was used. Each of these steps was carried out under high vacuum (10−4–10−8 torr). Helium gas was used as a carrier gas at a constant flow rate of 1 ml/min. Injector and mass transfer line temperatures were set at 250 and 280 °C, respectively. The components of fruit peel extracts were identified after comparison with those available online libraries like, NIST02 and AMDIS provided by National Institute of Standards and Technology (NIST) attached to the GC–MS instrument and reported.
Acute oral toxicity
The safe dose of aqueous methanol LP extract was calculated according to the Organization for Economic Co-operation and Development (OECD) guidelines 423 (adopted in 2001). Female Wistar rats were supplemented with LP extract at a dosage of 5000 mg/kg body weight and were monitored for 2 d to observe any immediate toxic effects. At the end of the 14th day, the animals were sacrificed and blood was collected by cervical decapitation under anesthetic condition. Blood collected with anticoagulant was used for hematological analyses, while blood without anticoagulant was centrifuged at 4000 rpm in 4 °C to separate the serum. Serum parameters such as albumin, protein, creatinine, urea, calcium, aspartate transaminase, alanine transaminase, alkaline phosphatase, and cholesterol were quantified and hemoglobin concentration, WBC, and RBC counts were also checked along with other hematological parameters.
Experimental design
All animal experiments and maintenance were carried out according to the ethical guidelines suggested by the Institutional Animal Ethics Committee (Registration No-1333/c/10/CPCSEA; Approval number: SBST/IAEC no./19/2010). Animals were housed in polypropylene cages and maintained under standard conditions of 12 h dark/light cycle at 27 ± 1 °C. The rats were supplied with regular pellets and water ad libitum in the VIT Animal House, Vellore.
To study the effect of lemon peel extract on preventing and curing the lithogenesis condition, 24 rats were divided into four independent groups with six animals in each group. Group 1 animals received 0.9% saline orally and were considered as normal. Group 2 animals received 0.75% ethylene glycol mixed with drinking water for 7 weeks. Group 3 animals were supplemented with ethylene glycol as mentioned above and were orally administered with 100 mg/kg body weight of aqueous methanol extract of lemon peel as a preventive group from the beginning of the study. Group 4 animals were also orally supplemented with EG as mentioned and administered with LP extract from the 4th week to the 7th week of the experimental study as a treatment group.
Urinary investigations
At the end of the 7th week, rats were housed in metabolic cages and urine samples were collected under acidified conditions. The collected samples were centrifuged at 2500 rpm (REMI, R24, Mumbai, India) for 5 min and the supernatant was estimated for calcium, urea, creatinine, and protein by using the commercially available kit reagents. Oxalate was measured by the method proposed by Hodgkinson and Williams (Citation1972), phosphate by that of Goldenberg and Fernandez (Citation1966), and citrate was estimated using penta bromoacetone proposed by Rajagopal (Citation1984). The values are expressed as mg/24 h urine.
Serum investigations
The rats were anaesthetized and blood was collected from the retro-orbital region and centrifuged at 10 000 rpm for 10 min to separate serum. The serum was analyzed for levels of calcium, urea, phosphate, creatinine, and protein and results were expressed as mg/dL. Calcium, urea creatinine, and protein were estimated using commercially available reagents and phosphorus was measured using method proposed by Goldenberg and Fernandez (Citation1966). The serum analyses were performed after seven weeks of study.
Tissue investigations
After 7 weeks of study, the animals were sacrificed under anesthesia to avoid pain and stress. Kidney tissue was removed carefully and washed in phosphate buffered saline. Then the tissue was trimmed off connective tissues and 10% tissue homogenate was prepared using 0.01 M Tris-HCl buffer, pH 7.4. Tissue homogenate was used for estimating catalase activity by Sinha’s (Citation1972) method, superoxide dismutase activity by Marklund and Marklund method (1974) and level of lipid peroxidation was determined by method proposed by Ohkawa et al. (Citation1979).
Histopathological examinations
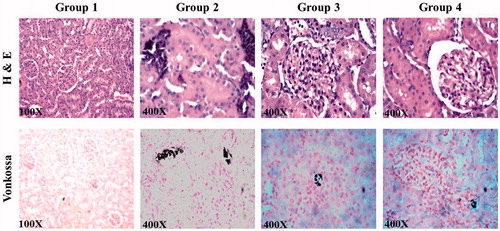
A part of kidneys was removed and washed with phosphate buffered saline (PBS). The kidneys were then fixed in 10% neutral buffered formalin and the tissues were processed and embedded in paraffin. Sections were cut to a thickness of 4 μm using Leica RM 2126 microtome (Leica Inc., Allendale, NJ) and mounted on slides. The slides were stained with haematoxylin and eosin (H&E) and Von Kossa stains for histopathological analyses. The sections were photographed under microscope (Olympus BX51; Olympus optical, Tokyo, Japan) at a magnification of ×400. H and E staining was used to study the tubular damage and crystal deposition while Von Kossa staining was performed to observe the intratubular calcium deposition.
Immunohistochemical analyses
Paraffin sections (4 μm thick) were cut, mounted on slides, dewaxed in xylene, and rehydrated in graded alcohol. Endogeneous peroxidase activity was blocked by incubation with 3% H2O2 for 15 min. After washing with PBS containing 0.1% Tween 20, the slides were incubated overnight with primary antibody for THP (1:200 dilutions) and NF-κB (1:200 dilutions) at 4 °C. The immunoreactivity was performed using horseradish peroxidase conjugated with goat-anti-rabbit IgG antibody by incubating for 30 min at room temperature. The detection step was performed by treatment with 3,3′-diaminobenzidine (Dako, Carpinteria, CA) as chromogen. Slides were counter stained with haematoxylin, rinsed with tap water, dehydrated, placed in xylene, and mounted. The sections were then photographed at a magnification of ×400.
Statistical analysis
The data were analyzed on Graph Pad Prism 5.01 software (GraphPad Software, Inc., La Jolla, CA) and expressed as mean ± SD (n = 6). Statistical analysis was performed by one-way ANOVA followed by Dunnett’s test to compare the diseased and the LP extracts supplemented groups. At the same time, the toxicity study was analyzed by un-paired t-test. The results were considered statistically significant, if p < 0.05.
Results
GC–MS analysis
The phytochemical composition of aqueous methanol LP extract was analyzed by GC–MS and the compounds present in the LP extract are shown in and the structures of these compounds are given in ).
Table 1. Major compounds obtained from GC–MS analyses of the LP extract.
Acute oral toxicity
The animals did not show any signs of toxicity at the end of 14 d after administration of 5000 mg/kg body weight of animals. The serum and hematological parameters of the extract supplemented rats showed no significant alterations when compared with the control rats ( and ). Although there was a major change in the platelet count between control and the LP extract-supplemented rats, the values are well within the normal physiological range. The results of the toxicity study clearly suggested that the LD50 cut off must be greater than 5000 mg/kg of body weight of animals and according to the Globally Harmonized Classification System (GHS), the LP extract falls under the category 5 or unclassified category of LD50 range. Hence, it is safer to use the extract at a concentration of 100 mg/kg of body weight for the prevention and treatment of kidney stone disease.
Table 2. Serum parameters of LP extract supplemented and control rats.
Table 3. Haematological parameters of LP extract supplemented and control rats.
Serum and urine parameters
Significant changes in the urinary and serum constituents were observed in the animals supplemented with ethylene glycol as shown in and . The major inorganic constituent calcium was elevated in urine and reduced in serum (8.39 mg/dL) of the urolithic rats compared with the control animals. But supplementation of aqueous methanol LP extract had shown significant preventive and curative effect in Group 3 and 4 animals, respectively, at the significant level (p < 0.05). LP extract manages the serum calcium level in order to prevent the progression of hypercalciuria to urolithiasis. Hypercalciuria was accompanied by hyperoxaluria in EG-supplemented rats and an increase of oxalate up to 18.86 mg in 24 h urine samples was observed on the 7th week. Oral administration of LP extract throughout the study period has decreased the excretion of oxalate to 4.24 mg/24 h (p < 0.05). Treatment strategy with LP extract showed a urinary oxalate concentration of 7.07 mg which can be reported as a significant reduction (p < 0.05) compared with the urolithic animals. Phosphate was found to be another urinary constituent that significantly increased in urine, but at the same time, it decreased in serum of EG-supplemented animals (7.77 mg/24 h), relative to control animals. LP extract limits the phosphorus leak in the serum at 7.82 and 7.66 mg in Groups 3 and 4, respectively. This is obviously reflected in the urinary phosphorus level which was reduced to 5.6 and 5.7 mg/24 h urine sample of the experimental group relative to urolithic animals. Citrate is well-known inhibitor for CaOx crystal aggregation, which decreased drastically in urolithic rats, but supplementation of LP extract enhances citrate level significantly as shown in .
Table 4. Effect of LP extract on urinary parameters of control and experimental groups.
Table 5. Effect of LP extract on serum parameters of control and experimental groups.
Levels of urinary and serum creatinine, urea, and protein are the best indicators of renal damage. As shown in , at the end of 7 weeks, the levels of serum creatinine and urea in Groups 3 and 4 were close to normal, when compared with urolithic animals. Urinary excretion of creatinine and urea decreased in EG-challenged rats (), while LP extract significantly normalized the excretion of renal damage markers. This was further validated by the urinary protein level which was reduced to 2.03 and 2.13 mg in 24 h urine by LP treatment in Groups 3 and 4, when compared with EG administration (Group 2). These results showed that LP extract alleviated the membrane damage caused by hyperoxaluria and CaOx crystals in Group 4 animals and also managed to protect the Group 3 animals from oxalate challenge. The above-mentioned results exhibited the efficacy of LP extract in prevention and treatment of urolithiasis. Histopathological examination of the kidney tissues excised from the control and other experimental animals was performed to validate the improvement in urinary and serum parameters.
Tissue antioxidant enzymes
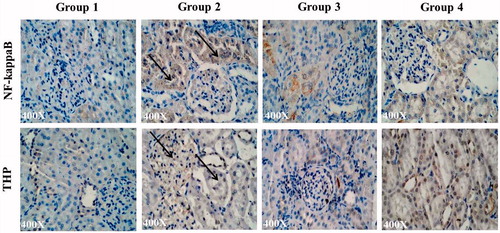
In uroliothic rats (Group 2), crystal-induced oxidative damage was obviously demonstrated by high lipid peroxidation level and increased antioxidant enzyme levels (). Superoxide dismutase activity was reduced to 11.27 and 10.6 units/mg of protein in LP extract-supplemented rats, which is comparable with control values (). reveals that LP extract administration significantly reduced the catalase activity (17.16 and 12.46 units/mg of protein) and follows a similar trend like SOD activity. While the antioxidant enzymes were being normalised, the ability of LP extract in nephroprotection was also clearly exhibited by the reduction in lipid peroxidation process which was about 0.47 and 0.55 mg of MDA/mg of protein (). The diseased rats showed MDA level as high as 1.12 mg/mg of protein, which shows that the LP extract has shown significant renal membrane protection and which resulted in reduction of antioxidant enzyme levels.
Figure 2. Effects of LP extract on tissue antioxidant enzymes. (a) Superoxide dismutase (SOD); (b) catalase, and (c) lipid peroxidation. The values are expressed as mean ± SD. The results were statistically analyzed by one-way ANOVA with Bonferroni’s multiple comparison post test (n = 6). The comparisons were made as ‘a’ – Group 1 versus Group 2; ‘b’ – Group 2 versus Groups 3; ‘c’ – Group 2 versus Groups 4. ***p < 0.001, **p < 0.01, *p < 0.05.
Histology and immunohistochemistry
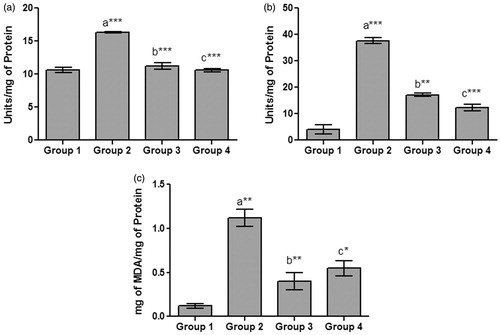
Histological examination of kidney sections showed prominent deposition of crystals and tubular degeneration in urolithic rats. An increased tubular necrosis with interstitial edema was observed at the end of 7 weeks, which shows the complete progression of the disease (). Lemon peel extract was found to be effective in preventing the crystal deposition and renal damage. Group 3 animals that received LP extract from day one, showed a mild tubular degeneration and almost a normal histology. Animals from Group 4 showed a moderate tubular necrosis and reduction of calcium deposits compared with the Group 2. The absence of calcium oxalate crystals in Group 3 animals demonstrates the protective effect of LP extract, while the kidney of Group 4 animals showed significant improvement in regeneration of tubules proving the ability of the extract to dissolve the preformed and aggregated crystals. The presence of calcium containing stones was confirmed by Von Kossa staining of kidney sections of diseased animals, where calcium deposits were observed as dark intratubular aggregates (). Reduction of these dark spots in LP extract-treated rats showed that it was effective in interfering with crystal deposition process.
Figure 3. Figure shows kidney sections of control and various experimental groups stained with haematoxylin and eosin and Von Kossa (×400).
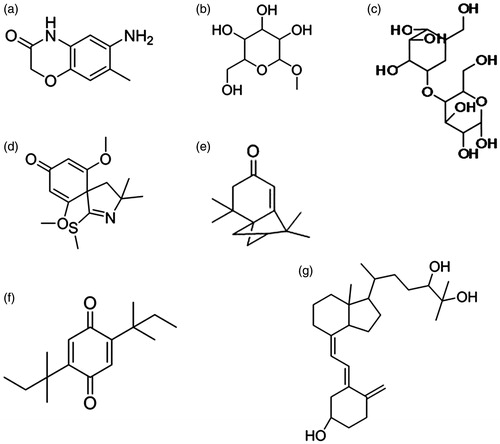
Immunohistochemical studies confirmed the presence of Tamm–Horsfall protein (THP) in the distal part of the nephron () in the control kidney. But, EG-supplemented rats showed a prominent increase (>50% increase than control) in the expression of THP on the 7th week and it was prominently expressed in distal tubule and occasionally in the glomerulus. THP was localized primarily in distal tubules and no glomerular expression was observed in the LP extract supplemented rats. Preventive therapy with LP extract completely reduced the THP expression to normal, while a 10% increase in expression was found in Group 4. Expression of NF-κB was also found to be significantly increased in the EG-supplemented animals and localized in the proximal and distal tubular regions (). Group 3 animals with LP extract fed from day one had a very low expression of this pro-inflammatory cytokine and observed to be near normal, while Group 4 animals showed an increased expression of NF-κB compared with control, but significantly low compared to diseased animals. This shows that LP extract has brought the homeostasis in the renal environment after initial oxalate and CaOx insult.
Discussion
Management of kidney stone disease and its recurrence require balanced diet, lifestyle modification, and a safer medical therapy. Single therapy that can handle all the risk factors for CaOx stone formation has been the prime concern. So, if the drugs focus on certain primary risk factors like supersaturation, crystal deposition and membrane damage that will assist management of urolithiasis. Various folk medicines are being used as a treatment for kidney stone disease including Costus igneus N.E.Br. (Costaceae), banana stem, Boerhavia diffusa L. nom. cons. (Nyctaginaceae), Salix taxifolia H.B.K-Kunth. (Salicaceae), etc. (Manjula et al., Citation2012; Pareta et al., Citation2011; Poonguzhali & Chegu Citation1994; Vargas & Pérez Citation2002). In the search for a safer and alternate antiurolithic agent, we targeted the Citrus limon (lemon) peel, which is composed of bioflavonoids like hesperidin, eriocitrin, narigenin, rutin, etc. These compounds have a large spectrum of biological activity including antibacterial, antifungal, antidiabetic, anticancer, and antiviral activities (Burt, Citation2004). These flavonoids can function as direct antioxidants, free radical scavengers, and have the capacity to modulate enzymatic activities and protect membranes (Ortuno et al., Citation2006). The diversity of experimental results obtained in this study, which demonstrated the effect of Citrus limon peel extract on urinary lithogenicity, offered inevitable data for the management of calcium oxalate stone disease. This study provided therapy in the form of LP extract, which provides a reasonable alkali load and promotes hypercitraturia that can reduce the propensity of calcium oxalate crystal deposition and removal of pre-deposited crystals in the kidney of urolithic rats.
Preparation of aqueous methenolic LP extract was directed towards identification of bioflavonoids in lemon peel. The extraction started with removal of essential oils by n-hexane and chloroform was used to remove other non-polar compounds. The remaining polar compounds and flavonoids were extracted with ethanol at a temperature of about 90 °C and partitioned with aqueous methanol and n-hexane as performed by Cai et al. (Citation2010). In order to observe the mechanism behind the antilithogenic role of lemon peel, we studied the composition of the LP extracts using GC–MS analysis before exhibiting its antilithogenic potential.
There are several reports on chemical composition of various Citrus species which includes identification and quantification of many volatile compounds and essential oils. On one hand, Limonine is one of the abundant essential oils present in the Citrus fruit peel (Qiao et al., Citation2008; Singh et al., Citation2010). On the other hand, flavonoids and other phenolic compounds were testified to be extracted efficiently by polar solvents (Karimi et al., Citation2012). In our study, the extraction procedure mainly focussed on the obtaining the flavonoids and other phenolic compounds and the GC–MS analysis demonstrates that we have been successful in extracting important phenolic compounds that are rich in the Citrus peel. As shown in , the compound 1 represents the oxazine derivatives that are highly established bioactive compounds reported to have antioxidant and antimicrobial properties (Chylińska et al., Citation1971). This group of compounds also assists in the smooth muscle contraction of urinary bladder (Imai et al., Citation2001). The presence of glycosides detected (compounds 2 and 3) in the mass spectrum was the prime evidence for the presence of flavonoids in the LP extract. The hydroquinone derivative (compound 6) which was also identified in the extract is well known for its ability to scavenge the lipid peroxyl radical, which prevents the membrane damage (Yamaguchi et al., Citation2006). Compounds 1 and 6 are the major constituents of the extract and can be acclaimed for performing the role of membrane protection. These compounds can also be assumed to show its hypotensive property, which leads to increased diuresis that dissolves and flushes the crystals out, similar to the activity of thiazides and α-blockers (Seitz et al., Citation2009).
Many in vivo models have been built up to explore the effect of various therapeutic agents on progression of the disease. Rats are the most frequently used animals in the models of the CaOx deposition in the kidneys, a process that mimics the etiology of kidney stone formation in humans (Atmani et al., Citation2004). Rat models of CaOx urolithiasis induced by either EG alone or in combination with other drugs such as ammonium chloride are often used to study the pathogenesis of kidney crystal deposition. Sood et al. (Citation2010) studied the role of lemon peel in treatment of peptic ulcer in Wistar rats with various concentrations starting from 200 mg/kg of body weight. The lack of effective dosage information about the LP extract for animal supplementation with respect to urolithiasis study led us to perform a pilot study on toxicity of the extract ( and ). Based on the preliminary investigation, we assigned 100 mg/kg of bodyweight as optimal and effective dose for oral supplementation.
Hyperoxaluria is the primary risk factor for calcium oxalate deposition in the kidney, and at the end of 20 d, EG-supplemented animals excreted elevated amounts of oxalate in the urine. This was mainly due to the increased availability of precursor for the oxalate biosynthesis leading to a positive regulation of enzymes involved in the pathway (Thamilselvan et al., Citation2003). As reported by Selvam et al. (Citation2001), the increased oxalate in EG-supplemented animals led to elevated levels of calcium and phosphorous in urine. Our results also demonstrated increased excretion of calcium and inorganic phosphorus in urolithic rats, supported by hyperoxaluria. This hyperoxaluric and hypercalciuric state was corrected by supplementing LP extract which limit excess excretion of these major urolithic components. Hypocitraturia, another predisposing factor in calculogenesis, was maintained till 7 weeks in diseased animals. The urinary excretion of citrate increased in LP extract administered animals and probably helped in chelation with calcium ions in urine. This helps in avoiding the vital step of crystal nucleation and further agglomeration. Apart from being a calcium chelator, the presence of citrate enhances the alkaline condition and improves the pH of the urine after challenging the rats with EG (Zuckerman & Assimos, Citation2009).
These data were further supported by urinary and serum renal damage markers like protein, urea and creatinine which were normalized in the urolithic rats, co-supplemented with LP extract in the Groups 3 and 4. Proteinuria is a marker for the tubular damage and dysfunction, which was found to be obvious in urolithic rats (Resnick & Boyce, Citation1979). The components of LP extract assisted to decrease tubular damage and it was reflected as diminution in the urinary protein excretion of the experimental animals. Obstruction of urinary tract by stones results in decreased GFR, followed by the accumulation of waste products like urea and creatinine in the serum (Bayir et al., Citation2011). The study conducted by Touhami et al. (Citation2007) proved that administration of lemon juice to urolithic animals restored urinary renal damage marker, as shown in our study where LP extract exhibited restoration of blood urea nitrogen, serum creatinine, and urinary protein to near normal. Our results indicate that LP extract ameliorated the outcome of CaOx crystal growth or agglomeration, and helped in increasing the urine clearance rate in diseased rats.
Superoxide dismutase and catalase are the major cellular antioxidant enzymes that keep the ROS and oxidative stress under threshold constantly in our system. Change in ROS level due to various disease conditions or toxic reactions significantly alter the level of these antioxidant enzymes in order to balance the redox status (Rashed et al., Citation2004). Hyperoxaluria and calcium oxalate crystallization generated a large amount of ROS and RNS. This triggered the antioxidant switch in the renal cells which was observed as a raise in catalase and SOD activity in urolithic rats. LP extract being a reservoir of biologically active compounds joins the antioxidant system in the kidney to restore the redox balance. Our results were in correlation with the observations of Gandhi et al. (Citation2013). As an outcome of increased ROS production in the renal system due to crystals, lipid peroxidation was observed in urolithic rats and LP extract helped in neutralizing the free radicals and preventing LPO formation and membrane damage to a significant extent (Selvam, Citation2002).
The study conducted by Grases et al. (Citation1994) concluded that medicinal plants had little effect on the urinary chemistry of urolithiasis. But in our study, we proved that urinary and serum urolithic constituents like calcium, oxalate, phosphorous, etc., were significantly normalized in EG-supplemented experimental animals which are co-administered with Citrus limon peel extract, both as preventive and curative agent. The concurrency in the results shown by our urine and serum parameters to the reports of Soundararajan et al. (Citation2006) and various other established research groups infer that the bioflavonoids and other bioactive compounds in LP extract played a vital role in minimizing the calcium oxalate stone formation by reducing urinary risk factors and membrane protection (Aggarwal et al., Citation2010; Grases et al., Citation1995; Lin et al., Citation2012).
Von Kossa and H & E staining of kidney sections from diseased animals at the end of the 7th week were subjected to microscopic examination which confirmed that nephrolithic rats showed intratubular and interstitial crystal deposits consistent of other investigator findings (Khan et al., Citation2010; Shukla et al., Citation2013). The presence of such deposits is evidence of adhesion and retention of calcium oxalate crystal within the renal tubules. LP extract showed a prophylactic activity as it restores the normal kidney architecture of the animals. As reported by Dhanavade et al., (Citation2011) antioxidant potential of the extract and its bioactive components is the only possible reason to validate the results showing nephroprotection from hyperoxaluric and CaOx insult.
Urinary macromolecular expression and secretion always play a role in innate protection against this disease (Wesson et al., Citation2003). Hyperoxaluria and CaOx crystals have the ability to damage the renal tubules through inflammatory process. Our results showed the changes in urolithic kidney with higher tubular expression of NF-κB, a pro-inflammatory cytokine that manages the expression of other inflammatory cytokines. Prevention and treatment with LP extract has significantly reduced NF-κB expression, showing a decline in inflammation-mediated cellular damage, through various flavonoids and other polyphenols that can possess anti-inflammatory properties. Tamm–Horsfall protein (THP), being a potent crystal inhibitor, has a tendency to get expressed more in the diseased state in order to interfere with crystal aggregation process and preventing them from adhering to the tubules (Mo et al., Citation2004). Decrease in its expression of THP shows LP extract has managed to attenuate the progression of urolithiasis.
Conclusion
The results obtained from the study conclude that LP extract is potent in preventing kidney stone formation and can effectively cure the progression of the disease. The extract effectively decreases excess excretion of calcium, oxalate, phosphate, and citrate in urine and accumulation of creatinine and urea in serum. Histological examinations reveal the decrease in tubular damage, which may be due to bio-flavonoids in the LP extracts which reduces the oxidative stress and maintains the membrane integrity so that CaOx crystal deposition process can be evaded. Above-mentioned findings are the evidences for antiurolithic and nephroprotective role of crude LP extract in the management of urolithiasis. Hence, further studies are warranted to explore and isolate the active principle responsible for antilithogenic role of LP extract, which can be subsequently used for reducing the incidence of recurrent calcium oxalate stone formation in human.
Acknowledgements
Authors are grateful to Dr. B. Angeline Vijayakumari, The Head, Department of Botany, Voorhees College, Vellore, for validating the plant specimen. Authors would like to acknowledge Dr. Prashanth Kumar N. S., Assistant Professor (Senior), School of Social Science and Languages, VIT University. Authors are thankful to VIT University for providing instrumentation and other resources to carry out the project.
Declaration of interest
The authors report that they have no conflicts of interest. T. M. Shiju and S. Badrinathan are thankful to Council of Scientific and Industrial Research and Indian Council of Medical Research, respectively for providing fund in the form of Senior Research Fellowship
Reference
- Agarwal MM, Shwaran K, Singh SK, et al. (2011). Preventive fluid and dietary therapy for urolithiasis: An appraisal of strength, controversies and lacunae of current literature. Indian J Urol 27:310–19.
- Aggarwal A, Tandon S, Singla SK, Tandon C. (2010). Diminution of oxalate induced renal tubular epithelial cell injury and inhibition of calcium oxalate crystallization in vitro by aqueous extract of Tribulus terrestris. Int Braz J Urol 36:480–9.
- Atmani F, Slimani Y, Mimouni M, et al. (2004). Effect of aqueous extract from Herniaria hirsuta L. on experimentally nephrolithiasic rats. J Ethanopharmacol 95:87–93.
- Bayir Y, Halici Z, Kelesc MS, et al. (2011). Helichrysum plicatum DC. subsp. plicatum extract as a preventive agent in experimentally induced urolithiasis model. J Ethanopharmacol 138:408–14.
- Boruczkowska A. (1994). Effect of urine alkalization on excretion of renal citrate and degree of urine saturation with calcium oxalate in patients with calcium-oxalate urolithiasis and in healthy subjects. Pol Arch Med Wewn 91:77–83.
- Burt SA. (2004). Essential oils: Their antibacterial properties and potential applications in foods: A review. Int J Food Microbiol 94:223–53.
- Cai W, Gu X, Tang J. (2010). Extraction, purification, and characterisation of the flavonoids from Opuntia milpa alta Skin. Czech J Food Sci 28:108–16.
- Chylińska JB, Janowiec M, Urbański T. (1971). Antibacterial activity of dihydro-1,3-oxazine derivatives condensed with aromatic rings in positions 5, 6. Br J Pharmacol 43:649–57.
- Coe FL, Evan A, Worcester E. (2005). Kidney stone disease. J Clin Invest 5:2598–608.
- Dhanavade MJ, Jalkute CB, Ghosh JS, Sonawane KD. (2011). Study antimicrobial activity of lemon (Citrus lemon L.) peel extract. Br J Pharmacol Toxicol 2:119–22.
- Gandhi M, Aggarwal M, Puri S, Singla SK. (2013). Prophylactic effect of coconut water (Cocos nucifera L.) on ethylene glycol induced nephrocalcinosis in male Wistar rat. Int Braz J Urol 39:108–17.
- Goldenberg H, Fernandez A. (1966). Simplified method for estimation of inorganic phosphorus in body fluids. Clin Chem 12:871–82.
- Grases F, Melero G, Costa-Bauzá A, et al. (1994). Urolithiasis and phytotherapy. Int Urol Nephrol 26:507–11.
- Grases F, Ramis M, Costa-Bauzá, March JG. (1995). Effect of Herniaria hirsuta and Agropyron repens on calcium oxalate urolithiasis risk in rats. J Ethnopharmacol 45:211–14.
- Hodgkinson A, Williams A. (1972). An improved colorimetric procedure for oxalate. Clin Chim Acta 36:127–32.
- Ilbey YO, Ozbek E, Simsek A, et al. (2009). Effects of pomegranate juice on hyperoxaluria-induced oxidative stress in the rat kidneys. Renal Failure 31:522–31.
- Imai T, Okamoto T, Yamamoto Y, et al. (2001). Effects of different types of K+ channel modulators on the spontaneous myogenic contraction of guinea-pig urinary bladder smooth muscle. Acta Physiol Scand 174:323–33.
- Kalaiselvi P, Selvam R. (2001). Effect of experimental hyperoxaluria on renal calcium oxalate monohydrate binding proteins in the rat. Br J Urol Int 87:110–16.
- Karimi E, Oskoueian E, Hendra R, et al. (2012). Phenolic compounds characterization and biological activities of Citrus aurantium bloom. Molecules 17:1203–18.
- Khan NI, Shinge JS, Naikwade NS. (2010). Antilithiatic effect of Helianthus annuus Linn. leaf extract in ethylene glycol and ammonium chloride induced nephrolithiasis. Int J Pharm Pharm Sci 2:180–4.
- Kundusen S, Haldar PK, Gupta M, et al. (2011). Evaluation of antihyperglycemic activity of Citrus limetta fruit peel in streptozotocin-induced diabetic rats. ISRN Endocrinol [online]. Available from: http://www.hindawi.com/journals/isrn/2011/869273/abs. Epub 2011 Jul 21.
- Li WM, Chou YH, Li CC, et al. (2009). Association of body mass index and urine pH in patients with urolithiasis. Urol Res 37:193–6.
- Lin WC, Lai MT, Chen HY, et al. (2012). Protective effect of Flos carthami extract against ethylene glycol-induced urolithiasis in rats. Urol Res 40:655–61.
- Manjula K, Rajendran K, Eevera T, Kumaran S. (2012). Effect of Costus igneus stem extract on calcium oxalate urolithiasis in albino Wistar rats. Urol Res 40:499–10.
- Marklund S, Marklund G. (1974). Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–74.
- Mo L, Huang HY, Zhu XH, et al. (2004). Tamm–Horsfall protein is a critical renal defence factor protecting against calcium oxalate crystal formation. Kidney Int 66:1159–66.
- Nogata Y, Ohta H, Ishii T, Sekiya K. (2007). Isolation of eriocitrin (eriodictyol 7-O-rutinoside) as an arachidonate lipoxygenase inhibitor from Lumie fruit (Citrus lumia) and its distribution in citrus species. J Sci Food Agric 87:82–9.
- Ohkawa H, Ohisi N, Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–8.
- Organisation for Economic Co-operation and Development (OECD). (2001). Guidelines for the Testing of Chemicals: 423. Acute Oral Toxicity-Acute toxic class method. (Adopted in December 2001), 1–14.
- Ortuno A, Baidez P, Gomez P, et al. (2006). Citrus paradisi and Citrus sinensis flavonoids: Their influence in the defence mechanism against Penicillium digitatum. Food Chem 98:351–8.
- Pareta SK, Patra KC, Mazumder PM, Sasmal D. (2011). Aqueous extract of Boerhaavia diffusa root ameliorates ethylene glycol-induced hyperoxaluric stress and renal injury in rat kidney. Pharm Biol 49:1224–33.
- Poonguzhali PK, Chegu H. (1994). The influence of banana stem extract on urinary risk factors for stones in normal and hyperoxaluric rats. Br J Urol 74:23–5.
- Pourmand G, Nasseh H, Sarrafnejad A, et al. (2005). Urinary Tamm–Horsfall protein and citrate: A case-control study of inhibitors and promoters of calcium stone formation. Urol J 2:79–85.
- Qiao Y, Xie BJ, Zhang Y, et al. (2008) Characterization of aroma active compounds in fruit juice and peel oil of Jinchen Sweet Orange fruit (Citrus sinensis L. Osbeck) by GC–MS and GC-O. Molecules 13:1333–44.
- Rajagopal G. (1984). A simple colorimetric procedure for estimation of citric acid in urine. Indian J Exp Biol 22:391–2.
- Rashed T, Menon M, Thamilselvan S. (2004). Molecular mechanism of oxalate-induced free radical production and glutathione redox imbalance in renal epithelial cells: Effect of antioxidants. Am J Nephrol 24:557–68.
- Rathod NR, Biswas D, Chitme HR, et al. (2012). Antiurolithiatic effects of Punica granatum in male rats. J Ethnopharmacol 140:234–8.
- Resnick MI, Boyce WH. (1979). Low molecular weight urinary proteins and renal lithiasis. Invest Urol 16:270–3.
- Seitz C, Liatsikos E, Porpiglia F, et al. (2009). Medical therapy to facilitate the passage of stones: What is the evidence? Eur Urol 56:455–71.
- Selvam R. (2002). Calcium oxalate stone disease: Role of lipid peroxidation and antioxidants. Urol Res 30:35–47.
- Selvam R, Kalaiselvi P, Govindaraj A, et al. (2001). Effect of Aerva lanata leaf extract and Vediuppu chunnam on the urinary risk factors of calcium oxalate urolithiasis during experimental hyperoxaluria. Pharmacol Res 43:89–93.
- Shukla AB, Mandavia DR, Barvaliya MJ, et al. (2013). Antiurolithiatic effect of cow urine ark on ethylene glycol-induced renal calculi. Int Braz J Urol 39:565–71.
- Singh P, Shukla R, Prakash B, et al. (2010). Chemical profile, antifungal, antiaflatoxigenic and antioxidant activity of Citrus maxima Burm. and Citrus sinensis (L.) Osbeck essential oils and their cyclic monoterpene, dl-limonene. Food Chem Toxicol 48:1734–40.
- Sinha AK. (1972). Colorimetric assay of catalase. Anal Biochem 47:389–94.
- Sood S, Arora B, Bansal S, et al. (2009). Antioxidant, anti-inflammatory and analgesic potential of the Citrus decumana L. peel extract. Inflammopharmacology 17:267–74.
- Sood S, Muthuraman A, Gill NS, et al. (2010). Effect of Citrus karna peel extract on stress induced peptic ulcer on rat. J Biol Sci 10:231–6.
- Soundararajan P, Mahesh R, Ramesh T, Begum VH. (2006). Effect of Aerva lanata on calcium oxalate urolithiasis in rats. Indian J Exp Biol 44:981–6.
- Thamilselvan S, Khan SR, Menon M. (2003). Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: Effect of antioxidants. Urol Res 31:3–9.
- Touhami M, Laroubi A, Elhabazi K, et al. (2007). Lemon juice has protective activity in a rat urolithiasis model. BMC Urol 7:18–27.
- Vargas R, Pérez RM. (2002). Antiurolithic activity of Salix taxifolia aqueous extract. Pharm Biol 40:561–3.
- Wesson JA, Johnson RJ, Mazzali M, et al. (2003). Osteopontin is a critical inhibitor of calcium oxalate crystal formation and retention in renal tubules. J Am Soc Nephrol 14:139–47.
- Yamaguchi LF, Lago JH, Tanizaki TM, et al. (2006). Antioxidant activity of prenylated hydroquinone and benzoic acid derivatives from Piper crassinervium Kunth. Phytochemistry 67:1838–43.
- Zuckerman JM, Assimos G. (2009). Hypocitraturia: Pathophysiology and medical management. Rev Urol 11:134–44.