?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Context: Fungal infections caused by fluconazole-resistant Candida albicans are an intractable clinical problem, calling for new efficient antifungal drugs. Kaempferol, an active flavonoid, has been considered a potential candidate against Candida species.
Objective: This work investigates the resistance reversion of kaempferol in fluconazole-resistant C. albicans and the underlying mechanism.
Materials and methods: The antifungal activities of fluconazole and/or kaempferol were assessed by a series of standard procedures including broth microdilution method, checkerboard assay and time-kill (T-K) test in nine clinical strains as well as a standard reference isolate of C. albicans. Subsequently, the morphological changes, the efflux of rhodamine 6G, and the expressions of CDR 1, CDR 2, and MDR 1 were analysed by scanning electron microscope (SEM), inverted fluorescence microscope and quantitative reverse transcription polymerase chain reaction (qRT-PCR) in C. albicans z2003.
Results: For all the tested C. albicans strains, the minimum inhibitory concentrations (MICs) of fluconazole and kaempferol ranged 0.25–32 and 128–256 μg/mL with a range of fractional inhibitory concentration index of 0.257–0.531. In C. albicans z2003, the expression of both CDR 1 and CDR 2 were decreased after exposure to kaempferol alone with negligible rhodamine 6G accumulation, while the expression of CDR 1, CDR 2 and MDR 1 were all decreased when fluconazole and kaempferol were used concomitantly with notable fluorescence of rhodamine 6G observed.
Discussion and conclusion: Kaempferol-induced reversion in fluconazole-resistant C. albicans might be likely due to the suppression of the expression of CDR1, CDR2 and MDR1.
Introduction
Candida albicans is currently the most medically important fungi leading to high risks ranging from superficial mucosal infections to life-threatening systemic diseases. Candidiasis caused by C. albicans accounts for 40–60% mortality in immunocompromised patients (Tobudic et al. Citation2012). Due to long-term use of triazoles, such as fluconazole (FLC), in clinical settings, fluconazole-resistant C. albicans occurs with increasing prevalence. The available anticandidal drugs are limited. Therefore, the search for novel effective agents to reverse the resistance of fluconazole-resistant C. albicans becomes urgent. Many traditional antioxidant, anti-inflammatory, and antibacterial agents have been considered to have favorable antifungal potential alone and/or in combination with FLC against fluconazole-resistant C. albicans (Zhou et al. Citation2012; Letscher-Bru et al. Citation2013).
Several chemicals from traditional herbs, such as tetrandrine (Zhang et al. Citation2013) and allicin (Khodavandi et al. Citation2011), have been recognised as alternatives to enhance the antifungal activity of FLC. Flavonoids are a group of chemicals, and have also been reported to have broad-spectrum antibacterial and antifungal activities (Friedman Citation2007; Orhan et al. Citation2010; Peralta et al. Citation2012). Kaempferol (KAE), one of the active flavonoids, widely exists in many fruits and plants with broad-spectrum antioxidant, anti-inflammatory and anticancer properties (Rajendran et al. Citation2014). As demonstrated previously, KAE and its derivatives (especially kaempferol rhamnoside) have been considered to possess satisfactory antibacterial and antifungal capabilities (Kataoka et al. Citation2001; Tatsimo et al. Citation2012; Martino et al. Citation2014; Pinho et al. Citation2014). Recently, KAE was reported to present synergistic antifungal activities with histone deacetylase (HDAC) inhibitor against C. tropicalis in spite of its mild activity against C. albicans (Rajasekharan et al. Citation2014). To our knowledge, KAE as an agent to treat fluconazole-resistant C. albicans has received little attention, and relevant antifungal mechanisms of KAE remain unclear.
In this study, broth microdilution method, checkerboard assay, time-kill (T-K) test and scanning electron microscope (SEM) were used to evaluate the antifungal activities of KAE and/or FLC, and the quantitative reverse transcription polymerase chain reaction (qRT-PCR) was employed to analyse the expression of CDR1, CDR2, and MDR1.
Materials and methods
Strains and cultivation
Candida albicans SC 5314 was kindly provided by Prof. YuanYing Jiang from College of Pharmacy, Second Military Medical University, Shanghai, China. Candida albicans z2003, z1007, z1105, z1110, 2144, 2019, 2209, sp1927, and 215 were kindly provided by Prof. HuaiWei Lu, Clinical Laboratory, Anhui Provincial Hospital, Hefei, China. All strains were stored in YPD medium (1% yeast extract, 2% peptone, and 2% dextrose) and 20% glycerol at −80 °C. Then they were subcultured on Sabouraud dextrose broth (SDB) for 24 h at 37 °C. The cells were harvested by centrifugation at 3000g, washed twice with sterile phosphate-buffered saline (PBS), resuspended in RPMI-1640 medium (Invitrogen, Carlsbad, CA), and calculated by using a hemocytometer.
Susceptibility test
The minimum inhibitory concentrations (MICs) of FLC and KAE were determined by the broth microdilution method based on the CLSI M27-A3 (Clinical and Laboratory Standards Institute Citation2008). Briefly, the fungal cells were adjusted to 2 × 103 CFU/ml in RPMI-1640 medium. The suspensions were added into a 96-well bottom-flat polystyrene microtiter plate incubated with KAE and/or FLC at 37 °C for 48 h. The final concentrations of KAE were two-fold diluted serially ranging from 4 to 2048 μg/mL (4, 8, 16, 32, 64, 128, 256, 512, 1024, and 2048 μg/mL), and the concentrations of FLC ranged from 0.125 to 2048 μg/mL (0.125, 0.25, 0.5, 1, 2, 4, 8, 16, 32, 64, 128, 256, 512, 1024, 2048 μg/mL). The control contained fungal cells and broth medium with no drug. The MIC90 value was defined as the lowest concentration of FLC or KAE to cause 90% OD reduction at the wavelength of 490 nm using a spectrophotometer (SpectraMax M2/M2e, Wang Lab, Sunnyvale, CA) compared with the control (Khodavandi et al. Citation2014). The checkerboard assay was used to assess the in vitro interactions of KAE and FLC according to their respective MICs used alone. Briefly, the fungal cells were diluted to 2 × 103 CFU/ml in RPMI-1640 medium. The FLC and KAE were prepared to the final concentrations of 0.0625–64 μg/mL (0.0625, 0.125, 0.25, 0.5, 1, 2, 4, 8, 16, 32, and 64 μg/mL) and 16–1024 μg/mL (16, 32, 64, 128, 256, 512, and 1024 μg/mL), respectively. The fractional inhibitory concentration index (FICI) was equal to (MICFLC in combination/MICFLC alone) + (MICKAE in combination/MICKAE alone), in which synergism was interpreted as FICI ≤ 0.5, indifference was defined as 0.5 <FICI ≤ 4.0, and antagonism was FICI > 4.0 (Odds Citation2003).
Time-kill test
The 10 C. albicans strains were all adjusted to 1 × 106 CFU/mL by RPMI 1640. A fraction of 5 mL of each suspension was mixed separately with 2 mL FLC, KAE, and FLC + KAE at 37 °C for 48 h in a 96-well bottom-flat polystyrene microtiter plate. The concentrations of FLC and KAE were set at a level equal to the MICs when they were used in combination in the 10 selected strains. At each time point within 48 h incubation (8, 16, 24, 32, 40, and 48 h) at 37 °C, 100 μL of each suspension was pipetted out, serially diluted 100-fold in sterile PBS, and plated on Sabouraud dextrose agar (SDA). The viable fungal cells were determined at 37 °C for 48 h (Khodavandi et al. Citation2014). The control contained fungal cells and broth medium with no drug. According to the previous report, synergism was defined as an increase of no less than 100-fold in killing for the combined agents compared with the most active agent used alone at 48 h, and antagonism was defined as a decrease of no less than 100-fold in killing for the combined agents compared with the most active agent alone at 48 h. Less than 100-fold in killing for the combined agents compared with any agents used was considered as indifference (Eliopoulos and Eliopoulos Citation1988).
Efflux test of rhodamine-6G
A volume of Ca z2003 culture (2 mL) was adjusted to 1 × 108 cells/mL in RPMI 1640. The suspension was then mixed separately with the same volumes of FLC, KAE, and FLC + KAE to the final concentrations of 32 μg/mL, 128 μg/mL, and 32 + 128 μg/mL at 37 °C for 5 h of incubation. After centrifugation at 2000g, the supernatant was discarded, mixed with 2 mL sterile PBS for 2 h of incubation at 37 °C with an agitation of 200 rpm. Subsequently, rhodamine-6G was added to a final concentration of 10 μmol/L for 2 h of incubation at 37 °C. The solution was centrifuged at 3000g, and the supernatant was discarded. The pellets were washed three times by sterile PBS. As efflux of rhodamine-6G from Candida cells required the presence of glucose, glucose dissolved in PBS was coincubated with the pellets to the final concentration of 2 mmol/L at 37 °C 200 rpm for 1 h. The suspension was centrifuged at 3000 g. An aliquot of supernatant (100 μL) was removed and detected at an excitation wavelength of 525 nm and an emission wavelength of 550 nm with an inverted fluorescence microscope IX71 (Olympus, Tokyo, Japan) (Peralta et al. Citation2012).
CDR1, CDR2, and MDR1 expression analysed by qRT-PCR
The procedures of qRT-PCR analysis were described in a previous study of our group (Shao et al. Citation2014). Briefly, total RNA samples from Ca z2003 were extracted according to the instructions of MagExtractor-RNA kit (ToyoBo, Tokyo, Japan). The extracted total RNA (6 μL) was incubated with 2 μL 4 × DNA Master I (with gDNA Remover) and 2 μL 5RT-Master Mix II, and reverse-transcribed into cDNA as recommended by ReverTra Ace qPCR RT Master Mix with gDNA Remover kit (ToyoBo, Tokyo, Japan) with procedures as follows: initial 5 min of RNA denaturation at 65 °C and 1 min at 4 °C, then 15 min at 37 °C, 5 min at 50 °C, 5 min at 98 °C, and 1 min at 4 °C. The prepared cDNA was diluted 10-fold prior to the use for RT-PCR. Primers for CDR1, CDR2, MDR1, and ACT1 () were designed by Primer Premier 5.0 and synthesised by Sangon Biotech (Shanghai, China). Real-time PCR mixture (= 25 μL) was composed of 12.5 μL of 2 × SYBR Green Real-time PCR, 1 μL of PCR forward primer, 1 μL of PCR reverse primer, 0.5 μL of cDNA, and 10 μL of ddH2O. A ABI7000 fluorescent quantitative PCR system (Applied Biosystem, Foster City, CA) was used with following cycles: 95 °C for 60 s, and then 95 °C for 15 s, 55 °C for 15 s, 72 °C for 45 s for total of 40 cycles. All data were normalised to housekeeping gene ACT1 as the internal reference gene. The relative target-gene expression was calculated as a fold change of 2−ΔCt value, in which as previously described.
Table 1. The primers of CDR1, CDR2, MDR1, and ACT1 for qRT-PCR.
Scanning electron microscope (SEM)
After FLC or/and KAE treatments, the samples was fixed by 2.5% glutaraldehyde overnight, and dehydrated by 30, 50, 70, and 100% ethanol for 10 min each. After air drying, the samples were sputter coated with gold in a vacuum evaporator, and the morphological observation was performed by a scanning electron microscope (SEM, JSM-6700F, JEOL, Tokyo, Japan) as described previously (Staniszewska et al. Citation2013).
Statistical analysis
All experiments were performed in triplicate. The results were reported as mean ± standard deviation (n = 3) and calculated by SPSS 17.0 (SPSS Inc., Chicago, IL). The data among groups were analysed by one-way ANOVA, in which p < 0.05 was considered as statistically significant.
Results
Susceptibility of C. albicans strains to FLC and/or KAE
The antifungal activity of KAE alone was relatively weak against the ten C. albicans strains with MIC90 of KAE ranging 256-512 μg/mL (). After combination, MIC90 of both KAE and FLC were lowered by 2–4 fold ranging 128–256 μg/mL and 2–128 fold ranging 0.25–32 μg/mL respectively. Three clinical fluconazole-resistant C. albicans strains showed synergism with FICI of 0.257 (). However, the FICIs were little higher than 0.5 in the remained eight strains which could be interpreted as indifference. Therefore, T–K test was employed to further evaluate the antifungal activities of FLC/KAE alone and in combination by using the MIC90s of FLC/KAE determined in the checkerboard assay. It could be observed that neither KAE nor FLC presented enough inhibition against the isolates compared with the drug-free control (p > 0.5) within 48 h. Obviously, the concomitant use of FLC/KAE displayed strong inhibition on the growth of fungal cell after 24-h of medication in comparison with FLC/KAE alone (p < 0.5). Moreover, FLC and KAE showed synergism when they were used concomitantly for 48 h in the 10 strains () according to the definitions for T–K test described in the Materials and methods section. In addition, representative morphological changes of C. albicans z2003 observed by SEM also demonstrated synergistic antifungal activity of KAE/FLC as there were no visual hyphae and yeasts left ().
Figure 1. Time–kill curves of Ca 215, Ca z2003, and Ca z1110 after the treatments of no drugs (control), 32 μg/mL FLC, 128 μg/mL KAE, 32 μg/mL FLC + 128 μg/mL KAE, those of Ca z1007 and Ca 2019 after the treatments of no drugs (control), 16 μg/mL FLC, 128 μg/mL KAE, 16 μg/mL FLC + 128 μg/mL KAE, those of Ca 2144 and Ca 2209 after the treatments of no drugs (control), 32 μg/mL FLC, 256 μg/mL KAE, 32 μg/mL FLC + 256 μg/mL KAE, that of Ca z1105 after the treatments of no drugs (control), 2 μg/mL FLC, 128 μg/mL KAE, 2 μg/mL FLC + 128 μg/mL KAE, that of Ca sp1927 after the treatments of no drugs (control), 1 μg/mL FLC, 256 μg/mL KAE, 1 μg/mL FLC + 256 μg/mL KAE, and that of Ca SC5314 after the treatments of no drugs (control), 0.25 μg/mL FLC, 128 μg/mL KAE, 0.25 μg/mL FLC + 128 μg/mL KAE. FLC, fluconazole; KAE, kaempferol.
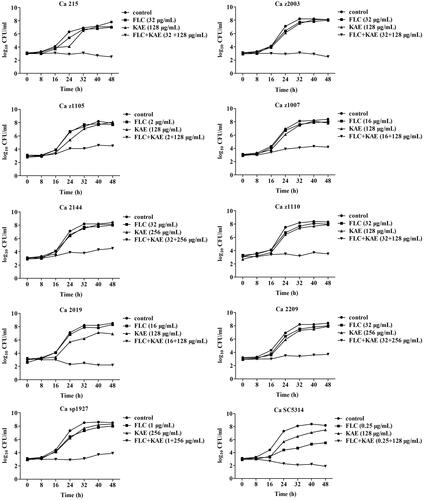
Figure 2. Scanning electron microscope of Ca z2003 after the treatment of (A) no drugs (control), (B) 32 μg/mL FLC, (C) 128 μg/mL KAE, and (D) 32 μg/mL FLC + 128 μg/mL KAE. Bar: 20 μm. FLC, fluconazole; KAE, kaempferol.
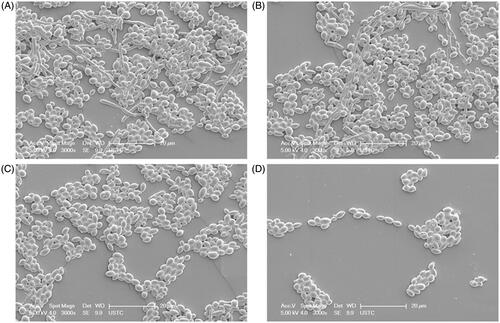
Table 2. Interactions between FLC and KAE alone and in combination against Candida albicans strains.
The effects of KAE or/and FLC on the functions of efflux pump and the expression of CDR1, CDR2, and MDR1
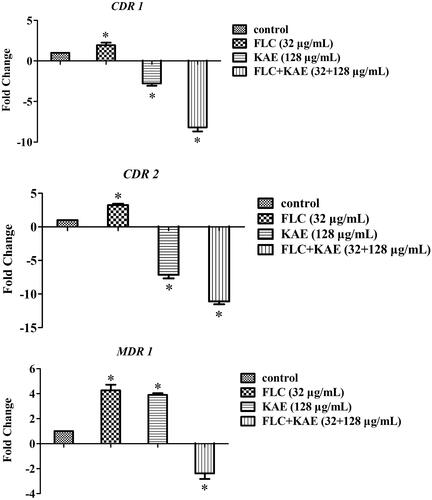
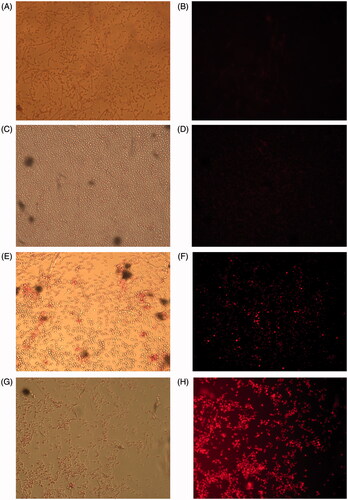
To further investigate the mechanisms of action of KAE/FLC alone and in combination, we used C. albicans z2003 to study the fluorescence efflux of rhodamine-6G and three critical gene expressions related to efflux pump. Compared with , it could be observed that (i) FLC alone was unable to improve rhodamine-6G accumulation (), (ii) the internal concentration of rhodamine-6G was increased by KAE with several appearances of red dots in the cell (), and (iii) a majority of rhodamine-6G remained in the cytosol as notable red dots could be seen after the combined treatment of KAE/FLC (). According to qRT-PCR results in , it was evident that (i) the expressions of CDR1, CDR2, and MDR1 were all upregulated when FLC was used alone (p < 0.05); (ii) the expressions of CDR1 and CDR2 were downregulated by 2.78- and 7.14-fold (p < 0.05), while that of MDR1 was upregulated by 3.9-fold when KAE was used alone (p < 0.05); (iii) the combined use of KAE and FLC exerted inhibition on the expression of CDR1, CDR2, and MDR1 with 8.2-, 11.1-, and 2.38-fold decreases (p < 0.05).
Figure 3. Effluxes of rhodamine-6G after the treatments of (A) no drugs (control), (C) 32 μg/mL FLC, (E) 128 μg/mL KAE, (G) 32 μg/mL FLC + 128 μg/mL KAE at light field, and of (B) no drugs (control), (D) 32 μg/mL FLC, (F) 128 μg/mL KAE, (H) 32 μg/mL FLC + 128 μg/mL KAE at an excitation wavelength of 525 nm and an emission wavelength of 550 nm on Ca z2003. FLC, fluconazole; KAE, kaempferol.
Discussion
Fluconazole-resistant C. albicans is responsible for most prevalent nosocomial fungal infections and has resulted in many clinical treatment failures in immunocompromised patients, leading to the search for novel antifungal agents urgent (Schulz et al. Citation2011; Abdelmegeed and Shaaban Citation2013). KAE was considered a promising candidate to enhance the efficacy of traditional antifungal drug (such as FLC). However, a previous study as well as the present work ( and ) demonstrated relatively mild antifungal activity of KAE (MIC = 256–512 μg/mL) alone against Candida species (Rajasekharan et al. Citation2014). Due to the limited antifungal efficacy of KAE, the synergistic effect of KAE with traditional antifungals such as FLC was performed. As demonstrated in our experiment, the uses of KAE and FLC were synergistic to only three of Candida isolates with FICI <0.5, but the FICIs were all less than 0.55 which could be interpreted as indifference in the remained seven strains in the checkerboard assay (). Subsequently, T–K test was introduced to evaluate the reliability of FICI definition for the 10 strains used in the current study including C. albicans z2003 (FICI = 0.515) which was employed for further investigation of the antifungal mechanism of action. Interestingly, KAE in combination with FLC could synergistically inhibit all the strains after 48 h of incubation in the T–K test () including C. albicans z2003, indicating that it was useful for T–K test to further assess the interpretations acquired in a checkerboard assay when FICI was a little higher than 0.5.
The upregulations of multidrug efflux pump controlled by Cdr1p, Cdr2p belonging to ATP-binding cassette superfamily (APC transporter) and Mdr1p, a member of major facilitator superfamily (MFS) were implicated in most fluconazole-resistant C. albicans strains (Niimi et al. Citation2004; Holmes et al. Citation2012; Prasad and Rawal Citation2014) as FLC was a substrate for CDR1, CDR2, and MDR1 (Kohli et al. Citation2001; Nakamura et al. Citation2001). Rhodamine 6G was the most common used fluorescent dye of ABC pump substrate requiring ATP as energy to observe transporter-mediated FLC resistance (Lamping et al. Citation2007; Peralta et al. Citation2012). Our experiments showed that FLC alone failed to lead to rhodamine 6G accumulation in the intracellular space in C. albicans z2003 due to active efflux pump (). The result was further proved by RT-PCR, in which the expression of MDR1 increasing by 4.26-fold was more than those of CDR1 and CDR2 increasing by 3.22- and 1.94-fold when FLC was used alone (). Although KAE alone had relatively weak antifungal activity, it did enhance rhodamine 6G accumulation in intracellular space of C. albicans z2003 cell (). This could be explained by reduced activity of transporter-mediated efflux pump, especially by the downregulated expressions of CDR1 and CDR2 (). Interestingly, KAE alone caused the upregulation of MDR1 expression, correlated with the fact that CDR1, CDR2, and MDR1 controlled possibly by two transcriptional pathways contributed differentially to antifungal resistance in C. albicans (Cannon et al. Citation2009). The combination of KAE/FLC could promote the intracellular accumulation of rhodamine 6G, implying that KAE might assist FLC by suppressing the efflux pump. There were several reports to give a clue that the role of CDR1 was more crucial than CDR2 in the regulation of transporter-mediated efflux pump to confer resistance to FLC (Xu et al. Citation2007; Holmes et al. Citation2008). In this study (), however, both KAE alone and KAE/FLC in combination appeared to more profoundly inhibit the expressions of CDR2 (7.14- and 11.1-fold) than CDR1 (2.78- and 8.2-fold). In summary, the synergistic effect of KAE with FLC was most likely due to the inhibition on the expressions of efflux pump gene, especially on the expression of CDR1 than CDR2 in clinical fluconazole-resistant C. albicans strains ( and ).
A number of flavonoids have been shown to possess favorable antifungal activity; however, most of them including KAE barely achieve satisfactory antifungal effect in vitro when used alone. Intriguingly, they can enhance the antifungal activities of traditional agents (such as FLC) against fluconazole-resistant C. albicans and other non-Candida albicans Candida (NCAC) species, as demonstrated by several authors (Hirasawa and Takada Citation2004; da Silva et al. Citation2014). There have already been enough evidences to support the involvement of efflux pump in the antifungal mechanism of action of KAE. KAE derivative (such as kaempferol rhamnoside) could inhibit the multidrug efflux transporter NorA of Staphylococcus aureus conferring multidrug resistance to a broad spectrum of compounds (Holler et al. Citation2012). Moreover, some flavonoids (such as baicalein) have been proved to combat against C. albicans and NACA via disrupting the expressions of efflux pump genes, especially CDR1, CDR2, and MDR1 (Huang et al. Citation2008; Peralta et al. Citation2012; Tsang et al. Citation2015). It is known that the mutation of efflux pump gene is the main responsible cause for the prevalence of fluconazole-resistant C. albicans, which effectively reduces the intracellular concentration of antifungal agent and ultimately makes these drugs including FLC ineffective. As a result, the use of KAE, which is an efflux pump inhibitor, in combination with FLC to enhance antifungal effect of FLC, is of choice for the treatment of clinical fluconazole-resistant C. albicans strains.
In this experiment, KAE was able to lower the susceptibility of fluconazole-resistant C. albicans to FLC probably via inhibiting multidrug efflux pump, especially decreasing the expression of CDR1 and CDR2. More studies are needed to investigate the antifungal mechanism of KAE in in vivo and the mechanism of action of KAE against other NCAC species. Furthermore, it is also necessary to evaluate the antibiofilm capability of KAE as biofilm is another common factor to cause resistance of C. albicans in clinical settings.
Declaration of interest
The authors report that they have no conflicts of interest. This work was supported by National Natural Science Foundation of China (No. 81073127), Natural Science Foundation of Anhui Province (Nos. 1408085MH165, 1508085MH163, and 1508085QH193), Talent Fund of Anhui University of Chinese Medicine (2013RC001), Natural Science Foundation of Anhui University of Chinese Medicine (2013zr009).
References
- Abdelmegeed E, Shaaban MI. 2013. Cyclooxygenase inhibitors reduce biofilm formation and yeast-hypha conversion of fluconazole resistant Candida albicans. J Microbiol. 51:598–604.
- Cannon RD, Lamping E, Holmes AR, Baret PV, Keniya MV, Tanabe K, Niimi M, Goffeau A, Monk BC. 2009. Efflux-mediated antifungal drug resistance. Clin Microbiol Rev. 22:291–321.
- Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts – third edition: approved standard M27-A3. Wayne, PA: CLSI.
- da Silva CR, de Andrade Neto JB, de Sousa Campos R, Figueiredo NS, Sampaio LS, Magalhaes HI, Cavalcanti BC, Gaspar DM, de Andrade GM, Lima IS. 2014. Synergistic effect of the flavonoid catechin, quercetin, or epigallocatechin gallate with fluconazole induces apoptosis in Candida tropicalis resistant to fluconazole. Antimicrob Agents Chemother. 58:1468–1478.
- Eliopoulos G, Eliopoulos C. 1988. Antibiotic combinations: should they be tested? Clin Microbiol Rev. 1:139–156.
- Friedman M. 2007. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol Nutr Food Res. 51:116–134.
- Hirasawa M, Takada K. 2004. Multiple effects of green tea catechin on the antifungal activity of antimycotics against Candida albicans. J Antimicrob Chemother. 53:225–229.
- Holler JG, Christensen SB, Slotved HC, Rasmussen HB, Gúzman A, Olsen CE, Petersen B, Molgaard P. 2012. Novel inhibitory activity of the Staphylococcus aureus NorA efflux pump by a kaempferol rhamnoside isolated from Persea lingue Nees. J Antimicrob Chemother. 67:1138–1144.
- Holmes AR, Keniya MV, Ivnitski-Steele I, Monk BC, Lamping E, Sklar LA, Cannon RD. 2012. The monoamine oxidase A inhibitor clorgyline is a broad-spectrum inhibitor of fungal ABC and MFS transporter efflux pump activities which reverses the azole resistance of Candida albicans and Candida glabrata clinical isolates. Antimicrob Agents Chemother. 56:1508–1515.
- Holmes AR, Lin YH, Niimi K, Lamping E, Keniya M, Niimi M, Tanabe K, Monk BC, Cannon RD. 2008. ABC transporter Cdr1p contributes more than Cdr2p does to fluconazole efflux in fluconazole-resistant Candida albicans clinical isolates. Antimicrob Agents Chemother. 52:3851–3862.
- Huang S, Cao YY, Dai BD, Sun XR, Zhu ZY, Cao YB, Wang Y, Gao PH, Jiang YY. 2008. In vitro synergism of fluconazole and baicalein against clinical isolates of Candida albicans resistant to fluconazole. Biol Pharm Bull. 31:2234–2236.
- Kataoka M, Hirata K, Kunikata T, Hirata K, Kunikata T, Ushio S, Iwaki K, Ohashi K, Ikeda M, Kurimoto M. 2001. Antibacterial action of tryptanthrin and kaempferol, isolated from the indigo plant (Polygonum tinctorium Lour.), against Helicobacter pylori-infected Mongolian gerbils. J Gastroenterol. 36:5–9.
- Khodavandi A, Alizadeh F, Vanda NA, Karimi G, Chong PP. 2014. Possible mechanisms of the antifungal activity of fluconazole in combination with terbinafine against Candida albicans. Pharm Biol. 52:1505–1509.
- Khodavandi A, Harmal NS, Alizadeh F, Harmal NS, Alizadeh F, Scully OJ, Sidik SM, Othman F, Sekawi Z, Ng KP, Chong PP. 2011. Comparison between allicin and fluconazole in Candida albicans biofilm inhibition and in suppression of HWP1 gene expression. Phytomedicine 19:56–63.
- Kohli A, Gupta V, Krishnamurthy S, Hasnain SE, Prasad R. 2001. Specificity of drug transport mediated by CaMDR1: a major facilitator of Candida albicans. J Biosci. 26:333–339.
- Lamping E, Monk BC, Niimi K, Holmes AR, Tsao S, Tanabe K, Niimi M, Uehara Y, Cannon RD. 2007. Characterization of three classes of membrane proteins involved in fungal azole resistance by functional hyperexpression in Saccharomyces cerevisiae. Eukaryotic Cell. 6:1150–1165.
- Letscher-Bru V, Obszynski C, Samsoen M, Sabou M, Waller J, Candolfi E. 2013. Antifungal activity of sodium bicarbonate against fungal agents causing superficial infections. Mycopathologia 175:153–158.
- Martino R, Canale F, Sülsen V, Alonso R, Davicino R, Mattar A, Anesini C, Micalizzi B. 2014. A fraction containing kaempferol-3,4'-dimethylether from Larrea divaricata Cav. induces macrophage activation on mice infected with Candida albicans. Phytother Res. 28:917–924.
- Nakamura K, Niimi M, Niimi K, Holmes AR, Yates JE, Decottignies A, Monk BC, Goffeau A, Cannon RD. 2001. Functional expression of Candida albicans drug efflux pump Cdr1p in a Saccharomyces cerevisiae strain deficient in membrane transporters. Antimicrob Agents Chemother. 45:3366–3374.
- Niimi M, Niimi K, Takano Y, Holmes AR, Fischer FJ, Uehara Y, Cannon RD. 2004. Regulated overexpression of CDR1 in Candida albicans confers multidrug resistance. J Antimicrob Chemother. 54:999–1006.
- Odds F. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 52:1.
- Orhan DD, Ozcelik B, Ozgen S, Ergun F. 2010. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol Res. 165:496–504.
- Peralta MA, Calise M, Fornari MC, Ortega MG, Diez RA, Cabrera JL, Perez C. 2012. A prenylated flavanone from Dalea elegans inhibits rhodamine 6 G efflux and reverses fluconazole-resistance in Candida albicans. Planta Med. 78:981–987.
- Pinho E, Ferreira IC, Barros L, Carvalho AM, Soares G, Henriques M. 2014. Antibacterial potential of northeastern Portugal wild plant extracts and respective phenolic compounds. BioMed Res Int. 2014:1–8.
- Prasad R, Rawal MK. 2014. Efflux pump proteins in antifungal resistance. Front Pharmacol. 5:1–13.
- Rajasekharan SK, Ramesh S, Bakkiyaraj D. 2014. Synergy of flavonoids with HDAC inhibitor: new approach to target Candida tropicalis biofilms. J Chemother. [Epub ahead of print]. doi:10.1179/1973947814Y.0000000186.
- Rajendran P, Rengarajan T, Nandakumar N, Palaniswami R, Nishigaki Y, Nishigaki I. 2014. Kaempferol, a potential cytostatic and cure for inflammatory disorders. Eur J Med Chem. 86:103–112.
- Schulz B, Weber K, Schmidt A, Borg-von Zepelin M, Ruhnke M. 2011. Difference in virulence between fluconazole-susceptible and fluconazole-resistant Candida albicans in a mouse model. Mycoses 54:e522–e530.
- Shao J, Wang T, Yan Y, Shi G, Cheng H, Wu D, Wang C. 2014. Matrine reduces yeast-to-hypha transition and resistance of a fluconazole-resistant strain of Candida albicans. J Appl Microbiol. 117:618–626.
- Staniszewska M, Bondaryk M, Swoboda-Kopec E, Siennicka K, Sygitowicz G, Kurzatkowski W. 2013. Candida albicans morphologies revealed by scanning electron microscopy analysis. Braz J Microbiol. 44:813–821.
- Tatsimo SJ, Tamokou JD, Havyarimana L, Csupor D, Forgo P, Hohmann J, Kuiate JR, Tane P. 2012. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res Notes. 5:158.
- Tobudic S, Kratzer C, Lassnigg A, Presterl E. 2012. Antifungal susceptibility of Candida albicans in biofilms. Mycoses 55:199–204.
- Tsang PW, Chau KY, Yang HP. 2015. Baicalein exhibits inhibitory effect on the energy-dependent efflux pump activity in non-albicans Candida fungi. J Chemother. 27:61–62.
- Xu D, Jiang B, Ketela TLemieux S, Veillette K, Martel N, Davison J, Sillaots S, Trosok S, Bachewich C, 2007. Genome-wide fitness test and mechanism-of-action studies of inhibitory compounds in Candida albicans. PLoS Pathog. 3:e92.
- Zhang X, Guo H, Gao LSong Y, Li S, Zhang H, 2013. Molecular mechanisms underlying the tetrandrine-mediated reversal of the fluconazole resistance of Candida albicans. Pharm Biol. 51:749–752.
- Zhou Y, Wang G, Li Y, Liu Y, Song Y, Zheng W, Zhang N, Hu X, Yan S, Jia J. 2012. In vitro interactions between aspirin and amphotericin B against planktonic cells and biofilm cells of Candida albicans and C. parapsilosis. Antimicrob Agents Chemother. 56:3250–3260.