Abstract
Context: The antihyperlipidemic, antiarrhythmic, neuroprotective and hepatoprotective effects of Melissa officinalis L. (Lamiaceae) have been reported. However, no study has examined its effects on the resistance of the heart to stressful conditions.
Objective: The objective of this study is to evaluate the effects of aqueous extract of M. officinalis aerial parts on Wistar rat heart with/without cardiac injury.
Materials and methods: Animals were grouped as control, isoproterenol (ISO), M. officinalis without (M50, M100, and M200) and with isoproterenol (M50 + ISO, M100 + ISO, and M200 + ISO). The aqueous extract of M. officinalis was orally administered at dosages of 50, 100, and 200 mg/kg/d, respectively, for 7 consecutive days. On the 6th and 7th day, ISO, M50 + ISO, M100 + ISO, and M200 + ISO groups received 85 mg/kg of isoproterenol for myocardial injury induction. On day 8, hemodynamic parameters were recorded and samplings were done.
Results: The extract (50, 100, and 200 mg/kg) significantly reduced the heart rate (264 ± 5, 259 ± 5 and 281 ± 3 versus 377 ± 13 in control group, p < 0.01). Blood pressure was significantly decreased in M50 + ISO (75 ± 5) versus M50 (110 ± 6) and M100 + ISO (72 ± 6) versus M100 (105 ± 5 mmHg, p < 0.01). The malondialdehyde levels of the injured hearts were lower in M50 + ISO and M100 + ISO groups than in the ISO group (p < 0.05). Serum cardiac troponin I was higher in the M200 + ISO group (5.1 ± 1.7) than in the ISO group (2.7 ± 0.7 ng/ml, p < 0.05).
Conclusion: The lower dose of extract, by improving the balance of the redox system and by reducing the heart rate, may increase the heart resistance to injury. However, the higher doses of extract may intensify the injury of ischemic heart.
Introduction
Melissa officinalis L. Lamiaceae or Labiatae, belongs to a genus which includes five species of perennial herbs native to Europe, central Asia, and Iran. Melissa officinalis subsp. officinalis and M. officinalis subsp. altissima are two famous subspecies of this plant (Meyers Citation2007). Melissa officinalis is a well-known herbal drug in the Eastern and Western societies. In traditional medicine, it has been recommended to treat depression (Inge Citation1986; Meyers Citation2007), bronchitis, asthma (Mamedov and Craker Citation2001), menstrual problems, hypertension, migraines, vertigo, fever (Lawless Citation1992; Duke Citation2002; Meyers Citation2007), snake bite (Duke Citation2002), eczema (Lawless Citation1992), and gout (Gunther Citation1959). It can also cause an emotional boost of happiness (Culpeper Citation1652; Payne Citation2006; Meyers Citation2007). Experimental research has indicated the antioxidant (Mimica-Dukic et al. Citation2004; Ferreira et al. Citation2006), antianxiety (Cases et al. Citation2011), antiviral, antibacterial, antifungal (Mimica-Dukic et al. Citation2004; Ulbricht et al. Citation2005; Mazzanti et al. Citation2008), antihyperlipidemic (Bolkent et al. Citation2005), and antitumoral effects (de Sousa et al. Citation2004; Encalada et al. Citation2011) of M. officinalis. It has also been shown that M. officinalis has hepatoprotective (Bolkent et al. Citation2005) and neuroprotective effects as well (Ferreira et al. Citation2006; López et al. Citation2009; Hassanzadeh et al. Citation2011). The German Commission E has approved the use of M. officinalis for the treatment of sleep disturbance and functional gastrointestinal disorders. This drug has also been recommended by ESCOP (European Scientific Cooperative on Phytotherapy) for the treatment of some gastrointestinal and neurological disorders (Goldberg et al. Citation2000).
In modern scientific research, however, less attention has been devoted to the cardiovascular effects of M. officinalis. The mild antiarrhythmic effect of M. officinalis was shown in our previous study (Joukar et al. Citation2014b). Gazola et al. (Citation2004) demonstrated that the M. officinalis aqueous extract significantly decreased the heart rate, but had no effect on the contractility of isolated rat heart. However, no study has yet dealt with the effects of M. officinalis consumption on the heart in the presence of ischemic stressful conditions. This study was conducted to assess the pre-treatment effects of this herb on physiological, biochemical, and histological indices of rat heart with/without myocardial injury.
Materials and methods
Drugs, kits, and M. officinalis extract
These items were purchased, respectively: sodium thiopental from Sandoz, Kundl, Austria; isoproterenol (ISO) from Sigma, Welwyn Garden City, UK; GPX assay kit from Randox Laboratory Ltd., Crumlin, UK; and troponin I assay kit from BioMerieux, Lyon, France.
M. officinalis was collected during the Spring of 2012 from Sirjan area (Kerman province, Iran) and it was identified by the Department of Botany, Bahonar University of Kerman. A voucher specimen (KF1429-1) of the plant was deposited in the Herbarium Center of the Faculty of Pharmacy at Kerman University of Medical Sciences. The fresh aerial parts of the plant were dried and were cleaned to remove dirt and ground manually and powdered to particle sizes of about 0.5 mm. Then, boiling distilled water (100 ml) was added to the 500 mg powder for 10 min to brew the M. officinalis. The mixture was then filtered, and the liquid evaporated under vacuum at 45–50 °C. The material was then dried completely at 70 °C as reported previously (Hassanzadeh et al. Citation2011; Joukar et al. Citation2014b). Finally, the dried prepared extract was stored in glass vials at −20 °C prior to use. During the treatment period, the needed amounts of dry extract were weighed daily and dissolved in water and were fed to animals.
Experimental design
This experiment was carried out on male Wistar rats that were 3 months old and weighed 250–300 g. The study was conducted according to the national guidelines for animal studies (Ethic committee permission no. 91/152, Kerman University of Medical Sciences). The animals were divided into eight groups (18 each) and were kept in a controlled room temperature with 12 h light/dark cycle. The animal groups were treated for 7 d in the following manner: The control (CTL) group was orally administrated with 1 mL of distilled water, an equal volume to extract for 7 d. The test groups were orally administrated with the aqueous extract of M. officinalis 50 (M50), 100 (M100), 200 (M200) mg/kg, respectively, for 7 d. The isoproterenol (ISO) group orally received 1 mL of distilled water daily while the test groups were administrated with the aqueous extract of M. officinalis 50 (M50 + ISO), 100 (M100 + ISO), and 200 (M200 + ISO) mg/kg, respectively, every day. On the 6th and 7th day of the study, these groups (ISO, M50 + ISO, M100 + ISO, and M200 + ISO) received 85 mg/kg isoproterenol (s.c.) for the induction of cardiac injury, while the corresponding control groups received equal volume of normal saline (s.c.). Two hours after the last isoproterenol or saline injection, 1.5 mL blood sample was taken under light ether anesthesia by retroorbital puncture and then centrifuged. The samples were stored at −20 °C for measurement of cardiac troponin I – a reliable biochemical marker of myocardial injury.
On the 8th day, the animals were anesthetized with sodium thiopental (50 mg/kg i.p.). The surgical preparation and the method for parameters recording have been described in the previous studies (Joukar et al. Citation2012a,Citation2014a). Briefly, the trachea of animals was cannulated, which allowed spontaneous breathing throughout the experiment. The left common carotid artery was cannulated with a filled catheter (saline with 15 IU/ml heparin) and connected to a pressure transducer of a PowerLab analog to digital converter (AD Instruments, Lexington, Australia) system for heart rate (HR) and arterial blood pressure (BP) recording. The other cannula was inserted into the left ventricle through the right carotid artery for left ventricular pressure (LVP) recording.
Hemodynamic and biochemical parameters
The hemodynamic and heart performance indices were recorded in half of the animals in each group. The mean arterial pressure (MAP) was calculated by “MAP = Pd + (Ps − Pd)/3 formula”, where Pd = diastolic arterial pressure and Ps = systolic arterial pressure. The maximum velocity of contraction (+dp/dt max) and the maximum velocity of relaxation (−dp/dt max) of the left ventricle were calculated from the left ventricular pressure trace (Joukar et al. Citation2012a,Citation2014a). At the end of the experiment, the animals were sacrificed. The hearts of the other half of the animals in each group were removed and washed with cold saline. Later a piece of the hearts’ apex was dissected, weighed and homogenized in 5 ml of 0.1 M Tris–HCl buffer (pH 7.4) under ice-cold conditions. After centrifuging, the clear supernatant solution was taken for the biochemical analysis. The malondialdehyde (MDA) level, an index of lipid peroxidation, was estimated by the concentration of the thiobarbituric acid reactive substances (TBARS) (Ohkawa et al. Citation1979). The glutathione peroxidase (GPX) in the heart tissue was determined by using the Randox assay kit according to the protocol of the manufacturer (Joukar et al. Citation2012b). In order to measurement of MDA and GPX per mg protein, the total protein was also measured by using the Lowry et al. (Citation1951) method. The level of plasma cardiac Troponin I is measured by VIDAS Troponin I ultra assay (TINU, 30448 BioMerieux, Lyon, France) which is an enzyme-linked fluorescent immunoassay-based method (Joukar et al. Citation2014a). The remaining portion of the heart was fixed by using the 10% buffered formalin (pH 7.4). After the paraffin molding of the tissues, 5 μm thick sections which were stained with hematoxylin and eosin (H&E) were prepared, and later examined microscopically by a pathologist who was blind to the animal groupings. The lesions were graded as follows: (0) nil; (1) minimum = very mild (focal myocytes damage); (2) mild (small multifocal degeneration with slight degree of inflammatory process); (3) moderate (extensive myofibrillar degeneration and/or diffuse inflammatory process); and (4) severe (necrosis with diffuse inflammatory process) (Joukar et al. Citation2012a,Citation2014a).
Statistical analysis
The quantitative data are expressed in the form of mean ± SEM and the comparisons were made by one-way ANOVA followed by Turkey’s post hoc test. The histopathological findings are reported qualitatively as the number of animals with different grades of myocardial lesions in each group. The statistical analysis was performed using the non-parametric Kruskal–Wallis and pair wise differences measured by the Mann–Whitney U-test. p-values < 0.05 were considered as statistically significant.
Results
Hemodynamic
Pretreatment with three different doses of M. officinalis individually had no significant effect on the mean arterial pressure (MAP) in comparison with the CTL group. Isoproterenol administration was associated with only insignificant decrease in the blood pressure of the ISO group. However, all the M. officinalis groups which were exposed to cardiac injury induced by isoproterenol showed a reduction in MAP but, this reduction was only significant in the M100 + ISO and M50 + ISO groups versus the M100 and M50 groups, respectively (p < 0.05) (). In the presence or absence of cardiac injury, the different doses of M. officinalis significantly reduced the heart rate in comparison with the control and ISO groups. In comparison with the control group, injection of isoproterenol did not show significant effect on heart rate (). Similar to blood pressure, pretreatments with M. officinalis had no significant effect on the left ventricular systolic pressure (LVSP) compared with the control group. However, LVSP was significantly reduced in the M50 + ISO and M100 + ISO groups (p <0.05 versus its corresponding groups, the M50 and M100 groups) (). The left ventricular end diastolic pressure (LVEDP) was lower in the animals which received M. officinalis without isoproterenol; however, it was only significant in the M200 group than in the CTL group (p <0.05). Also, this parameter increased significantly in the ISO group (p < 0.05 compared with the CTL group). Pretreatments with three different doses of M. officinalis attenuated the effect of isoproterenol on LVEDP, such that this index was lower in the M50 + ISO, M100 + ISO, and M200 + ISO groups than in the ISO group (p <0.01) (). Max dp/dt (+dp/dt maximum) and Min dp/dt (−dp/dt maximum) were significantly higher in the animals which were pretreated with 50 and 100 mg/kg of M. officinalis in comparison with the CTL group. In the presence of myocardial injury, these parameters were reduced in all groups in comparison with the related control groups except for the M200 + ISO group ( and ).
Figure 1. Left ventricular end-diastolic pressure (LVEDP) in different animal groups. n = 7–8. Values are mean ± SEM. CTL: control, M50: animal group which received 50 mg/kg/d of M. officinalis extract, M100: animal group which received 100 mg/kg/d of M. officinalis extract, M200: animal group which received 200 mg/kg/d of M. officinalis extract, ISO: isoproterenol. *p < 0.05 versus the CTL group, ♦ p < 0.01 versus all other groups except the CTL group, ○p < 0.05 versus the M200 group.
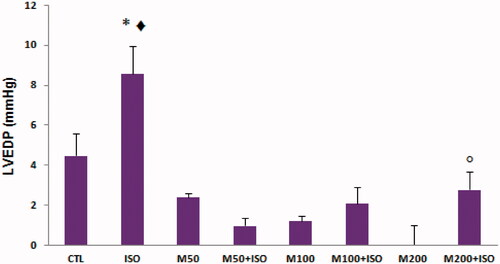
Figure 2. Max dp/dt (+dp/dt maximum) in different animal groups. n = 7–8. Values are mean ± SEM. CTL, control; M50, the animal group which received 50 mg/kg/d of M. officinalis extract, M100, the animal group which received 100 mg/kg/d of M. officinalis extract; M200, the animal group which received 200 mg/kg/d of M. officinalis extract; ISO, isoproterenol. ▾p < 0.01 versus the M100 group, + p < 0.01 versus the ISO group, ** p < 0.01 versus CTL and M200 groups, • p < 0.01 versus CTL and M50 groups.
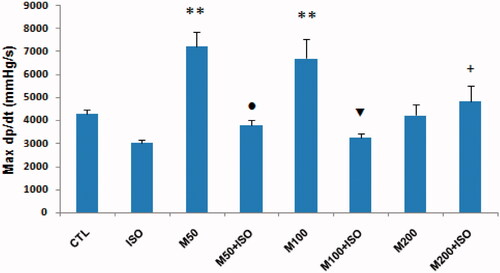
Figure 3. Min dp/dt (−dp/dt maximum) in different animal groups. n = 7–8. Values are mean ± SEM. CTL, control; M50, the animal group which received 50 mg/kg/d of M. officinalis extract; M100, the animal group which received 100 mg/kg/d of M. officinalis extract; M200, the animal group which received 200 mg/kg/d of M. officinalis extract; ISO, isoproterenol. **p < 0.01 versus CTL, M200, and M50 + ISO groups, * p < 0.05 versus CTL, M200, and M100 + ISO groups, Φ p < 0.05 versus M200 and M100 + ISO groups, ¤ p < 0.01 versus the ISO group.
Table 1. Mean arterial blood pressure, heart rate, and left ventricular systolic pressure in different animal groups.
Biochemical parameters
The M. officinalis consumption alone had no significant effect on the serum level of cardiac troponin I (cTnI). As shown in , the serum level of cTnI showed a significant increase in the ISO group (p < 0.05 versus CTL, M50 and M100 and M200 groups). Seven days consumption of 50 mg/kg of M. officinalis non-significantly decreased the cTnI level in the M50 + ISO group when compared with the ISO group. The use of 100 mg/kg of M. officinalis had no effect on the cTnI level in the animals with myocardial injury. However, the consumption of the 200 mg/kg of M. officinalis was associated with the highest level of serum cTnI in the animals subjected to heart injury (p < 0.05 versus the ISO and M100 + ISO groups and p < 0.01 versus CTL, M50, M100, M200, and M50 + ISO groups) ().
Table 2. Serum cardiac troponin I (cTnI), glutathione peroxidase (GPX), and MDA (malondialdehyde) of heart tissue in animal groups.
Three doses of M. officinalis consumption alone or along with isoproterenol had no significant effect on the GPX level of the heart tissue. This parameter insignificantly decreased in the ISO group and recovered in the presence of ISO plus M. officinalis (). In addition, an increase in the MDA levels of heart was observed in the ISO group (p < 0.01 than CTL, M50, M100, and M200). Pretreatments with 50 and 100 mg/kg of M. officinalis reduced the effect of isoproterenol on the MDA level of the animals’ hearts (p < 0.01 and p < 0.05, respectively versus the ISO group). However, pretreatments with 200 mg/kg of M. officinalis did not change the effect of isoproterenol on the MDA level ().
Histopathological findings
The myocardial tissue examination revealed normal microscopic appearance of CTL and focal myocytes damage in some animals in the M50, M100, and M200 groups ( and ). Isoproterenol induced moderate to severe cardiac damage; 50% of the animals showed extensive myofibrillar degeneration and/or diffused inflammatory process and 50% of them showed necrosis with diffused inflammatory process.
Figure 4. H&E-stained sections of heart tissue in different animal groups. The magnification of is ×200. (a) The CTL group heart sections showing normal appearance of cardiac myofibers. (c, e, and g) Sections of animals’ heart from M50, M100, and M200 groups are shown, respectively. In these groups, heart muscle appearance is associated with mild interstitial edema especially in the M200 group. (b) The ISO group section showing severe myodegeneration and necrosis of muscle fibers, interstitial edema, and inflammatory cell infiltration. (d, f, and h) Sections of heart tissues from M50 + ISO to M100 + ISO and M200 + ISO groups, respectively are shown. CTL, control; M50, the animal group which received 50 mg/kg/d of M. officinalis extract; M100, the animal group which received 100 mg/kg/d of M. officinalis extract; M200, the animal group which received 200 mg/kg/d of M. officinalis extract; ISO, isoproterenol.
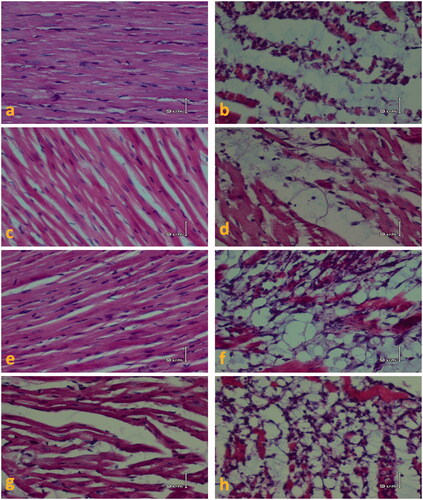
Table 3. Histopathological scores with different degrees of injury in each animal group.
The consumption of different doses of M. officinalis did not significantly prevent the destructive effects of isoproterenol on the heart. The score of lesions was non-significantly lower in the M50 + ISO group while non-significantly higher in the M200 + ISO in comparison with the ISO group ( and ).
Discussion
The present study examined the pretreatment effect of M. officinalis aqueous extract on blood pressure, heart rate, heart contractility, cardiac muscle structure, and cardiac resistance to ischemia in the rats exposed to high doses of isoproterenol-induced cardiac injury.
Isoproterenol is a nonselective beta agonist causing infarct-like lesions in the heart in high doses. This effect is caused by sudden increase in the heart rate, a drop in the blood pressure and disruption in the myocardial oxygen supply and the demand balance (Rona et al. Citation1959; York et al. Citation2007). In addition, isoproterenol disrupts the oxidant/antioxidant system’s balance, increasing oxidative damage to heart cells (Singal et al. Citation1982; Sharma et al. Citation2001). These effects appeared as an increase in the level of serum cardiac troponin I and also cardiac lesions and cardiac dysfunction (Rona et al. Citation1959; York et al. Citation2007).
In agreement with the previous reports, the significant increase of LVEDP, troponin I, and MDA values as well as histopathological findings in the ISO group in the present study confirmed the adverse effects of isoproterenol. The animals treated with isoproterenol showed moderate to severe damage of their heart cells, and also an increased level of serum cardiac troponin I and the cardiac tissue MDA (as an index of cells membrane lipid peroxidation). Additionally, in line with the previous studies (Joukar et al. Citation2013), the high dose of isoproterenol also led to a raise in the left ventricular end-diastolic pressure (LVEDP), an index of cardiac dysfunction. In this study, isoproterenol yielded an insignificant reduction in MAP, LVSP, Min dp/dt, and Max dp/dt which corresponds to our previous studies (Joukar et al. Citation2011,Citation2012c). The consumption of M. officinalis on its own or with isoproterenol decreased the heart rate of the animals in comparison with the control group and/or the ISO group. Moreover, the results of this study indicated that pretreatments with different doses of M. officinalis caused the mean arterial pressure (MAP) to drop in the groups exposed to heart injury (their arterial pressure was lower than the ISO group and corresponding control groups).
An in vitro experimental study showed that the aqueous extract of M. officinalis significantly reduces the heart rate (Gazola et al. Citation2004). Traditional medicine has also mentioned the beneficial effects of M. officinalis on palpitations (Hosseini & Davazdahemami Citation2007). In line with the previous studies and findings, our study also showed that M. officinalis decreased the heart rate in either the presence or absence of heart injury. As far as we know, no experimental study has been carried out to examine the effects of M. officinalis on blood pressure. However, in some traditional medicine texts, it has been suggested that M. officinalis can be effective in reducing hypertension (Wood Citation2008; Taylor Citation2012). In the present research, M. officinalis had no effect on the normal blood pressure; but in the presence of isoproterenol-induced heart injury, it significantly intensified the blood pressure drop. The hypotensive effect of M. officinalis in the presence of isoproterenol may arise from the mild relaxant peripheral vascular effect of this plant (Taylor Citation2012). This effect can also be ascribed to the negative chronotropic effect of M. officinalis which was observed both in this and previous studies (Gazola et al. Citation2004; Hosseini & Davazdahemami Citation2007). On the contrary, indices of contractility (Max dp/dt) and the speed of left ventricular relaxation (Min dp/dt) showed a significant increase only in animals that received 50 and 100 doses of M. officinalis (M50 and M100 groups) in comparison with the CTL group. Under heart injury conditions, pretreatments with 200 mg/kg of M. officinalis only increased these indices in contrast to the isoproterenol group. 50 mg/kg and 100 mg/kg of M. officinalis also led to a significant drop in the left ventricular systolic pressure under heart injury conditions, but a 200 mg/kg dose of M. officinalis did not show this effect. Cardiac injury also increased the left ventricular end-diastolic pressure significantly. The consumption of M. officinalis significantly decreased this adverse effect of isoproterenol in all three doses. This desired effect was more prominent in the M50 + ISO group. The significant reduction of systolic pressure of the left ventricle in the some animal groups (M50 + ISO and M100 + ISO) may be related to low arterial pressure and hence the low after load of left ventricle. Therefore, the heart regulates its pressure proportional to the level of its after load. Yet, in the M200 + ISO group, the drop of the left ventricular systolic pressure was insignificant, which may be due to the fact that the ventricle is more effective in terms of contractility in this group since in the presence of heart injury, only this group of injured animals had a higher index of Max dp/dt and Min dp/dt than that of the isoproterenol group. The reason as to why the high dose of M. officinalis (200 mg/kg) was accompanied with increased cardiac contractile force in damaged heart requires further research. However, it is likely that in stressful conditions M. officinalis prevents the cardiac cells from entering into stunning conditions (which weakens cardiac contractile force), and therefore it maintains cardiac contractile force. Regardless of involving mechanisms, in the beginning, this effect may help prevent the heart failure and stabilizes arterial blood pressure and also delays the increase in the end-diastolic pressure of the injured heart, however, it can intensify cell damage in ischemic conditions due to the increase of myocardial demand to oxygen. Biochemical and histopathological findings of this study supported this idea and showed that pretreatments with high dose of M. officinalis (200 mg/kg) did not decrease the level of cardiac troponin I, and the index of lipid peroxidation (MDA) was also high in this group similar to the isoproterenol group and the severity of the heart injury was higher in this group as well.
As it was mentioned, isoproterenol can disrupt the balance of oxidant/antioxidant system, which this was proved by the increased level of MDA in this study. A low dose of M. officinalis (50 mg/kg) led to a sharp decline in MDA in cardiac ischemic conditions. This effect was repeated in the pretreatment with M. officinalis (100 mg/kg) significantly; however, 200 mg/kg of M. officinalis could not reduce MDA. The changes in MDA in the current research were consistent with the level of serum cardiac troponin I among the different groups, which thus confirmed that low doses of M. officinalis can, to a certain degree, reduce isoproterenol-induced heart injury through sustaining the redox system's balance. In agreement of this finding, other researchers have also confirmed the antioxidant role and free radical scavenging activity of M. officinalis (Ferreira et al. Citation2006; Martins et al. Citation2012; EMA Citation2013).
Conclusion
The results revealed that, first, M. officinalis has negative chronotropic effects on rats under normal conditions and after heart injury. Second, its consumption was associated with minimum degree of heart cells lesion even in normal animals, so more research is needed to determine its safety. Third, a combination of isoproterenol induced heart injury and M. officinalis can lead to the prominent drop of blood pressure in rat. Fourth, M. officinalis in low doses (50 mg/kg) is more effective against the isoproterenol-induced cardiac injury to some extent by reducing the arterial pressure, the heart rate, and also by helping to balance the redox system. Finally, high doses of this medication may, to some degree, intensify isoproterenol-induced cardiac injury under myocardial ischemia conditions. It may result from increasing of cardiac contractility, increasing of myocardial oxygen demand, and hence more risk of cardiac injury. Overall, one can conclude that under heart injury conditions, the negative effects of M. officinalis in high doses are more than its positive effects, which may even intensify the severity of heart injury in rat. Therefore, regarding the recommendation of this agent for some therapeutic purposes (EMA Citation2013), its cardiovascular side effects especially in patients with heart disease should be considered.
Declaration of interest
The authors report that they have no conflicts of interest. The authors are thankful to the Vice Chancellor of Research, Kerman University of Medical Sciences, for financial support.
References
- Bolkent S, Yanardag R, Karabulut-Bulan O, Yesilyaprak B. 2005. Protective role of Melissa officinalis L. extract on liver of hyperlipidemic rats: a morphological and biochemical study. J Ethnopharmacol. 99:391–398.
- Cases J, Ibarra A, Feuillère N, Roller M, Sukkar SG. 2011. Pilot trial of M. officinalis L. leaf extract in the treatment of volunteers suffering from mild-to-moderate anxiety disorders and sleep disturbances. Med J Nutr Metab. 4:211–218.
- Culpeper N. 1652. The English physitian: or an astrologo-physical discourse of the vulgar herbs of this nation. London: Peter Cole; [cited 2006 Aug 7]. Available from: http://www.info.med.yale.edu/library/historical/culpeper/b.htm.
- de Sousa AC, Alviano DS, Blank AF, Alves PB, Alviano CS, Gattass CR. 2004. Melissa officinalis L. essential oil: antitumoral and antioxidant activities. J Pharm Pharmacol. 56:677–681.
- Duke JA. 2002. Handbook of medicinal herbs. USA: CRC Press.
- European Medicines Agency (EMA). 2013. Assessment report on Melissa officinalis L. folium; [cited 2015 Apr 16]. Available from: http://www.ema.europa.eu/docs/enGB/documentlibrary/HerbalHMPCassessmentreport/2013/08/WC500147187.pdf, Accessed16April2015.
- Encalada MA, Hoyos KM, Rehecho S, Berasategi I, de Ciriano MG, Ansorena D, Astiasarán I, Navarro-Blasco I, Cavero RY, Calvo MI. 2011. Anti-proliferative effect of Melissa officinalis on human colon cancer cell line. Plant Foods Hum Nutr. 66:328–334.
- Ferreira A, Proença C, Serralheiro ML, Araújo ME. 2006. The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J Ethnopharmacol. 108:31–37.
- Gazola R, Machado D, Ruggiero C, Singi G, Macedo Alexandre M. 2004. Lippia alba, Melissa officinalis and Cymbopogon citratus: effects of the aqueous extracts on the isolated hearts of rats. Pharmacol Res. 50:477–480.
- Goldberg B, Goldberg M, Goldberg A, Brinckmann J. 2000. Herbal medicine: expanded commission E monographs. Newton, MA: Integrative Medicine Communications.
- Gunther R. 1959. The Greek herbal of dioscorides. New York: Hafner Publishing.
- Hassanzadeh G, Pasbakhsh P, Akbari M, Shokri S, Ghahremani M, Amin G, Kashani I, Azami Tameh A. 2011. Neuroprotective properties of Melissa officinalis L. extract against ecstasy-induced neurotoxicity. Cell J. 13:25–30.
- Hosseini M, Davazdahemami S. 2007. Agriculture and manufacturing plants, herbs and spices. Tehran, Iran: Institute of Tehran University Publications.
- Inge N. 1986. Magic and medicine of plants. Pleasantville, NY: The Reader's Digest Association Inc.
- Joukar S, Bashiri H, Dabiri S, Ghotbi P, Sarveazad A, Divsalar K, Joukar F, Abbaszadeh M. 2012a. Cardiovascular effects of black tea and nicotine alone or in combination against experimental induced heart injury. J Physiol Biochem. 68:271–279.
- Joukar S, Najafipour H, Dabiri S, Sheibani M, Sharokhi N. 2014a. Cardioprotective effect of mumie (shilajit) on experimentally induced myocardial injury. Cardiovasc Toxicol. 14:214–221.
- Joukar S, Najafipour H, Dabiri S, Sheibani V, Esmaeili-Mahani S, Ghotbi P, Amanallahi F, Joukar F. 2011. The effect of chronic co-administration of morphine and verapamil on isoproterenol-induced heart injury. Cardiovasc Hematol Agents Med Chem. 9:218–224.
- Joukar S, Najafipour H, Mirzaeipour F, Nasri H, Ahmadi MYH, Badinloo M. 2013. Modulatory effect of semelil (angipars™) on isoproterenol induced cardiac injury. EXCLI J. 12:122–129.
- Joukar S, Shahouzehi B, Najafipour H, Gholamhoseinian A, Joukar F. 2012b. Ameliorative effect of black tea on nicotine induced cardiovascular pathogenesis in rat. EXCLI J. 11:309–317.
- Joukar S, Sheibani M, Joukar F. 2012c. Cardiovascular effect of nifedipine in morphine dependent rats: hemodynamic, histopathological, and biochemical evidence. Croat Med J. 53:343–349.
- Joukar S, Zarisfi Z, Sepehri G, Bashiri A. 2014b. Efficacy of Melissa officinalis in suppressing ventricular arrhythmias following ischemia–reperfusion of the heart: a comparison with amiodarone. Med Princ Pract. 23:340–345.
- Lawless J. 1992. The encyclopedia of essential oils. Shaftesbury, Dorset: Element Books.
- López V, Martín S, Gómez-Serranillos MP, Carretero ME, Jäger AK, Calvo MI. 2009. Neuroprotective and neurological properties of Melissa officinalis. Neurochem Res. 34:1955–1961.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem. 193:265–275.
- Mamedov N, Craker LE. 2001. Medicinal plants used for the treatment of bronchial asthma in Russia and Central Asia. J Herbs Spices Med Plants. 8:91–117.
- Martins EN, Pessano NT, Leal L, Roos DH, Folmer V, Puntel GO, Rocha JB, Aschner M, Ávila DS, Puntel RL. 2012. Protective effect of Melissa officinalis aqueous extract against Mn-induced oxidative stress in chronically exposed mice. Brain Res Bull. 87:74–79.
- Mazzanti G, Battinelli L, Pompeo C, Serrilli AM, Rossi R, Sauzullo I, Mengoni F, Vullo V. 2008. Inhibitory activity of Melissa officinalis L. extract on Herpes simplex virus type 2 replication. Nat Prod Res. 22:1433–1440.
- Meyers M. 2007. Lemon balm: An Herb Society of America Guide; [cited 2013 Aug 17]. Available from: http://www.herbsociety.org/factsheets/Lemon%20Balm%20Guide.pdf
- Mimica-Dukic N, Bozin B, Sokovic M,Simin N. 2004. Antimicrobial and antioxidant activities of Melissa officinalis L. (Lamiaceae) essential oil. J Agric Food Chem. 52:2485–2489.
- Ohkawa H, Ohishi N, Yagi K. 1979. Assay of lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal Biochem. 95:351–358.
- Payne B. 2006. Modest Melissa. Herbs 31:10–11.
- Rona G, Chappel CI, Balazs T, Gaudry R. 1959. An infarct-like myocardial lesion and other toxic manifestations produced by isoproterenol in the rat. AMA Arch Pathol. 67:443–455.
- Sharma M, Kishore K, Gupta SK, Joshi S, Arya DS. 2001. Cardioprotective potential of Ocimum sanctum in isoproterenol induced myocardial infarction in rats. Mol Cell Biochem. 225:75–83.
- Singal PK, Kapur N, Dhillon KS, Beamish RE, Dhalla NS. 1982. Role of free radicals in catecholamine-induced cardiomyopathy. Can J Physiol Pharmacol. 60:1390–1397.
- Taylor L. 2012. Topical plant database, Lemonbalm (Melissa officinalis); [cited 2013 Oct 4]. Available from: http://www.rain-tree.com/lemonbalm.htm.
- Ulbricht C, Brendler T, Gruenwald J, Kligler B, Keifer D, Abrams TR, Woods J, Boon H, Kirkwood CD, Hackman DA. 2005. Natural Standard Research Collaboration. Lemon balm (M. officinalis L.): an evidence-based systematic review by the Natural Standard Research Collaboration. J Herb Pharmacother. 5:71–114.
- Wood M. 2008. Sunny Field Herb Farm, The Indispensable Nerve Sedative, Melissa officinalis (Lemonbalm). [cited 2013 Oct 4]. Available from: http://www.woodherbs.com/LemonBalm.html.
- York M, Scudamore C, Brady S, Chen C, Wilson S, Curtis M, Evans G, Griffiths W, Whayman M, Williams T. 2007. Characterization of troponin responses in iso-proterenol-induced cardiac injury in the Hanover Wistar rat. Toxicol Pathol. 35:606–617.
