Abstract
Context: Breast cancer seriously harms the health of women and there are currently few therapeutic options for patients with breast cancer.
Objective: Effects of ginsenoside compound K (CK) in combination with cisplatin (DDP) on the proliferation, apoptosis, and epithelial mesenchymal transition (EMT) of MCF-7 cells were studied.
Materials and methods: MCF-7 cells were divided into CK (50 μmol/L) group, DDP (10 mg/L) group, CK (50 μmol/L) +DDP (10 mg/L) group, and control (CON) group. The cells in the CON group were not treated with any drugs. Proliferation, apoptosis, expression of E-cadherin, N-cadherin, vimentin, protein kinase B (Akt), phosphorylated Akt (p-Akt), and level of fibronectin (FN) in MCF-7 cells were detected by methyl thiazolyl tetrazolium (MTT), flow cytometry, western blotting, and enzyme-linked immuno sorbent assay (ELISA), respectively.
Results: The proliferation inhibition rates in CK, DDP, and CK + DDP groups at 48 h were 19.18 ± 2.25, 21.34 ± 2.84, and 43.37 ± 5.62, respectively. The apoptosis rates were 2.85 ± 0.56, 13.37 ± 2.28, 20.04 ± 2.92, and 30.78 ± 4.64 at 24 h and 3.14 ± 0.72, 20.36 ± 3.28, 27.58 ± 4.09, and 41.62 ± 5.83 at 48 h in CON, CK, DDP, and CK + DDP groups, respectively. CK or DDP alone and their combination all could reduce the levels of N-cadherin, vimentin, p-Akt/Akt, and FN and elevate level of E-cadherin.
Discussion and conclusion: Both CK and DDP can inhibit the proliferation, EMT, and induce the apoptosis in MCF-7 cells, which may be related to the PI3K/Akt pathway. In addition, the combination of CK with DDP can produce a better effect.
Keywords:
Introduction
Breast cancer is a common malignancy that seriously harms the health of women; there are 1.2 million women suffering from breast cancer and 0.5 million die of it each year worldwide (Siegel et al. Citation2015). The number of patients with breast cancer is rapidly increasing and showing a younger trend, seriously affecting women's health and lives (Zghair et al. Citation2014). Currently, the treatment means of breast cancer include surgery, chemotherapy, radiotherapy, and endocrine therapy (Joy et al. Citation2015). Given that most breast cancer has metastasized by the time it is diagnosed, and chemotherapy can eradicate residual tumor cells in the body and thus improve the cure rate of surgery, chemotherapy is very important for the treatment of breast cancer (Cancello et al. Citation2015). Chemotherapeutic drugs used early for breast cancer, such as cisplatin, cyclophosphamide, and methotrexate, and those used recently, such as doxorubicin and paclitaxel, all have shown a good effect on breast cancer (Shimada et al. Citation2015). However, due to the complexity and uncertainty of the pathogenesis of cancers, and the poor selectivity of chemotherapeutic drugs, the therapy can often induce nausea, vomiting, bone marrow suppression, and other serious side effects when some therapeutic effects are achieved, and these side effects can not only reduce the lifetime and life quality of patients with cancer, but also offset the benefit of treatment, forcing the patients to delay or terminate the treatment, and even directly accelerate the death of the patients (Saxena Citation2006; Mailliez et al. Citation2010; Caffo et al. Citation2015). Thus, exploring the effects of different chemotherapeutic drugs on breast cancer and the related mechanisms is of great significance for the clinical treatment of breast cancer.
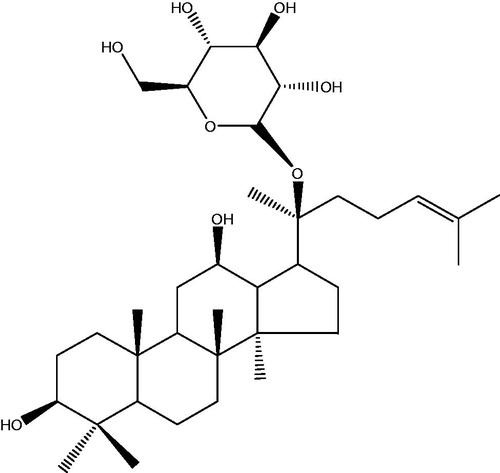
In recent years, to look for safe and effective antitumor substances with less severe side effects or lead compounds from natural plants has become an important area of research (Lim et al. Citation2014). Ginsenoside is one of the main active ingredients of ginseng (Panax ginseng Meyer), and the chemistry, pharmacology, and other aspects of ginseng have been studied systemically and in-depth in modern medicine. So far, more than 40 ginsenosides have been isolated from the whole plant and processed ginseng, which have been demonstrated to have some biological activities, such as immune regulation, antiaging, antidementia, and improvement of cerebral ischemia-reperfusion injury (Cheng et al. Citation2005; Wang et al. Citation2011; Kim et al. Citation2012); of which, ginsenoside Rg3, Rg1, Rh1, Rb1, and other components show a significant antitumor effect mainly by promoting the apoptosis of tumor cells, blocking the metastasis of tumor cells, inhibiting the angiogenesis of tumor, reversing the multidrug resistance of tumors, and affecting the expression of cancer genes related to the tumor signal transduction, and particularly, play a prominent role in reducing side effects of the chemotherapy and improving the sensitivity of tumor cells to chemotherapeutic drugs in cancer patients (Lee et al. Citation2003; Lau et al. Citation2009; Guo et al. Citation2014; Park et al. Citation2014). CK, i.e., 20 (S)-O-β-d-glucoside protopanaxadiol (the chemical structure is shown in ) is an active metabolite of ginsenosides in vivo, having shown some significant effects, such as a hypoglycemic effect, anti-inflammatory activity, improving immunity, liver protection, and antitumor (Guo et al. Citation2014; Lee et al. Citation2014; Mathiyalagan et al. Citation2014; Wu et al. Citation2014). DDP is a common drug used for the chemotherapy of malignant tumors with a strong and broad-spectrum anticancer effect and can inhibit the DNA replication process of cancer cells, but it is common for tumor cells to develop a resistance to it in clinical chemotherapy (Seto et al. Citation2015; Watanabe et al. Citation2015). In this study, human breast cancer MCF-7 cells were used as the object to investigate effects of CK alone and CK combined with DDP on the proliferation, apoptosis and EMT of MCF-7 cells. In addition, their underlying molecular mechanisms were explored.
Materials and methods
Materials and chemicals
CK (purity > 98%) was provided by Weikeqi Biological Technology Co. (Shichuan, China). DDP was purchased from Haosen Pharmaceutical Co., Ltd. (Jiangsu, China). Human breast cancer cell line MCF-7 cells were purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). RPMI-1640 medium, foetal bovine serum, penicillin, and streptomycin were purchased from Hyclone Co. (Logan, UT). MTT and sodium dodecyl sulphate (SDS) were purchased from Sigma-Aldrich Co. (St. Louis, MO). Annexin-FITC/PI apoptosis detection reagent box was purchased from BD Biosciences Co. (Franklin Lakes, NJ). E-Cadherin and N-cadherin polyclonal antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA). Vimentin, Akt, and P-Akt polyclonal antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, TX).
Detection of proliferative activity
MCF-7 cells were placed in RPMI-1640 medium under conventional culture conditions, namely 10% foetal bovine serum, 1 × 105 U/L penicillin, and 100 mg/L streptomycin, 37 °C, 5% CO2 and in saturated humidity, and the medium was changed every 2–3 d. Logarithmic growth phase MCF-7 cells digested with trypsin were prepared into a single cell suspension. The cells were seeded at 1 × 106 cells/well in 96-well culture plates and treated with 50 μmol/L CK or 10 mg/L DDP alone or 50 μmol/L CK in combination with 10 mg/L DDP, respectively, after they were stably cultured for 24 h. The MCF-7 cells were divided into four groups: CON group, CK group, DDP group, and CK + DDP group. The cells seeded in the media in the CON group were not treated with any drugs; after they were cultured under the conventional conditions for 24, 48, 72, and 96 h, 10 μL of 5 mg/mL MTT solution were added to each well, which was incubated for 4 h and then the absorbance values were tested at 492 nm wavelength with an automatic microplate reader. Three wells were set for the each concentration and the experiment was repeated three times. The inhibition rate of proliferation was calculated according to the following formula:
Flow cytometry
MCF-7 cells were seeded at 1 × 106 cells/well in culture plates, and grouped and treated as above described accordingly. The cells were collected after the treatment for 24 and 48 h, washed with PBS, fixed with cold ethanol and treated with RNase in turn, and the subsequent operations were carried out according to the instructions of apoptosis detection kit. Annexin V/FITC and PI were added to the samples for the dark staining for 15 min, and then the apoptosis rate was measured by flow cytometry.
Western blotting
The cell lysate was added to cells collected after the treatment for 48 h in an ice bath, which was centrifuged by high-speed centrifugation at 12 000 rpm for 30 min after the lysis. The supernatant was collected and the protein concentration in it was measured with BCA assay. An equal amount of the protein was electrophoresed by SDS-PAGE gel. After the transfer of protein by semi-dry transfer method, according to the membrane area, an appropriate amount of the first antibody of E-cadherin, N-cadherin, vimentin, Akt, and p-Akt (1:100 except that E-cadherin was 1:200) was in turn added to the membrane, which was incubated at 4 °C overnight; then, the secondary antibody (1:3000) was added to it, which was incubated with shaking at room temperature for 2 h. After the chemiluminescence development, the optical density of the strip after exposure was analysed with IPP 6.0 software (Media Cybernetics, Inc., Bethesda, MD), and the final results indicated as the ratio of the optical density of each band and the internal reference GADPH (Santa Cruz Biotechnology, Inc., Santa Cruz, TX).
ELISA
FN levels in supernatants of cells treated for 24, 48, 72, and 96 h in each group were measured according to the operating steps described in the instructions of a FN test kit.
Statistical analysis
The data in this study were analysed with SPSS 19.0 software (SPSS Inc., Chicago, IL). The data were expressed as mean ± SD. The results from multiple groups were compared using one-way analysis of variance (ANOVA) and pairwise comparisons were conducted using the Bonferroni method. p < 0.05 was considered statistically significant.
Results
Effect of CK combined with cisplatin on the proliferation of MCF-7 cells
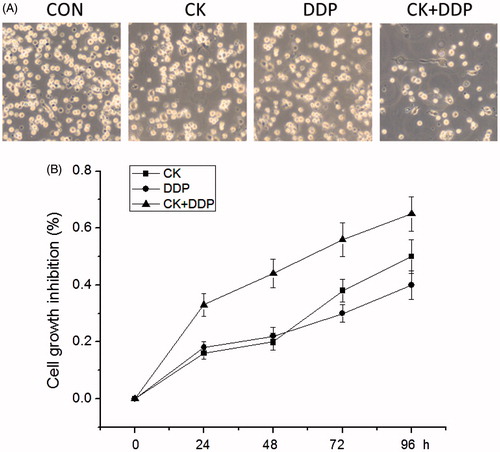
The detection with MTT assay showed that compared with those in the CON group, proliferation inhibition rates in CK, DDP and CK + DDP groups increased significantly within the treatment duration of 24–96 h in a time-dependent manner and the inhibition rates at 48 h were 19.18 ± 2.25, 21.34 ± 2.84, and 43.37 ± 5.62, respectively; meanwhile, those in the CK + DDP group were significantly higher than those in CK and DDP groups (p < 0.05). The results are shown in .
Figure 2. Effect of CK combined with DDP on the inhibition rate of MCF-7 cell proliferation. (A) MCF-7 cells were treated with CK, DDP, CK + DDP for 48 h; (B) the inhibition rate of cell proliferation was measured with MTT assay for 24, 48, 72, and 96 h. Each value is presented as mean ± SD (n = 3).
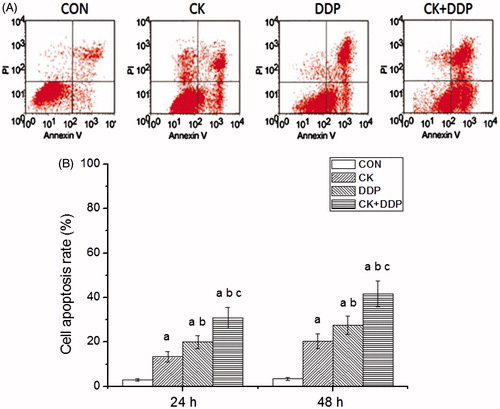
Effect of CK combined with cisplatin on the apoptosis of MCF-7 cells
As shown in , the detection with Annexin-FITC/PI double staining found that the apoptosis rates were 2.85 ± 0.56, 13.37 ± 2.28, 20.04 ± 2.92, and 30.78 ± 4.64 at 24 h and 3.14 ± 0.72, 20.36 ± 3.28, 27.58 ± 4.09, and 41.62 ± 5.83 at 48 h in CON, CK, DDP, and CK + DDP groups, respectively. Compared with those in the CON group, the apoptosis rates after the treatment for 24 and 48 h in CK, DDP, and CK + DDP groups increased significantly (p < 0.05). The apoptosis rates after the treatment for 24 and 48 h in the CK + DDP group were significantly higher than those in CK and DDP groups and after the treatment for 48 h were higher than those after the treatment for 24 h in CK, DDP and CK + DDP groups (p < 0.05).
Figure 3. Effect of CK combined with DDP on the apoptosis rate of MCF-7 cells. (A) Flow cytometric analysis after 48 h; (B) cell apoptosis rate after 24 and 48 h. Each value is presented as mean ± SD (n = 3). ap < 0.05 compared with those in the CON group, bp < 0.05 compared with those in the CK group, cp < 0.05 compared with those in the DDP group.
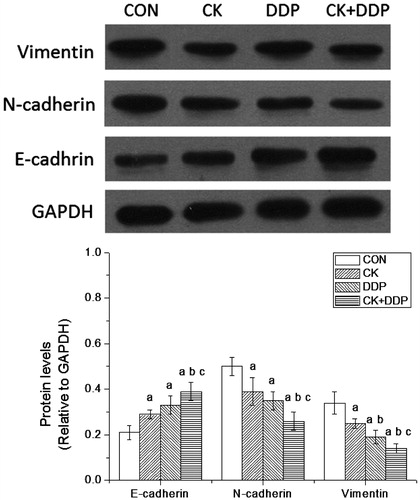
Effect of CK combined with cisplatin on EMT-related markers
The western blotting analysis showed that compared with those in the CON group, E-cadherin levels after the treatment for 48 h increased, and N-cadherin and vimentin levels decreased significantly in CK, DDP, and CK + DDP groups (p < 0.05); E-cadherin levels in the CK + DDP group were significantly higher than those in CK and DDP groups, and N-cadherin and vimentin levels in the CK + DDP group were significantly lower than those CK and DDP groups (p < 0.05); vimentin levels in DDP group were significantly lower than those in the CK group (p < 0.05), while E-cadherin and N-cadherin levels did not change significantly (p > 0.05). The results are shown in .
Figure 4. Effect of CK combined with DDP on the expressions of E-cadherin, N-cadherin and vimentin in MCF-7 cells. MCF-7 cells were treated with CK, DDP, and CK + DDP for 48 h. Then the expressions of target protein were evaluated by western blot assay. Each value is presented as mean ± SD (n = 3). ap < 0.05 compared with those in the CON group, bp < 0.05 compared with those in the CK group, cp < 0.05 compared with those in the DDP group.
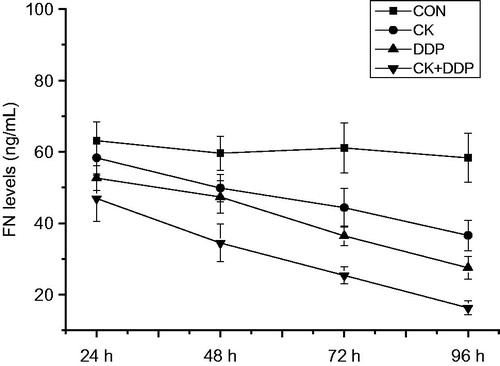
Effect of CK combined with cisplatin on FN levels in the supernatant of MCF-7 cell
As shown in , the detection by ELISA showed that compared with those in the CON group, FN levels within the treatment for 24–96 h were significantly decreased in CK, DDP and CK + DDP groups (p < 0.05); those in the CK + DDP group were significantly lower than those in CK and DDP groups (p < 0.05); those in the DDP group were lower than those in the CK group, and with the increase in the treatment time, FN levels were reduced in CK, DDP, and CK + DDP groups (p < 0.05), in a time-dependent manner.
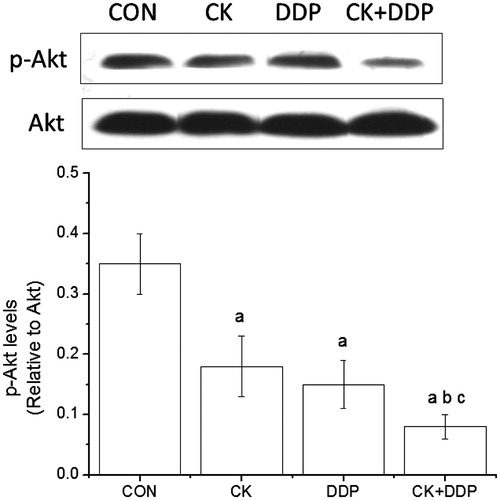
Effect of CK combined with cisplatin on PI3K/Akt signalling pathway in MCF-7 cells
As shown in , the detection by western blotting showed that p-Akt/Akt values in CK, DDP, and CK + DDP groups were significantly lower than those in the CON group, and those in the CK + DDP group were lower than those in the CK and DDP groups (p < 0.05), but there was no significant difference in p-Akt/Akt values between the CK and DDP groupc (p > 0.05).
Figure 6. Effect of CK combined with DDP on the PI3K/Akt signaling pathway in MCF-7 cells. MCF-7 cells were treated with CK, DDP, and CK + DDP for 48 h. Then the expression of p-Akt and Akt were evaluated by western blot assay. Each value is presented as mean ± SD (n = 3). ap < 0.05 compared with those in the CON group, bp < 0.05 compared with those in the CK group, cp < 0.05 compared with those in the DDP group.
Discussion
DDP, a cell-cycle non-specific agent with the bifunctional group similar to alkylating agents, can interfere with DNA replication by acting on bases of intracellular DNA chain to change the normal function of template replication (Fuertes et al. Citation2003). Although the effective rate of DDP monotherapy on advanced breast cancer is low, therapies of DDP combined with paclitaxel or vinorelbine or gemcitabine, etc., have achieved significant effects, with an effective rate of 56.7–69.0% (Sledge et al. Citation1988; Rosati et al. Citation2000; Ahn et al. Citation2005). Related studies have shown that CK has a certain inhibition on hepatocellular carcinoma, nasopharyngeal cancer, colon cancer, bladder cancer, etc., but there are few reports on the inhibition of CK and CK in combination with cisplatin on breast cancer (Kang et al. Citation2013; Kim et al. Citation2013; Wang et al. Citation2013; Zheng et al. Citation2014; Kwak et al. Citation2015). For this reason, in this study, human breast cancer MCF-7 cells were treated with CK or DDP alone or CK combined with DDP, confirming that both CK and DDP alone could significantly inhibit the proliferation of MCF-7 cells; in addition, the study also found out that after the treatment of CK combined with DDP, the inhibition on MCF-7 cells would be better and the inhibitory rate could increase with the prolonging of time.
It has been verified that CK exerts its antitumor effect primarily by inducing the apoptosis of cancer cells. Lee et al. (Citation2011) confirmed in their study on the induction of apoptosis of human promyelocytic leukemia cell line HL-60 cells by CK that caspase-8 could be involved in the regulation of apoptosis by the direct activation of caspase-3 or by the indirect Bid excision, cytochrome C release and caspase-9 activation pathways. Li et al. (Citation2009) found that CK could induce the apoptosis of prostate cancer PC-3 cells, in which CK could significantly reduce the membrane potential of mitochondria, compromise the integrity of mitochondrial membrane, increase the release of cytochrome C, then activate caspase-9, and further activate caspase-3. In this study, it was found that the apoptosis rate of MCF-7 cells treated with CK was significantly elevated, further confirming that CK could induce the apoptosis of MCF-7 cells; in addition, the results of the study also showed that the combination of CK with DDP could more effectively induce the apoptosis of MCF-7 cells.
EMT is an important step in the invasion and metastasis of cancer cells, and a physiological and pathological phenomenon, characterised by the loss of epithelial features and the acquisition of intercellular characteristics (Liu et al. Citation2015). In the process of EMT, the expression of E-cadherin, a membrane protein mediating the tight junction between epithelial cells, is downregulated, and the expression of connexin between mesenchymal cells, such as N-cadherin and vimentin, is upregulated (Liu et al. Citation2014). The results showed that CK or DDP alone or CK in combination with DDP could increase the level of epithelial marker molecule E-cadherin, and at the same time, reduce the level of mesenchymal markers N-cadherin and vimentin, indicating that both CK and DDP can inhibit the EMT process in MCF-7 cells. FN is an extracellular matrix glycoprotein involved in the adhesion and migration between cells and matrix, and among cells. Studies have shown that a lower FN level can suppress the occurrence of EMT (Jeon et al. Citation2015). In this study, the dynamic changes in FN levels were investigated by ELISA, it was also found that CK or DDP alone or their combination could reduce the level of FN, further indicating that the two drugs can inhibit the EMT process, and the effect of their combination should be stronger than that of either alone of them.
PI3K/Akt signal pathway plays an important role in the process of cell proliferation and apoptosis, and PI3K/Akt signal pathway in most tumour cells is activated (Chia et al. Citation2015). PI3K can cause the phosphorylation of Akt. If PI3K/Akt signal pathway is activated, the ratio of the p-Akt will increase, vise versa. Studies have shown that a variety of chemotherapeutic drugs may exert their antitumor effects by blocking this pathway (Zhang et al. Citation2013; Ryu et al. Citation2014). In addition, a number of studies have shown that the activation of PI3K/Akt signalling pathway may promote the occurrence of EMT (Ha et al. Citation2013). Yang et al. (Citation2014) confirmed that inhibiting the activation of PI3K/Akt pathway could inhibit the occurrence of EMT in breast cancer MCF-7 and BT-20 cells. Our results showed that CK or DDP alone and their combination could reduce the level of p-Akt, which may be one of the mechanisms through which CK and DDP can inhibit the proliferation and EMT, and induce the apoptosis in MCF-7 cells.
Both CK and DDP can inhibit the proliferation, EMT and induce the apoptosis in MCF-7 cells, which may be related to the PI3K/Akt pathway. In addition, the combination of CK with DDP can produce a better effect.
Declaration of interest
This work was funded by a scientific research project from the Science and Technology Development Plan of Jilin Province.
References
- Ahn JH, Kim SB, Sohn HJ, Lee JS, Kang YK, Kun Kim W. 2005. Docetaxel and cisplatin combination chemotherapy in metastatic breast cancer patients with previous exposure to anthracyclines. Breast. 14:304–309.
- Caffo O, Lo RG, Sava T, Buti S, Sacco C, Basso U, Zustovich F, Lodde M, Perin A, Facchini G, et al. 2015. Intermittent docetaxel chemotherapy as first-line treatment for metastatic castration-resistant prostate cancer patients. Future Oncol. 11:965–973.
- Cancello G, Bagnardi V, Sangalli C, Montagna E, Dellapasqua S, Sporchia A, Iorfida M, Viale G, Barberis M, Veronesi P, et al. 2015. Phase II study with epirubicin, cisplatin, and infusional fluorouracil followed by weekly paclitaxel with metronomic cyclophosphamide as a preoperative treatment of triple-negative breast cancer. Clin Breast Cancer. 15:259–265.
- Cheng Y, Shen LH, Zhang JT. 2005. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol Sin. 26:143–149.
- Chia S, Gandhi S, Joy AA, Gorr M, Hopkins S, Kondejewski J, Ayoub JP, Califaretti N, Rayson D, Dent SF. 2015. Novel agents and associated toxicities of inhibitors of the PI3k/Akt/MTOR pathway for the treatment of breast cancer. Curr Oncol. 22:33–48.
- Fuertes MA, Castilla J, Alonso C, Pérez JM. 2003. Cisplatin biochemical mechanism of action: From cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr Med Chem. 10:257–266.
- Guo J, Chang L, Zhang X, Pei S, Yu M, Gao J. 2014. Ginsenoside compound K promotes β-amyloid peptide clearance in primary astrocytes via autophagy enhancement. Exp Ther Med. 8:1271–1274.
- Guo JQ, Zheng QH, Chen H, Chen L, Xu JB, Chen MY, Lu D, Wang ZH, Tong HF, Lin S. 2014. Ginsenoside Rg3 inhibition of vasculogenic mimicry in pancreatic cancer through downregulation of VE-cadherin/EphA2/MMP9/MMP2 expression. Int J Oncol. 45:1065–1072.
- Ha GH, Park JS, Breuer EK. 2013. TACC3 promotes epithelial–mesenchymal transition (EMT) through the activation of PI3K/Akt and ERK signaling pathways. Cancer Lett. 332:63–73.
- Jeon M, Lee J, Nam SJ, Shin I, Lee JE, Kim S. 2015. Induction of fibronectin by HER2 overexpression triggers adhesion and invasion of breast cancer cells. Exp Cell Res. 333:116–126.
- Joy AA, Ghosh M, Fernandes R, Clemons MJ. 2015. Systemic treatment approaches in her2-negative advanced breast cancer-guidance on the guidelines. Curr Oncol. 22:S29–42.
- Kang KA, Piao MJ, Kim KC, Zheng J, Yao CW, Cha JW, Kim HS, Kim DH, Bae SC, Hyun JW. 2013. Compound K, a metabolite of ginseng saponin, inhibits colorectal cancer cell growth and induces apoptosis through inhibition of histone deacetylase activity. Int J Oncol. 43:1907–1914.
- Kim AD, Kang KA, Kim HS, Kim DH, Choi YH, Lee SJ, Kim HS, Hyun JW. 2013. A ginseng metabolite, compound K, induces autophagy and apoptosis via generation of reactive oxygen species and activation of JNK in human colon cancer cells. Cell Death Dis. 4:e750
- Kim J, Han BJ, Kim H, Lee JY, Joo I, Omer S, Kim YS, Han Y. 2012. Th1 immunity induction by ginsenoside Re involves in protection of mice against disseminated candidiasis due to Candida albicans. Int Immunopharmacol. 14:481–486.
- Kwak CW, Son YM, Gu MJ, Kim G, Lee IK, Kye YC, Kim HW, Song KD, Chu H, Park BC, et at. 2015. A bacterial metabolite, compound K, induces programmed necrosis in MCF-7 cells via GSK3β. J Microbiol Biotechnol. 25:1170–1176.
- Lau WS, Chen WF, Chan RY, Guo DA, Wong MS. 2009. Mitogen-activated protein kinase (MAPK) pathway mediates the oestrogen-like activities of ginsenoside Rg1 in human breast cancer (MCF-7) cells. Br J Pharmacol. 156:1136–1146.
- Lee CS, Bae IH, Han J, Choi GY, Hwang KH, Kim DH, Yeom MH, Park YH, Park M. 2014. Compound K inhibits MMP-1 expression through suppression of c-Src-dependent ERK activation in TNF-α-stimulated dermal fibroblast. Exp Dermatol. 23:819–824.
- Lee ES, Choi JS, Kim MS, You HJ, Ji GE, Kang YH. 2011. Ginsenoside metabolite compound K differentially antagonizing tumor necrosis factor-α-induced monocyte-endothelial trafficking. Chem Biol Interact. 194:13–22.
- Lee YJ, Jin YR, Lim WC, Park WK, Cho JY, Jang S, Lee SK. 2003. Ginsenoside-Rb1 acts as a weak phytoestrogen in MCF-7 human breast cancer cells. Arch Pharm Res. 26:58–63.
- Li W, Liu Y, Zhang JW, Ai CZ, Xiang N, Liu HX, Yang L. 2009. Anti-androgen independent prostate cancer effects of ginsenoside metabolites in vitro: Mechanism and possible structure-activity relationship investigation. Arch Pharm Res. 32:49–57.
- Lim HK, Bae W, Lee HS, Jung J. 2014. Anticancer activity of marine sponge Hyrtios sp. extract in human colorectal carcinoma RKO cells with different p53 status. Biomed Res Int. 2014:413575.
- Liu CH, Tang WC, Sia P, Huang CC, Yang PM, Wu MH, Lai IL, Lee KH. 2015. Berberine inhibits the metastatic ability of prostate cancer cells by suppressing epithelial-to-mesenchymal transition (EMT)-associated genes with predictive and prognostic relevance. Int J Med Sci. 12:63–71.
- Liu T, Zhao L, Zhang Y, Chen W, Liu D, Hou H, Ding L, Li X. 2014. Ginsenoside 20(S)-Rg3 targets HIF-1α to block hypoxia-induced epithelial-mesenchymal transition in ovarian cancer cells. PLoS One. 9:e103887.
- Mailliez A, Baldini C, Van JT, Servent V, Mallet Y, Bonneterre J. 2010. Nasal septum perforation: a side effect of bevacizumab chemotherapy in breast cancer patients. Br J Cancer. 103:772–775.
- Mathiyalagan R, Subramaniyam S, Kim YJ, Kim YC, Yang DC. 2014. Ginsenoside compound K-bearing glycol chitosan conjugates: Synthesis, physicochemical characterization, and in vitro biological studies. Carbohydr Polym. 112:359–366.
- Park EH, Kim YJ, Yamabe N, Park SH, Kim HK, Jang HJ, Kim JH, Cheon GJ, Ham J, Kang KS. 2014. Stereospecific anticancer effects of ginsenoside Rg3 epimers isolated from heat-processed American ginseng on human gastric cancer cell. J Ginseng Res. 38:22–27.
- Rosati G, Riccardi F, Tucci A, De Rosa P, Pacilio G. 2000. A phase II study of paclitaxel/cisplatin combination in patients with metastatic breast cancer refractory to anthracycline-based chemotherapy. Tumori. 86:207–210.
- Ryu YL, Jung KH, Son MK, Yan HH, Kim SJ, Shin S, Hong S, Hong SS. 2014. Anticancer activity of HS-527, a novel inhibitor targeting PI3-kinase in human pancreatic cancer cells. Cancer Lett. 353:68–77.
- Saxena A. 2006. Cancer chemotherapy and its side effect management. Nurs J India. 97:109–110.
- Seto A, Sugitani I, Toda K, Kawabata K, Takahashi S, Saotome T. 2015. Chemotherapy for anaplastic thyroid cancer using docetaxel and cisplatin: report of eight cases. Surg Today. 45:221–226.
- Shimada K, Ishikawa T, Kita K, Narui K, Sugae S, Shimizu D, Tanabe M, Sasaki T, Chishima T, Ichikawa Y, et al. 2015. Neoadjuvant docetaxel/cyclophosphamide in triple-negative breast cancer: Predictive value of class III-β tubulin and non-basal subtype. Anticancer Res. 35:907–912.
- Siegel RL, Miller KD, Jemal A. 2015. Cancer statistics, 2015. CA Cancer J Clin. 65:5–29.
- Sledge GW, Loehrer PJ, Roth BJ, Einhorn LH. 1988. Cisplatin as first-line therapy for metastatic breast cancer. J Clin Oncol. 6:1811–1814.
- Wang H, Jiang D, Liu J, Ye S, Xiao S, Wang W, Sun Z, Xie Y, Wang J. 2013. Compound K induces apoptosis of bladder cancer T24 cells via reactive oxygen species-mediated p38 MAPK pathway. Cancer Biother Radiopharm. 28:607–614.
- Wang Y, Liu J, Zhang Z, Bi P, Qi Z, Zhang C. 2011. Anti-neuroinflammation effect of ginsenoside Rbl in a rat model of Alzheimer disease. Neurosci Lett. 487:70–72.
- Watanabe N, Niho S, Kirita K, Umemura S, Matsumoto S, Yoh K, Ohmatsu H, Goto K. 2015. Vinorelbine and cisplatin in patients with advanced non-small cell lung cancer with interstitial pneumonia. Anticancer Res. 35:1697–1701.
- Wu H, Chen J, Wang Q, Jia X, Song S, Yuan P, Liu K, Liu L, Zhang Y, Zhou A, et al. 2014. Ginsenoside metabolite compound K attenuates inflammatory responses of adjuvant-induced arthritis rats. Immunopharmacol Immunotoxicol. 36:124–129.
- Yang J, Zeng Z, Peng Y, Chen J, Pan L, Pan D. 2014. IL-7 splicing variant IL-7δ5 induces EMT and metastasis of human breast cancer cell lines MCF-7 and BT-20 through activation of PI3K/Akt pathway. Histochem Cell Biol. 142:401–410.
- Zghair AN, Sharma R, Sharma AK. 2014. Hormone responsive breast cancer and BRCA1 mutation: mechanism, regulation and iron-mediated effects. Curr Pharm Biotechnol. 15:1113–1124.
- Zhang X, Li XR, Zhang J. 2013. Current status and future perspectives of PI3K and mTOR inhibitor as anticancer drugs in breast cancer. Curr Cancer Drug Targets. 13:175–187.
- Zheng ZZ, Ming YL, Chen LH, Zheng GH, Liu SS, Chen QX. 2014. Compound K-induced apoptosis of human hepatocellular carcinoma MHCC97-H cells in vitro. Oncol Rep. 32:325–331.