Abstract
Context There have not been any conclusive studies of the effects of diosmin, a modified flavanone glycoside obtained from Teucrium gnaphalodes L’Her (Lamiaceae), on urolithiasis.
Objective To evaluate anti-urolithiatic effects of diosmin in ammonium chloride and ethylene glycol-induced renal stone in experimental animals.
Materials and methods Thirty Sprague-Dawley were divided into five groups (n=6) receiving the following treatments, respectively, p.o. for 15 consecutive days: distilled water, 0.75% v/v ethylene glycol + 2% w/v ammonium chloride, 0.75% v/v ethylene glycol + 2% w/v ammonium chloride + cystone® 750 mg/kg, 0.75% v/v ethylene glycol + 2% w/v ammonium chloride + diosmin 10 mg/kg or 0.75% v/v ethylene glycol + 2% w/v ammonium chloride + diosmin 20 mg/kg. Different biomarkers of urolithiasis in urine and serum were evaluated and histopathological examination of kidney was done.
Results Animals treated with diosmin (both 10 and 20 mg/kg) had significantly (p < 0.005) decreased in kidney weight, urinary pH, total urinary protein, urinary calcium, phosphorus, serum potassium, sodium, magnesium, creatinine, uric acid and blood urea nitrogen levels and significantly (p < 0.005) increased in urinary volume, urinary magnesium, potassium, sodium, creatinine, uric acid and serum calcium levels in comparison to animals treated with ethylene glycol and ammonium chloride. However, results were better with diosmin 20 mg/kg in comparison to the control group.
Conclusion Diosmin (10 and 20 mg/kg) has very good anti-urolithiatic activity similar to the standard drug cystone®.
Introduction
Renal stones, one of the most painful urologic disorders, have beset humans for centuries. Urinary calculi have been found in the tombs of Egyptian mummies dating back to 4000 BC and in the graves of North American Indians from 1500 to 1000 BC (Vijaya et al. Citation2013). Reference to stone formation was made even in the early Sanskrit documents in India between 3000 and 2000 BC. (Khan et al. Citation2010).
Urolithiasis is the third most common urinary tract disorder responsible for serious human suffering and economic cost to society (Adepu et al. Citation2014). It is a consequence of some complex physical processes. The major factors are super saturation of urine with the offending salt and crystallisation. Crystals retained in kidney become nucleus for stone formation (Tortora & Derrickson Citation2009).
An alarming high incidence of urolithiasis with varied chemical composition of calculi has been reported from various parts of India (Yagisawa et al. Citation1999). In North India, more than 80% of urinary calculi comprise calcium oxalate alone or in combination with calcium phosphate. Hyperoxaluriasis is the main initiating factor of human idiopathic calcium oxalate stone disease. Oxalate is a powerful crystallisation-driving factor present in the urine, retention of which enhances cell injury and causes early stages of lithogenesis (Evan 2010; Kumar et al. Citation2002).
The calculi causing urinary obstructions occur more commonly in males due to smaller diameter and greater length of urethra. Calculi resulting from the precipitation of stone constituents might be due to super saturation in urine also. Previous studies found that the incidence of upper urinary tract stones was many fold greater in men than in women, and stones were composed mainly of calcium oxalate mostly in males than in females (Bushinsky et al. Citation2002; Trinchieri Citation2008). Although the pathogenesis of urolithiasis appears to be multi-factorial and intricate, sex hormone-derived gender differences are thought to influence the process. In addition, several previous reports have concluded that testosterone promotes urolithiasis and oestrogen inhibits it (Yoshioka et al. Citation2010). Epidemiological studies also indicate many factors like age, sex, industrialisation, socioeconomic status, diet and environment influences urolithiasis. The prevalence of renal stone formation is approximately 2–3% in the general population.
Now, diosmin is a naturally occurring flavonoid glycoside and a member of the citrus flavonoid family and is a modified form of hesperidin. It can be found in Teucrium gnaphalodes L’Her (Lamiaceae), a plant endemic to the Iberian Peninsula (Barberán et al. Citation1985). It is a semi-synthetic drug that is used as an oral phlebotropic agent in the treatment of venous disease, like chronic insufficiency and haemorrhoid disease. Diosmin prolongs the vasoconstrictor effect of norepinephrine on the vein wall, increase the venous tone, and reduces the venous capacitance, distensibility and stasis (Tamamoto et al. Citation2010). Diosmin has also been found to be effective in mitigating hyperglycaemia in diabetic rats (Srinivasan & Pari Citation2012). Its anti-inflammatory and anti-apoptotic activity has also been demonstrated in neuronal cells.
However, until now, clinical studies of the effects of diosmin on renal diseases have not been conclusive. Furthermore, only a limited number of articles have been published and few articles are available on the use of diosmin in renal diseases, particularly urolithiais. Based on this background, this study was done to evaluate the protective effects of diosmin in ethylene glycol and ammonium chloride-induced urolithiasis in Sprague-Dawley rats.
Materials and methods
Experimental animals
This experiment was performed after getting approval of the Institutional Animal Ethics Committee, Swamy Vivekanandha College of Pharmacy, Tamil Nadu, India (CPCSEA Reg. no. 889/SVCP/IAEC/M.Pharm/04/2013).
Adult male Sprague Dawley rats, weighing 150–210 g, obtained from the central animal house facility of Swamy Vivekanandha College of Pharmacy, Tamil Nadu, India, were used. The animals were acclimatised for 2 weeks in the laboratory conditions before initiating the experiment. Throughout the acclimatisation and experimental period, animals were housed in polyprolylene cages in standard laboratory conditions with temperature 21 ± 2 °C, relative humidity 50–60% and lighting conditions with 12 h light/dark cycle and having provision of separating faecal and urine samples. The rats were provided standard pellet diet and water ad libitum throughout the experiment.
Chemicals
Ethylene glycol (Sigma Aldrich, Grade: anhydrous, Assay: 99.8%), ammonium chloride (Sigma Aldrich (Saint Louis, MO), Grade: for molecular biology, Assay: ≥99.5%), diosmin (Emcure Pharmaceuticals Ltd., Pune, India), carboxymethylcellulose (Sigma Aldrich) and cystone® (Himalaya Herbal Healthcare, Bangalore, India) were obtained commercially.
Experimental design
Renal calculi were induced using ethylene glycol and ammonium chloride (Sailaja et al. Citation2011; Kishore et al. Citation2013). Cystone® was used as the standard drug for treatment (Rai Citation1960; Kumar Citation1979; Karamakar & Patki Citation2008). The animals were divided in the five following groups with six animals in each group receiving the following treatments orally (p.o.) for 15 consecutive days:
Group 1: distilled water (negative control).
Group 2: 0.75% v/v ethylene glycol + 2% w/v ammonium chloride (for inducing urolithiasis).
Group 3: 0.75% v/v ethylene glycol + 2% w/v ammonium chloride + cystone® 750 mg/kg (standard drug).
Group 4: 0.75% v/v ethylene glycol + 2% w/v ammonium chloride + diosmin 10 mg/kg (test drug).
Group 5: 0.75% v/v ethylene glycol + 2% w/v ammonium chloride + diosmin 20 mg/kg (test drug).
Assessment of anti-urolithiatic activity
All animals were kept in individual cages and 24 h urine samples were collected on the 15th day. Total urine volume, urinary pH and total protein content of urine were measured. The animals then underwent overnight fasting and on the next morning, blood samples were collected in EDTA tubes from retro-orbital venous plexus and serum was separated by centrifugation. Different parameters as markers of urolithiais were measured in urine and serum by the processed described below. The animals were then euthanised by chloroform inhalational method and both the kidneys were removed from each rat for histopathological examination.
Analytical procedures
Total urinary protein was measured by pyrogallol red-molybdenum complex method by spectrophotometry (600 nm wave length) (Marshall & Williams Citation2003). Creatinine in urine and serum was measured by Jaffe's method with the formation of creatinine-picrate and estimated by spectrophotometry (600 nm wave length) (Jaffe Citation1886). Calcium in urine and serum was measured by O-cresolphthalein complex method by spectrophotometry (578 nm wave length) (Parentoni et al. Citation2001). Sodium in urine and serum was measured by the formation of uranyl magnesium sodium acetate complex and adding potassium ferrocyanide and estimated by spectrophotometry (550 nm wave length) (Stone & Goldzieher Citation1949). Potassium in urine and serum was measured by adding sodium tetraphenyl boron and estimated by spectrophotometry (630 nm wave length) (Tubino et al. Citation2004). Magnesium in urine and serum was measured by adding calmagnite, chelating agents and potassium ferrocyanide, and estimated by spectrophotometry (630 nm wave length) (Addison Citation2012).
Histopathological examination
Kidneys were dissected and checked for gross morphological abnormalities, then standard procedure for histopathological examination was undertaken. Kidney tissues were fixed in a buffered formalin solution and tissue dehydration was done with ascending grades of ethanol, followed by tissue clearing with xylene. The tissues were then transferred to molten paraffin for impregnation and embedded in paraffin blocks. After fine sectioning with microtome, staining was done with haematoxylin and eosin (H&E) stain and was examined under light microscope (Chakraborty & Chakraborty Citation2003).
Statistical analysis
All analyses were done using Microsoft Excel (Redmond, WA) and SPSS (Armonk, NY) (version 17). All values were expressed as mean ± SEM. Analysis of variance was done following Tukey’s multiple comparison test. p < 0.05 was considered as significant.
Results
In this study, administration of ethylene glycol + ammonium chloride aqueous solution to male Sprague Dawley rats resulted in hyperoxaluria and renal stones. Oxalate and calcium excretion were grossly increased in these animals as evident by demonstration of oxalate crystals in urine. Increased urinary calcium is a factor favouring the nucleation and precipitation of calcium oxalate and formation of calcium phosphate stones. The anti-urolithiatic effect of diosmin (LD50: 10 g/kg p.o. and ED50: 100 mg/kg as obtained from the literature) (Meyer Citation1994) has been described by evaluating different urinary, serum and other parameters as well as histopathological examination of the kidneys. Respective results are enumerated in .
Table 1. Effects of diosmin on kidney weight, urine volume, urinary pH, urinary protein in ethylene glycol + ammonium chloride induced urolithiasis.
Table 2. Effects of diosmin on various urinary parameters in ethylene glycol + ammonium chloride induced urolithiasis.
Table 3. Effects of diosmin on various serum parameters in ethylene glycol + ammonium chloride induced urolithiasis.
Kidney weight
The kidney weights of animals treated with ethylene glycol + ammonium chloride were significantly increased (1.138 ± 0.04) than in cystone® (0.735 ± 0.007), diosmin 10 mg/kg (0.824 ± 0.008), diosmin 20 mg/kg (0.746 ± 0.006) treated animals.
Urinary pH
The urine pH of animals treated with ethylene glycol + ammonium chloride controls groups was significantly increased (9.56 ± 0.16) than the rats treated with cystone® (5.3 ± 0.07), diosmin 10 mg/kg (6.35 ± 0.07), diosmin 20 mg/kg (5.38 ± 0.06).
Urine volume
The urine volume of animals treated with ethylene glycol + ammonium chloride control groups were significantly decreased (2.48 ± 0.10) than the animals treated with cystone® (7.36 ± 0.05), diosmin 10 mg/kg (6.57 ± 0.07), diosmin 20 mg/kg (7.49 ± 0.06).
Total urinary protein level
The urine total protein levels of animals treated with ethylene glycol + ammonium chloride controls groups were significantly increased (8.79 ± 0.14) than the animals treated with cystone® (3.62 ± 0.06), diosmin 10 mg/kg (5.70 ± 0.08), diosmin 20 mg/kg (4.27 ± 0.116).
Urine calcium level
The urine calcium levels of animals treated with ethylene glycol + ammonium chloride controls groups were significantly increased (8.01 ± 0.09) than the animals treated with cystone® (5.59 ± 0.04), diosmin 10 mg/kg (6.03 ± 0.06), diosmin 20 mg/kg (5.61 ± 0.08).
Urine magnesium level
The urine magnesium levels of animals treated with ethylene glycol + ammonium chloride were significantly decreased (2.151 ± 0.07) than the animals treated with cystone® (4.12 ± 0.05), diosmin 10 mg/kg (3.66 ± 0.11), diosmin 20 mg/kg (4.13 ± 0.04) treated animals.
Urine phosphorus level
The urine phosphorus levels of animals treated with ethylene glycol + ammonium chloride were significantly increased (7.82 ± 0.03) than the animals treated with cystone® (4.45 ± 0.05), diosmin 10 mg/kg (5.21 ± 0.08), diosmin 20 mg/kg (4.52 ± 0.039).
Urine potassium level
The urine potassium levels of animals treated with ethylene glycol + ammonium chloride were significantly decreased (30.41 ± 0.65), whereas those in animals treated with cystone® (42.l 8 ± 0.52), diosmin 10 mg/kg (38.88 ± 0.30), diosmin 20 mg/kg (41.82 ± 0.48) were increased.
Urine sodium level
The urine sodium levels of animals treated with ethylene glycol + ammonium chloride were significantly decreased (89.945 ± 0.350), whereas those in animals treated with cystone® (119.76 ± 0.56), diosmin 10 mg/kg (105.12 ± 0.78), diosmin 20 mg/kg (117.83 ± 0.67) were increased.
Urine creatinine level
The urine creatinine levels of positive control animals were significantly decreased (0.97 ± 0.006) than the animals treated with cystone® 750 mg/kg (2.65 ± 0.04), diosmin 10 mg/kg (2.07 ± 0.042), diosmin 20 mg/kg (2.52 ± 0.038).
Urine uric acid level
The uric acid levels of animals treated with ethylene glycol + ammonium chloride were significantly decreased, whereas those in cystone® 750 mg/kg (4.50 ± 0.018), diosmin 10 mg/kg (3.97 ± 0.04), diosmin 20 mg/kg (4.57 ± 0.028) treated animals were significantly increased.
Serum calcium
The serum calcium levels of animals treated with ethylene glycol + ammonium chloride control groups were significantly decreased (1.77 ± 0.01) than the animals treated with cystone® (4.88 ± 0.07), diosmin 10 mg/kg (3.97 ± 0.05), diosmin 20 mg/kg (4.76 ± 0.06).
Serum potassium
The serum potassium levels of animals treated with ethylene glycol + ammonium chloride control groups were significantly increased (11.1 ± 0.01) than the animals treated with cystone® (6.42 ± 0.03), diosmin 10 mg/kg (7.55 ± 0.04), diosmin 20 mg/kg (6.69 ± 0.09).
Serum sodium
The serum sodium levels of animals treated with ethylene glycol + ammonium chloride control groups were significantly increased (153.19 ± 0.60), whereas those in animals treated with cystone® (123.50 ± 0.27), diosmin 10 mg/kg (131.88 ± 0.80), diosmin 20 mg/kg (124.66 ± 0.35) were significantly decreased.
Serum magnesium
The serum magnesium levels of animals treated with ethylene glycol + ammonium chloride control groups were significantly increased (1.77 ± 0.048), whereas those in animals treated with cystone® (0.75 ± 0.055), diosmin 10 mg/kg (0.66 ± 0.026), diosmin 20 mg/kg (1.074 ± 0.03) were significantly decreased.
Serum creatinine
The serum creatinine levels of animals treated with ethylene glycol + ammonium chloride control groups were significantly increased (1.61 ± 0.03), whereas those in animals treated with cystone® (0.52 ± 0.01), diosmin 10 mg/kg (0.45 ± 0.02), diosmin 20 mg/kg (0.81 ± 0.03) were significantly decreased.
Serum uric acid level
The serum uric acid levels of animals treated with ethylene glycol + ammonium chloride control groups were significantly increased (25.81 ± 0.30), whereas those in animals treated with cystone® (19.57 ± 0.29), diosmin 10 mg/kg (22.20 ± 0.26), diosmin 20 mg/kg (20.09 ± 0.23) were significantly decreased.
Blood urea nitrogen
The blood urea nitrogen (BUN) levels of animals of positive control groups were significantly increased (2.24 ± 0.06) than in cystone® (1.42 ± 0.04), diosmin 10 mg/kg (1.79 ± 0.07), diosmin 20 mg/kg (1.57 ± 0.05) treated groups.
Histopathological findings
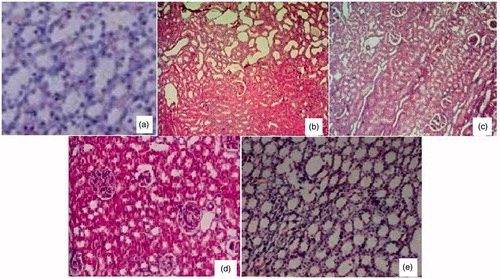
Kidneys of normal control animals showed normal organisation of tubular epithelial cells and glomeruli. There were no histopathological changes in renal tubules, glomeruli and blood vessels (). In the urolithiatic control group, histopathological study had shown moderate crystal deposition, tubular cystic dilation, cloudy swelling of proximal convoluted tubules and mild multifocal infiltration of lymphocytes in the interstitium with dilated collecting tubules (). In the standard cystone®-treated group, the kidneys showed mild congestion of the glomeruli. There were no changes in blood vessels and tubules (). Diosmin (10 mg/kg)-treated rats showed significant differences when compared to urolithiatic control rats. With mild cystic dilation of tubules, congestion of interstitum and mild inflammation were observed (). Diosmin (20 mg/kg)-treated animals showed only mild congestion of the glomeruli without any changes in blood vessels and tubules ().
Figure 1. Histopathological examination findings of rats of different groups (H&E × 10). Normal organisation of tubular epithelial cells and glomeruli in kidneys of untreated control animals showed (a). Moderate crystal depositions, tubular cystic dilation, cloudy swelling of proximal convoluted tubules and mild multifocal infiltration of lymphocytes in the interstitium with dilated collecting tubules in the urolithiatic control group (b). Mild congestion in glomeruli without any changes in blood vessel or tubules was seen in cystone® treated group (c). Mild cystic dilation of tubules, congestion of interstitum and mild inflammation were observed in rats treated with diosmin 10 mg/kg (d). And diosmin 20 mg/kg treated animals showed only mild congestion of the glomeruli without any changes in blood vessels and tubules (e).
Discussion
Diosmin is a type of citrus bio-flavonoid, mostly found in citrus fruits often used as a dietary supplement. It was historically considered to be a vascular-protecting agent used to treat chronic venous diseases. As a flavonoid, diosmin exhibit anti-inflammatory and free-radical scavenging activity too. Diosmin is safe for most people when used in short-term, for up to 3 months. It can cause some non-specific and mild side effects such as stomach, and abdominal pain, diarrhea and headache. Safety data in pregnancy and lactation are not available conclusively, so it is better to avoid in pregnant and lactating women (Craig Citation1999). The anti-urolithiatic activity of diosmin (10 and 20 mg/kg) was assessed in this study by evaluating different physical, biochemical and histological parameters in ethylene glycol- and ammonium chloride-induced urolithiasis in male Sprague-Dawley rats.
The increased weight of kidneys of the animals treated only with ethylene glycol + ammonium chloride was due to calculogenesis and urinary super saturation with respect to stone-forming constituents (Kore et al. Citation2011). Diosmin (20 mg/kg) reduced stone formation as reflected by the kidney weights. From the previous studies (Kore et al. Citation2011), it was evident that ethylene glycol + ammonium chloride-induced urolithiasis increases urinary pH. Generally, urine has acidic pH, which is useful to prevent crystallisation of stone-forming promoters by dissolving them into urine. But in case of chronic hyperoxaluric rats in diseased control group, urinary pH was found to be alkaline. Alkaline pH promotes the crystallisation in urine and thus stone formation. Since diosmin reduced the urine pH significantly compared to the positive control group, this suggests that the diosmin possesses very good anti-urolithiatic activity and diosmin 20 mg/kg shows more activity than diosmin 10 mg/kg. It is also evident from the previous studies (Ramesh & John Citation2010) that ethylene glycol + ammonium chloride-induced urolithiasis increased urine volume. Stones with smaller size can easily travel through the urinary system followed by excretion but large size stones may lead to obstruction in urine passage, so urine output decreases in urolithiasis.
Since diosmin increased urine output volume significantly compared to the positive control group, this suggests that the diosmin possesses very good anti-urolithiatic activity and diosmin 20 mg/kg shows more activity than diosmin 10 mg/kg. From other studies (Ramesh & John Citation2010) it was evident that ethylene glycol + ammonium chloride-induced urolithiasis increases total protein content in urine. Due to hemodynamic stress, capillary integrity gets damaged, which predisposes to leakage of proteins into the urine cause and increased urine protein levels. Diosmin decreased the urine output volume significantly compared to the ethylene glycol + ammonium chloride-treated group, this suggests that the diosmin 20 mg/kg possesses very good anti-urolithiatic activity. Increased urine calcium levels were observed in animals developing urolithiais (Ramesh & John Citation2010). An increased urinary calcium concentration is a factor favouring nucleation and precipitation of calcium oxalate from urine and subsequent crystal growth. This fact, combined with the increased urinary calcium leads to their super saturation in urine and finally stone formation. Since diosmin decreased the urine calcium levels significantly, this suggests that diosmin possesses very good anti-urolithiatic activity.
From some earlier studies it was evident that decreased urine magnesium levels were observed in animals developing urolithiais (Ramesh & John Citation2010). Magnesium is a well-known inhibitor of crystallisation – complexes with oxalate, thus reducing calcium oxalate super saturation in urine, as a consequence decreases the growth and nucleation rates of calcium crystals. Diosmin 20 mg/kg increased the urine magnesium levels significantly. Previous studies have shown that in urolithiasis, there is increased urine phosphorus level (Ramesh & John Citation2010). Increased urinary phosphorus excretion provides an environment appropriate for stone formation by forming calcium phosphate crystals, which induces calcium oxalate deposition. Since diosmin decreased the urine phosphorus levels significantly, it can be said that diosmin possesses very good anti-urolithiatic activity. Urolithiasis decreases the urine potassium level (Ramesh & John Citation2010). Potassium levels reduce due to the deposition of the crystals in the distal tubule of the kidney. Since diosmin increased urine potassium levels significantly, it can be said that diosmin possesses very good anti-urolithiatic activity and diosmin 20 mg/kg shows more activity.
From the previous studies (Preethi et al. Citation2010) it was evident that ethylene glycol + ammonium chloride-induced urolithiasis decreased urine sodium levels. Sodium levels are reduced due to the deposition of the crystals in the distal tubule of the kidney. Diosmin increased the urine sodium levels significantly compared to the positive control group, and this suggests diosmin possesses very good anti-urolithiatic activity. Previous study (Preethi et al. Citation2010) also demonstrated that in urolithiasis urine creatinine levels decrease as the glomerular filtration rate decreasse due to obstruction of urine flow by the stones in the urinary system. This causes impairment of renal function resulting decreased excretion of waste products, such as creatinine. Since diosmin increased the urine creatinine levels significantly compared to the ethylene glycol + ammonium chloride-treated group, it can be said that diosmin possesses very good anti-urolithiatic activity and diosmin 20 mg/kg showed more activity than diosmin 10 mg/kg.
From another study (Preethi et al. Citation2010) it was evident that in urolithiasis, decreased uric acid levels were observed. In urolithiasis, the glomerular filtration rate decreases due to obstruction of urine flow by the stones in the urinary system. This causes impairment of renal function resulting in decreased excretion of waste products, such as uric acid. Since diosmin increased the urine uric acid levels significantly compared to the ethylene glycol + ammonium chloride-treated group, this suggests that diosmin possesses very good anti-urolithiatic activity and diosmin 20 mg/kg showed more activity than diosmin 10 mg/kg. Previous studies (Kore et al. Citation2011) showed that urolithiasis decreases serum calcium level. Since diosmin increased serum calcium levels significantly compared to the positive control group, this suggests that diosmin possesses very good anti urolithiatic activity.
Also from the previous studies (Preethi et al. Citation2010), it was evident that urolithiasis increases serum potassium level. Since diosmin decreased serum potassium levels significantly compared to the positive control group, this suggests that diosmin possesses very good anti urolithiatic activity. Urolithiasis increases serum sodium level (Preethi et al. Citation2010). Diosmin decreased the serum sodium levels significantly compared to the positive control group, suggesting that it has a very good anti-urolithiatic activity. From the previous studies it was evident that urolithiasis increases serum magnesium level (Preethi et al. Citation2010). Diosmin decreased the serum magnesium levels significantly compared to the positive control group, suggesting that it has a very good anti-urolithiatic activity. It was earlier evident that ethylene glycol + ammonium chloride induces urolithiasis increased serum creatinine level (Preethi et al. Citation2010). Since diosmin decreased the serum creatinine levels significantly compared to the urolithiatic group, this suggests that diosmin possesses very good anti-urolithiatic activity and diosmin 20 mg/kg shows more activity than diosmin 10 mg/kg. It was also evident that ethylene glycol + ammonium chloride-induced urolithiasis increases serum uric acid level (Preethi et al. Citation2010). Since diosmin decreased the serum uric acid levels significantly compared to the urolithiatic group, this suggests that diosmin possesses very good anti-urolithiatic activity and diosmin 20 mg/kg shows more activity than diosmin 10 mg/kg.
From the previous studies (Ramesh & John Citation2010), it is evident that urolithiasis increases serum BUN. Diosmin 20 mg/kg decreased the serum BUN significantly compared to the ethylene glycol + ammonium chloride-treated group, reflecting its anti-urolithiatic activity. Histopathologically, diosmin (10 and 20 mg/kg)-treated animals also showed significant anti-urolithiatic activity than untreated controls.
So, it can be said that administration of diosmin, in a dose of 10 and 20 mg/kg to urolithiatic rats significantly recovered the elevated levels of serum parameters like creatinine, uric acid, magnesium, BUN, sodium, potassium, physiological parameters like kidney weights, urine pH and urinary parameters like urine calcium, phosphorus to normal level. It also recovered the decreased levels of urine parameters like creatinine, uric acid, magnesium, sodium, potassium, urine volume and serum parameters like calcium compared to ethylene glycol and ammonium chloride-treated group. Histopathological studies of the kidney samples confirmed this anti-urolithiatic activity too, and this was nearly equally efficacious with the standard drug cystone®.
In one previous study, it was shown that diosmin reduced calcium oxalate deposition and tissue degeneration in nephrolithiasis in rats (Noorafashan et al. Citation2013). In another study, it was shown that diosmin can inhibit calcium oxalate crystallisation (Saha & Verma Citation2015). Studies have also shown that flavonoids obtained from various plant sources are effective in urolithiasis (Soundarajan et al. Citation2006; Butterweck & Khan Citation2009; Brancalion et al. Citation2012). It may be pertinent here to mention that the observed anti-urolithiatic activity of diosmin might be due to its antioxidant, anti-inflammatory effects and protective effects of microcirculation. Exact mechanism underlying these effects is not clear, and hence further research is suggested to explore the exact mechanism behind such action of the drug.
Conclusion
It can be concluded from the above study that diosmin (10 and 20 mg/kg) has a very good anti-urolithiatic activity similar to the standard drug cystone® in chemical-induced urolithiasis in rats. More studies are warranted to explore the mechanism of action of the drug in this regard.
Declaration of interest
There are neither any conflicts of interests nor any financial interests in this study from the part of the contributing authors. No funding was received for this work.
References
- Adepu S, Kothandam H, Maguluru M, Idupuganti S. 2014. Evaluation of antiurolithiatic activity of aerial parts of Hibiscus vitifolius Linn. Int J Pharm Res Novel Sci. 1:60–67.
- Addison C. 2012. Magnesium (serum, plasma) [Internet]. © Association for Clinical Biochemistry; [cited 2014 Dec 15]. Available from: http://www.acb.org.uk/Nat%20Lab%20Med%20Hbk/Magnesium.pdf.
- Barberán FAT, Gil MI, Tomás F, Ferreres F, Arques A. 1985. Flavonoid aglycones and glycosides from Teucrium gnaphalodes. J Nat Prod. 48:859–860.
- Brancalion AP, Oliveira RB, Sousa JP, Groppo M, Berretta AA, Barros ME, Boim MA, Bastos JK. 2012. Effect of hydroalcoholic extract from Copaifera langsdorffii leaves on urolithiasis induced in rats. Urol Res. 40:475–481.
- Bushinsky DA, Asplin JR, Grynpas MD, Evan AP, Parker WR, Alexander KM, Coe FL. 2002. Calcium oxalate stone formation in genetic hypercalciuric stone-forming rats. Kidney Int. 61:975–987.
- Butterweck V, Khan SR. 2009. Herbal medicines in the management of urolithiasis: alternative or complementary? Planta Med. 75:1095–1103.
- Chakraborty P, Chakraborty G. 2003. Practical pathology. Kolkata: New Central Book Agency (P) Ltd.
- Craig WJ. 1999. Health promoting properties of common herbs. Am J Clin Nutr. 70:491–499.
- Evan AP. 2010. Physiopathology and etiology of stone formation in the kidney and the urinary tract. Pediatr Nephrol. 25:831–841.
- Jaffe M. 1886. Concerning the precipitate produced in normal urine by picric acid and a new reaction of creatinine. Phys Chem. 10:391–400.
- Karamakar D, Patki P. 2008. Evaluation of efficacy and safety of a herbal formulation cystone® in the management of urolithiasis: meta-analysis of 50 clinical studies. Internet J Alternat Med. 8.
- Khan NI, Hinge JS, Naikwade NS. 2010. Antilithiatic effect of Helianthus annuus Linn. leaf extract in ethylene glycol and ammonium chloride induced nephrolithiasis. Int J Pharm Pharm Sci. 2:180–184.
- Kishore DV, Moosavi F, Varma RK. 2013. Effect of ethanolic extract of Portulaca oleracea Linn. on ethylene glycol and ammonium chloride induced urolithiasis. Int J Pharm Pharm Sci. 5:134–140.
- Kore KJ, Shete RV, Jadhav PJ, Kabra MP. 2011. Antiurolithiatic effects of hydroalcoholic extract of Lawsonia inermis leaves. Int J Univ Pharm Life Sci. 1:81–92.
- Kumar R, Mukherjee M, Bhandari M, Kumar A, Sidhu H, Mittal RD. 2002. Role of Oxalobacter formigenes in calcium oxalate stone disease: a study from North India. Eur Urol. 41:318–322.
- Kumar P. 1979. Experiences with cystone in the management of urolithiasis. Probe XVIII:89–93.
- Marshall T, Williams KM. 2003. Aminoglycoside interference in the pyrogallol red-molybdate protein assay is increased by the addition of sodium dodecyl sulfate to the dye reagent. Clin Chem. 49:2111–2112.
- Meyer OC. (1994). Safety and security of Daflon 500 mg in venous insufficiency and in hemorrhoidal disease. Angiology 45:579–584.
- Noorafashan A, Doust SK, Karimi F. 2013. Diosmin reduces calcium oxalate deposition and tissue degeneration in nephrolithiasis in rats: a stereological study. Korean J Urol. 54:252–257.
- Parentoni LS, Pozeti RCS, Figueiredo JF, de Faria EC. 2001. The determination of total calcium in urine: a comparison between the atomic absorption and the ortho-cresolphtalein complexone methods. J Brasileiro Patol Med Lab. 37:235–238.
- Preethi G, Ahsan S, Gokul P, Teerthanath S. 2010. Evaluation of renoprotective effects of ethanolic extract of Morinda citrifolia L. in a murine model of gentamicin-induced nephrotoxicity. Int J Pharmacol Pharm Technol. 1:23–28.
- Rai SC. 1960. Clinical trials with cystone-in urolithiasis. Curr Med Pract. 9:484.
- Ramesh C, John WE. 2010. Antiurolithiatic activity of wood bark extracts of Cassia fistula in rats. JPBMS 2:1–9.
- Saha S, Verma RJ. 2015. Evaluation of hydro-alcoholic extract of Dolichos biflorus seeds on inhibition of calcium oxalate crystallization. J Herbal Med. 5:41–47.
- Sailaja B, Bharathi K, Prasad K. 2011. Protective effect of Tridax procunibens L. on calcium oxalate urolithiasis and oxidative stress. Pharmanest 2:9–13.
- Soundarajan P, Mahesh R, Ramesh T, Begum VH. 2006. Effect of Aerva lanata on calcium oxalate urolithiasis in rats. Indian J Exp Biol. 44:981–986.
- Srinivasan S, Pari L. 2012. Ameliorative effect of diosmin, a citrus flavonoid against streptozotocin-nicotinamide generated oxidative stress induced diabetic rats. Chem Biol Interact. 195:43–51.
- Stone GCH, Goldzieher JW. 1949. A rapid colorimetric method for the determination of sodium in biological fluids and particularly is serum. J Biol Chem. 181:511–521.
- Tamamoto LC, Schmidt SJ, Lee SY. 2010. Sensory profile of a model energy drink with varying levels of functional ingredients – caffeine, ginseng, and taurine. J Food Sci. 75:S271–S278.
- Tortora GJ, Derrickson B. 2009. Principles of anatomy and physiology. John Wiley & Sons, Inc.
- Trinchieri A. 2008. Epidemiology of urolithiasis: an update. Clin Cases Miner Bone Metab. 5:101–106.
- Tubino M, de Souza RL, Hoehr NF. 2004. Rapid quantitative turbidimetric spot test analysis of potassium in blood serum. J Braz Chem Soc. 15:635–639.
- Vijaya T, Kumar MS, Ramarao NV, Babu AN, Ramarao N. 2013. Urolithi-asis and its causes – short review. J Phytopharmacol. 2:1–6.
- Yagisawa T, Hayashi T, Yoshida A. 1999. Metabolic characteristics of the elderly with recurrent calcium oxalate stones. BJU Int. 9:924–928.
- Yoshioka I, Sujihata M, Momohara C, Akanae W, Nonomura N, Okuyama A. 2010. Effect of sex hormones on crystal formation in a stone-forming rat model. Urology 75:907–913.
