Abstract
Context Ixora megalophylla Chamch. (Rubiaceae) is a new plant species recently found in southern Thailand. Ethyl acetate extracts of its leaves and stems showed antimicrobial activities.
Objectives To isolate and identify the antimicrobial compounds from I. megalophylla leaves and stems.
Materials and methods The dried leaves (1.7 kg) and stems (3.5 kg) were consecutively extracted with petroleum ether (5 L × 4), ethyl acetate (5 L × 3) and ethanol (5 L × 4) under reflux conditions. The ethyl acetate extract was subjected to an antimicrobial assay guided isolation with Candida albicans and Streptococcus mutans. Compounds 1–10 were identified by 1H NMR, 13C NMR and EI-MS. Minimal lethal concentration (MLC) against C. albicans and Streptococcus spp. was determined using a broth microdilution method for 48 and 24 h, respectively.
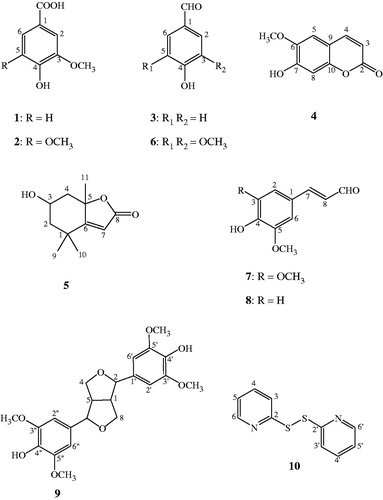
Results and discussion On the basis of the antimicrobial assay guided isolation, 10 known compounds, including vanillic acid (1), syringic acid (2), 4-hydroxy benzaldehyde (3), scopoletin (4), loliolide (5), syringaldehyde (6), sinapaldehyde (7), coniferaldehyde (8), syringaresinol (9) and 2,2′-dithiodipyridine (10), were identified. Compounds 1–5 were purified from the ethyl acetate extract of the leaves, while 6–9 and 10 were from the ethyl acetate and ethanol extracts of the stems, respectively. Among these isolates, 10 showed the strongest antibacterial activities against S. mutans and Streptococcus mitis, with minimum inhibitory concentrations (MICs) of 2–4 μg/mL, and MLC of 4 μg/mL, as well as having a weak antifungal activity against C. albicans (MIC of 125 μg/mL). This is the first report of the antimicrobial activities of 10.
Introduction
Oral diseases are a major public health problem worldwide (Petersen et al. Citation2005). They have large effects on individuals and communities as a result of the associated pain and suffering. The association between oral diseases and oral pathogens is well demonstrated. Various systemic and topical agents are commercially available for the treatment of oral infectious diseases. However, the use of these products has some limitations because they may be inactivated by food and saliva, or cause taste disturbances and mucosal irritation (Ciancio Citation1995), e.g. fluconazole has a high rate of developing drug resistance and drug interactions (Ghannoum & Rice Citation1999). Thus, a search for alternative natural occurring antifungal and antibacterial compounds for oral pathogens has been a major concern in recent years. Some medicinal plants have been extensively studied in order to find more effective and/or less toxic compounds. One such example is lawsone methyl ether, a potent antifungal agent from Impatiens balsamina L. (Balsaminaceae) leaves (Sakunphueak & Panichayupakaranant Citation2010), and has been used in a mouthwash for prophylaxis of oral candidiasis in HIV-infected patients and denture wearers, showed the same efficacy to a chlorhexidine mouthwash and no antifungal drug resistance was observed (Nittayananta et al. Citation2013).
There are 27 species of Ixora found in Thailand (Smitinand Citation2001), but only a few of them have been studied. Recently, there was a report of antibacterial activity in an ethanol extract from Ixora coccinea L. (Rubiaceae) roots against Staphylococcus aureus, Bacillus pumilius, Enterococcus faecalis, Escherichia coli, Salmonella typhi and Pseudomonas aeruginosa (Selvaraj et al. Citation2011). Our preliminary study found that a crude ethanol extract from the leaves and stems of Ixora megalophylla exhibited good antibacterial and antifungal activities against some oral pathogens. In addition, I. megalophylla is a new species of the genus Ixora (Chamchumroon Citation2014). Thus, there have been no previous reports on phytochemical studies of this plant or its traditional medical use. We were therefore interested in isolating antimicrobial compounds against some oral pathogens, i.e., S. mutans, S. mitis and C. albicans from the leaves and stems of I. megalophylla using the bioassay-guided isolation.
Materials and methods
Plant materials
Fresh leaves and stems of I. megalophylla were collected from Rajjaprabha Dam, Surat Thani Province, Thailand, in December 2012. The plant was identified by Voradol Chamchumroon and deposited at the Herbarium of the Southern Center of Traditional Medicine, Faculty of Pharmaceutical Sciences, Prince of Songkla University, Thailand, where the voucher specimens (Herbarium no. SKP 165 09 13 01) are kept. The plant materials were dried at 50–60 °C for 24 h in a hot-air oven, and then reduced to powders using a grinder. The powders were then passed through a sieve No. 45.
Preparation of plant extracts
Dried leaf powder (1.7 kg) was extracted with petroleum ether (5 L × 4), ethyl acetate (5 L × 3) and ethanol (5 L × 4), consecutively, under reflux conditions for 30 min, while a dried stem powder (3.5 kg) was extracted with petroleum ether (5 L × 2), ethyl acetate (5 L × 4) and ethanol (5 L × 5), consecutively, under similar conditions. The extracts obtained from each solvent were separately combined, and the solvents were evaporated under reduced pressure at 40 °C.
Microorganisms and media
Streptococcus mutans (DMST 26095), S. mitis (ATCC 49456T) and C. albicans (ATCC 90028) were obtained from the Department of Medical Sciences, Ministry of Public Health, Bangkok, Thailand. Brain–heart infusion (BHI), Sabouraud dextrose broth (SDB) and agar (SDA) were purchased from the Becton, Dickinson Company (Franklin Lakes, NJ).
Determination of the minimum inhibitory and minimal lethal concentration
Streptococcus mutans and S. mitis were incubated on BHI agar at 37 °C under microaerophilic conditions for 24 h, while C. albicans was incubated on SDA at 37 °C for 48 h. Inocula were prepared by mixing a few bacterial colonies with sterile ringer solution (0.85% NaCl) and the turbidity was compared with the standard 0.5 McFarland solution, which was claimed to be equivalent to 108 CFU/mL. The minimum inhibitory concentration (MIC) and minimal lethal concentration (MLC) were determined by the broth microdilution assay (CLSI Citation2008; NCCLS Citation2008; Kamble Citation2012). The sample solutions were prepared in 10% DMSO to the concentrations of 10 mg/mL for the extracts, and 800 or 1000 μg/mL for the pure compounds (stock solution), and serially diluted with BHI broth (or SDB in case of C. albicans) to the concentrations between 2.4 and 5000 μg/mL for the extract, and 0.1 and 400 or 500 μg/mL for the pure compounds. DMSO was used as a negative control, while standard ampicillin and clotrimazole were used as positive controls for the testing of antibacterial and antifungal activities, respectively. The MIC was defined as the lowest concentration of the compound to inhibit the growth of the microorganism, and the MLC was defined as the lowest concentration of the compound required to kill microorganisms.
Isolation of antimicrobial compounds from the leaf extracts
The ethyl acetate extract from the leaves (18 g) was fractionated on a silica gel column (10 cm × 100 cm) (1 g extract per 15 g silica gel) and eluted with a mixture of hexane and chloroform (1:1 v/v) followed by mixtures of chloroform and methanol (100:0, 50:1, 40:1, 30:1, 20:1, 15:1, 10:1, 7:1, 5:1, 3:1, 2:1 and 0:100 v/v) using a step-gradient elution. Based on the TLC chromatograms of each fraction (2 L), 13 pooled fractions (fractions 1–13) were obtained. The fractions were then subjected to an antimicrobial assay. The antimicrobial active fraction (fraction 5; 2.7 g) was further fractionated using an ODS column (3.5 cm × 36 cm). Mixtures of methanol and water (30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, 95 and 100% v/v methanol) were used for column elution, using a step-gradient elution to give 15 pooled fractions (fractions A–O). The fractions were then subjected to an antimicrobial assay. The antimicrobial fraction (fraction B) was further purified by preparative HPLC (ODS column 250 mm × 6 mm; 14% acetone containing 0.1% TFA, flow rate 1.6 mL/min, RI detector), to yield compound 1 (3.6 mg, tR 39.7 min), 2 (2.8 mg, tR 41.8 min), 3 (4.4 mg, tR 62.4 min), 4 (8.5 mg, tR 70.3 min) and 5 (5.7 mg, tR 82.5 min).
Isolation of antimicrobial compounds from the stem extracts
The ethyl acetate extract from the stems (45 g) was chromatographed on a silica gel column (10 cm × 100 cm) (1 g extract per 15 g silica gel) and fractionated (3 L for each fraction) using a mixture of hexane and chloroform (1:1 v/v) followed by mixtures of chloroform and methanol (100:0, 50:1, 40:1, 30:1, 20:1, 15:1, 10:1, 7:1, 5:1, 3:1, 2:1 and 0:100 v/v). Based on the TLC chromatograms of each fraction, 14 pooled fractions (fractions 1–14) were obtained. The fractions were then subjected to an antimicrobial assay. The antimicrobial fraction (fraction 6; 13 g) was further fractionated by an ODS column (3.5 cm × 36 cm) using a step gradient elution of mixtures of methanol and water (30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, 95 and 100% v/v methanol) to afford 15 pooled fractions (fractions A–O). The fractions were then subjected to an antimicrobial assay. Fractions B and C exhibited an antimicrobial activity. Fraction B was purified with a preparative HPLC (ODS column 250 mm × 10 mm; 21% acetone, flow rate 2.0 mL/min, RI detector), to yield compound 6 (5.7 mg, tR 19.6 min), 4 (6.7 mg, tR 25.3 min), 7 (6.1 mg, tR 33.8 min) and 8 (5.9 mg, tR 52.2 min), while fraction C was purified by a preparative HPLC (ODS column 250 mm × 6 mm; 23% acetone containing 0.1% TFA, flow rate 1.6 mL/min, RI detector), to afford compound 9 (4.9 mg, tR 24.2 min).
The ethanol extract from the stems (120 g) was subjected to partition between ethyl acetate (1.6 L) and water (2 L). The fractions were then subjected to an antimicrobial assay. The ethyl acetate fraction (27 g) was further fractionated on a Diaion HP-20 column (7.5 cm × 60 cm) (1 g extract per 20 g Diaion). Mixtures of methanol and water (50 and 100% v/v methanol) and mixtures of ethyl acetate and methanol (25, 50 and 100% v/v ethyl acetate) were used for column elution to give five fractions (1.1–1.5). The fractions were then subjected to an antimicrobial assay. Antimicrobial fraction 1.1 (19 g) was further purified on a silica gel column (7.5 cm × 60 cm) (1 g extract per 35 g silica gel). Mixtures of dichloromethane and ethyl acetate (100, 90, 80, …, 0% v/v dichloromethane) and mixtures of chloroform and methanol (100, 90, 80, 70, 60 and 50% v/v chloroform) were used for column elution to give 11 pooled fractions (fractions A–K). The fractions were then subjected to an antimicrobial assay. Antimicrobial fraction J (3 g) was further purified by a silica gel column (4 cm × 50 cm) (1 g extract per 35 g silica gel) eluted with mixtures of chloroform and methanol (90, 80, 70, … , 0% v/v chloroform) using a step gradient elution to afford eight pooled fractions (fractions J1–J8). The fractions were then subjected to an antibacterial assay. Antimicrobial active fraction J2 (170 mg) was further purified on a Sephadex LH-20 column (Amersham Pharmacia Biotech AB, Uppsala, Sweden) (3 cm × 80 cm) using methanol as an eluent to give five pooled fractions (fractions I–V). Antimicrobial fraction III (15 mg) was rechromatographed with the same column to produce three pooled fractions (fractions 3.1–3.3). A pale yellow crystal (compound 10, 3.7 mg) was obtained from the pooled antimicrobial fraction 3.2 after being purified using a preparative TLC (TLC silica gel 60 F254, Merck & Co., Inc., Germany; 90% chloroform in methanol, Rf 0.5).
Identification of compounds 1–10
Compounds 1–10 were structurally identified based on their spectral analyses including 1H NMR, 13C NMR and EI-MS by comparison with data in the literature (Yoshioka et al. Citation2004; Hu et al. Citation2007; Liu et al. Citation2008; Stanikunaite et al. Citation2009; He et al. Citation2010; Kusterer et al. Citation2010; Monthong et al. Citation2011; Zhu et al. Citation2012).
Vanillic acid (1): white solid; C8H8O4; 1H NMR (CD3OD, 600 MHz) δH: 3.79 (3H, s, –OCH3), 6.74 (1H, d, J=8.6 Hz, H-5), 7.45 (1H, s, H-2), 7.46 (1H, d, J=8.6 Hz, H-6); 13C NMR (CD3OD, 125 MHz) δC: 56.4 (–OCH3), 113.9 (C-2), 115.9 (C-5), 125.3 (C-6), 123.1 (C-1), 148.7 (C-3), 152.7 (C-4), 170.0 (–COOH); EI-MS m/z (rel. int. %): 168 (100), 153 (56), 114 (15), 97 (18), 72 (87).
Syringic acid (2): yellow amorphous powder; C9H10O5; 1H NMR (CD3OD, 600 MHz) δH: 3.78 (6H, s, –OCH3), 7.23 (2H, s, H-2, H-6); 13C NMR (CD3OD, 125 MHz) δC: 55.3 (3-OCH3, 5-OCH3), 106.9 (C-2, C-6), 147.4 (C-3, C-5), 120.5 (C-1), 140.3 (C-4), 168.5 (–COOH); EI-MS m/z (rel. int. %): 198 (78), 183 (28), 149 (31), 72 (100).
4-Hydroxy benzaldehyde (3): colourless solid; C7H6O2; 1H NMR (CD3OD, 600 MHz) δH: 6.75 (2H, d, J=8.5 Hz, H-3, H-5), 7.22 (2H, d, J=8.5 Hz, H-2, H-6), 9.75 (1H, s, H-7); 13C NMR (CD3OD, 125 MHz) δC: 115.8 (C-3, C-5), 130.5 (C-1), 129.0 (C-2, C-6), 158.8 (C-4), 192.8 (C-7); EI-MS m/z (rel. int. %): 122 (100), 93 (51), 65 (40).
Scopoletin (4): white needles; C10H8O4; 1H NMR (CD3OD, 600 MHz) δH: 3.81 (3H, s, –OCH3), 6.11 (1H, d, J=9.5 Hz, H-3), 7.76 (1H, d, J=9.5 Hz, H-4), 7.01 (1H, s, H-5), 6.67 (1H, s, H-8); 13C NMR (CD3OD, 125 MHz) δC: 56.9 (–OCH3), 164.1 (C-2), 112.6 (C-3), 146.1 (C-4), 110.0 (C-5), 147.1 (C-6), 152.9 (C-7), 104.0 (C-8), 112.59 (C-9), 151.5 (C-10); EI-MS m/z (rel. int. %): 192 (100), 177 (56), 164 (22), 149 (62), 121 (18), 67 (28).
Loliolide (5): colourless gum; C11H16O3; 1H NMR (CD3OD, 500 MHz) δH: 1.96 (1H, dt, J=14.5, 3 Hz, H-2), 1.52 (1H, dd, J=15.0, 3.0 Hz, H-2′), 4.13 (1H, m, H-3), 2.40 (1H, dt, J=13.6, 2.4 Hz, H-4), 1.73 (1H, dd, J=13.8, 4.0 Hz, H-4′), 5.65 (1H, s, H-7), 1.27 (3H, s, CH3), 1.44 (3H, s, CH3), 1.74 (3H, s, CH3); 13C NMR (CD3OD, 125 MHz) δC: 37.1 (C-1), 48.0 (C-2), 67.2 (C-3), 46.4 (C-4), 88.9 (C-5), 185.6 (C-6), 113.3 (C-7), 174.4 (C-8), 31.0 (C-9, CH3), 26.9 (C-10, CH3), 27.4 (C-11, CH3); EI-MS m/z (rel. int. %): 178 (38), 140 (33), 111 (100), 95 (21), 57 (12), 43 (75).
Syringaldehyde (6): yellow amorphous powder; C9H10O4; 1H NMR (CD3OD, 600 MHz) δH: 7.24 (2H, s, H-2, H-6), 3.92 (6H, s, CH3O-3, CH3O-5), 9.77 (1H, s, CHO); 13C NMR (CD3OD, 125 MHz) δC: 129.6 (C-1), 109.5 (C-2, C-6), 150.9 (C-3, C-5), 145.9 (C-4), 192.4 (–CHO), 57.9 (CH3O-3, CH3O-5); EI-MS m/z (rel. int. %): 182 (90), 167 (12), 126 (14), 111 (15), 81 (17), 72 (100).
Sinapaldehyde (7): yellow solid; C11H12O4; 1H NMR (CD3OD, 600 MHz) δH: 7.10 (2H, s, H-2, H-6), 7.52 (1H, d, J=15.7 Hz, H-7), 6.91 (1H, dd, J=7.7, 15.7 Hz, H-8), 9.84 (1H, d, J=7.7 Hz, H-9), 3.84 (6H, s, CH3O-3, CH3O-5); 13C NMR (CD3OD, 125 MHz) δC: 125.5 (C-1), 107.8 (C-2, C-6), 149.8 (C-3, C-5), 142.1 (C-4), 154.5 (C-7), 127.0 (C-8), 193.9 (CHO), 56.9 (CH3O-3, CH3O-5); EI-MS m/z (rel. int. %): 208 (100), 180 (28), 165 (33), 137 (25), 114 (14), 72 (83).
Coniferaldehyde (8): yellow oil; C10H10O3; 1H NMR (CD3OD, 600 MHz) δH: 7.07 (1H, d, J=1.9 Hz, H-2), 6.97 (1H, d, J=8.1 Hz, H-5), 7.02 (1H, dd, J=1.9, 8.1 Hz, H-6), 7.27 (1H, d, J=15.7 Hz, H-7), 6.62 (1H, dd, J=7.7, 15.7 Hz, H-8), 9.52 (1H, d, J=7.7 Hz, H-9), 3.54 (3H, s, -OCH3); 13C NMR (CD3OD, 125 MHz) δC: 124.9 (C-1), 110.3 (C-2), 147.7 (C-3), 150.6 (C-4), 115.5 (C-5), 123.1 (C-6), 152.3 (C-7), 124.8 (C-8), 192.1 (CHO), 56.5 (–OCH3); EI-MS m/z (rel. int. %): 178 (100), 161 (12), 147 (25), 135 (26), 107 (14), 77 (12).
Syringaresinol (9): yellow solid; C22H26O8; 1H NMR (CD3OD, 600 MHz) δH: 3.3 (2H, m, H-1, H-5), 4.99 (2H, d, J=3.8 Hz, H-2, H-6), 4.39 (2H, m, H-4, H-8), 4.07 (2H, dd, J=9.1, 6.8 Hz, H-4′, H-8′), 6.97 (4H, s, aromatic proton), 3.82 (12H, s, four CH3O–); 13C NMR (CD3OD, 125 MHz) δC: 55.4 (C-1, C-5), 87.1 (C-2, C-6), 72.5 (C-4, C-8), 132.7 (C-1′, C-1″), 105.2 (C-2′, C-2″), 149.8 (C-3′, C-3″), 137.7 (C-4′, C-4″), 149.8 (C-5′, C-5″), 105.2 (C-6′, C-6″), 56.9 (CH3O-3′, CH3O-3″, CH3O-5′, CH3O-5″); EI-MS m/z (rel. int. %): 418 (100), 181 (52), 167 (41), 69 (13).
2,2′-Dithiodipyridine (10): pale yellow crystals; C10H8N2S2; 1H NMR (CD3OD, 500 MHz) δH: 7.71 (2H, dd, J=1.71, 8.54 Hz, H-3, H-3′), 7.54 (2H, td, J=8.54, 1.22 Hz, H-4, H-4′), 7.41 (2H, td, J=6.34, 1.71 Hz, H-5, H-5′), 8.42 (2H, dd, J=6.34, 1.22 Hz, H-6, H-6′); 13C NMR (CD3OD, 125 MHz) δC: 150.3 (C-2, C-2′), 123.6 (C-3, C-3′), 130.5 (C-4, C-4′), 124.8 (C-5, C-5′), 140.4 (C-6, C-6′); HRMS: 220.0131, EI-MS: m/z (rel. int. %): 220 (100), 187 (24), 156 (74), 78 (38), 51 (17).
Results and discussion
Evaluations of the antimicrobial activities of the leaf and stem extracts from I. megalophylla revealed that the ethyl acetate extract from the leaves and the ethyl acetate and ethanol extracts from the stems possessed satisfactory antifungal activity against C. albicans with an MIC of 78 μg/mL, and antibacterial activity against S. mutans with MICs of 156, 78 and 312 μg/mL, respectively (). This result implied that the most active compounds had partial polarity, which dissolved better in ethyl acetate or ethanol. Petroleum ether may be less efficient in extracting the active compounds or was extracting different compounds with less activity. The ethyl acetate extract from the leaves and the ethyl acetate and ethanol extracts from the stems were therefore subjected to isolation and identification of antimicrobial compounds using anti-C. albicans and anti-S. mutans assay-guided isolation.
Table 1. Antimicrobial activities of the leaf and stem extracts from I. megalophylla.
Ten compounds, including vanillic acid (1), syringic acid (2), 4-hydroxybenzaldehyde (3), scopoletin (4), loliolide (5), syringaldehyde (6), sinapaldehyde (7), coniferaldehyde (8), syringaresinol (9) and 2,2′-dithiodipyridine (10), were identified (). Compounds 1–5 were purified from the ethyl acetate extract of the leaves, while 6–9 and 10 were from the ethyl acetate and ethanol extracts of the stems, respectively. Only 2, 5, 6, 7, 8 and 10 showed antimicrobial activities against the oral pathogenic microorganisms, including C. albicans, S. mitis and S. mutans (). Among these compounds, 10 exhibited the strongest anti-bacterial activities against S. mutans and S. mitis, with MICs of 2–4 μg/mL, and an MLC of 4 μg/mL. However, this compound exhibited weak antifungal activity against C. albicans with MIC and MLC values of 125 and 250 μg/mL, respectively. Compound 6 showed a stronger anti-C. albicans activity than 10, with an MIC of 62.5 μg/mL, but it had weaker antibacterial activities against S. mitis and S. mutans, with MIC values of 50 and 100 μg/mL, respectively. The chemical skeletal of 4-hydroxy-3,5-dimethoxybenzaldehyde may contribute to its anti-Candida activity. It has been reported that 6 possessed antifungal activity against C. guilliermondii (Kelly et al. Citation2008) as well as antioxidant and anti-inflammatory activities (Bortolomeazzi et al. Citation2007; Stanikunaite et al. Citation2009). In addition, the antibacterial activities of 6 against S. mitis and S. mutans agreed with the previous report by Meerungrueang and Panichayupakaranant (Citation2014).
Table 2. Antimicrobial activities of the isolated compounds and standard drugs.
Compound 10 was first identified as a naturally occurring compound in Allium stipitatum Regel (Alliaceae) by HPLC–MS/MS (Kusterer et al. Citation2010). In addition, it is known as ‘AldrithiolTM-2’ a commercially available product from Sigma-Aldrich (Munich, Germany). There have been no previous reports on biological activity of 10. This is the first report of the antimicrobial activities of 10 and this compound may be used as a new lead compound for development of a new antibacterial agent.
Acknowledgements
The authors thank Dr Brian Hodgson for assistance with the English.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the article. The authors thank the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission for support in the form of a research grant, and Thai Government Science and Technology PhD Scholarship from Ministry of Science and Technology, Thailand.
References
- Bortolomeazzi R, Sebastianutto N, Toniolo R, Pizzariello A. 2007. Comparative evaluation of the antioxidant capacity of smoke flavouring phenols by crocin bleaching inhibition, DPPH radical scavenging and oxidation potential. Food Chem. 100:1481–1489.
- Chamchumroon V. 2014. Two new species of Ixora (Rubiaceae) from Thailand. Thai For Bull (Bot). 42:85–90.
- Ciancio SG. 1995. Chemical agents: plaque control, calculus reduction and treatment of dentinal hypersensitivity. Periodontology 2000. 8:75–86.
- CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard. –3rd ed. CLSI document M27-A3. Wayne (PA): Clinical and Laboratory Standards Institute.
- Ghannoum MA, Rice LB. 1999. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev. 12:501–517.
- He Z, Zhang A, Ding L, Lei X, Sun J, Zhang L. 2010. Chemical composition of the green alga Codium divaricatum Holmes. Fitoterapia 81:1125–1128.
- Hu HB, Jian YF, Cao H, Zheng XD. 2007. Phenolic compounds from Elsholtzia bodinieri Van’t. J Chin Chem Soc. 54:1189–1194.
- Kamble VA. 2012. In vitro anticandidal activity of Pimenta dioica (allspice) essential oil against clinical isolates of Candida albicans and non-albicans Candida. Int J Life Sci Pharm Res. 2:L150–L158.
- Kelly C, Jones O, Barnhart C, Lajoie C. 2008. Effect of furfural, vanillin and syringaldehyde on Candida guilliermondii growth and xylitol biosynthesis. Appl Biochem Biotechnol. 148:97–108.
- Kusterer J, Vogt A, Keusgen M. 2010. Isolation and identification of a new cysteine sulfoxide and volatile sulfur compounds from Allium subgenus Melanocrommyum. J Agric Food Chem. 58:520–526.
- Liu Z, Li S, Han N, Sun D, Cao Y, Yin J. 2008. Studies on the chemical constituents of the vines of Streptocaulon juventus (Lour.) Merr. Asian J Trad Med. 3:193–198.
- Meerungrueang W, Panichayupakaranant P. 2014. Antimicrobial activities of some Thai traditional medical longevity formulations from plants and antibacterial compounds from Ficus foveolata. Pharm Biol. 52:1104–1109.
- Monthong W, Pitchuanchom S, Nuntasaen N, Pompimon W. 2011. (+)-Syringaresinol lignin from new species Magnolia thailandica. Am J Applied Sci. 8:1268–1271.
- NCCLS. 2008. Performance standards for antimicrobial susceptibility testing: ninth informational supplement. NCCLS document M100-S9. Wayne (PA): National Committee for Clinical Laboratory Standard.
- Nittayananta W, Pangsomboon K, Panichayupakaranant P, Chanowanna N, Chelae S, Vuddhakul V, Sukhumungoon P, Pruphetkaew N. 2013. Effects of lawsone methyl ether mouthwash on oral Candida in HIV-infected subjects and subjects with denture stomatitis. J Oral Pathol Med. 42:698–704.
- Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C. 2005. The global burden of oral diseases and risks to oral health. Bull World Health Organ. 83:661–669.
- Sakunphueak A, Panichayupakaranant P. 2010. Simultaneous determination of three naphthoquinones in the leaves of Impatiens balsamina L. by reversed-phase high performance liquid chromatography. Phytochem Anal. 21:444–450.
- Selvaraj N, Lakshmanan B, Mazumder PM, Karuppasamy M, Jena SS, Pattnaik AK. 2011. Evaluation of wound healing and antimicrobial potentials of Ixora coccinea root extract. Asian Pac J Trop Med. 4:959–963.
- Smitinand T. 2001. Flora of Thailand. Bangkok: Royal Forrest Department.
- Stanikunaite R, Khan SI, Trappe JM, Ross SA. 2009. Cyclooxygenase-2 inhibitory andantioxidant compounds from the truffle Elaphomyces granulates. Phytother Res. 23:575–578.
- Yoshioka T, Inokuchi T, Fujioka S, Kimura Y. 2004. Phenolic compounds and flavonoids as plant growth regulators from fruit and leaf of Vitex rotundifolia. Z Naturforsch. 59:509–514.
- Zhu Y, Mohammadi A, Ralph J. 2012. Facile synthesis of 4-hydroxycinnamaldehydes. Bioenerg Res. 5:407–411.