Abstract
Context We report the first ever chemical/biochemical study on Crocus mathewii Kerndorff (Iridaceae) – a Turkish endemic angiosperm. This plant has never been explored for its phytochemistry and bioactivities.
Objective This study explores C. mathewii corm and aerial parts for the chemical and biological properties of hexane, ethyl acetate, methanol and water fractions of the extracts.
Material and methods Plant material (20 g) was extracted by methanol (250 mL × 5, 3 days each) and fractioned into hexane, ethyl acetate, methanol and water. All fractions were subjected to β-carotene–linoleic acid, DPPH·, ABTS·+, CUPRAC, metal chelating and tyrosinase inhibition activities. Hexane fractions were submitted to GC–MS analysis.
Results Ethyl acetate fractions showed excellent IC50 values in DPPH· (aerial 36.21 ± 0.76 and corm 33.87 ± 0.02 mg/L) and ABTS·+ (aerial 33.01 ± 0.79 and bulb 27.87 ± 0.33 mg/L); higher than the IC50 of the standard α-tocopherol (DPPH 116.25 ± 1.97; ABTS 52.64 ± 0.37 mg/L), higher than BHA in DPPH (57.31 ± 0.25 mg/L), but slightly lower in ABTS (19.86 ± 2.73 mg/L). Methanol extract of aerial parts also showed higher activity than α-tocopherol in DPPH (85.56 ± 11.51 mg/L) but slightly less (72.90 ± 3.66 mg/L) than both the standards in ABTS. Linoleic (aerial 53.9%, corm 43.9%) and palmitic (aerial 22.2%, corm 18%) were found as the major fatty acids.
Discussion and conclusion Some fractions of C. mathewii showed higher antioxidant activities than the standards. There is a need to explore more about this plant.
Keywords:
Introduction
Crocus belongs to subfamily Crocoideae of Iridaceae, a family of around 80 genera and almost 1500 species (Goldblatt Citation1990; Bremer et al. Citation2009). Crocus is a genus of 90 flowering species that are grown from the corm (Wani & Mohiddin Citation2009); most of the species are cultivated for ornamental purposes. Crocus species are native to woodland, scrub and meadows. They grow from sea level to high altitudes in Europe, North Africa, Middle East, Aegean and China (Rudall Citation1994).
Crocus is derived from Greek word ‘krokos’ that means saffron (Crocus sativa L.). Crocus species are very important from both a historical and commercial point of view. Harvest and cultivation of C. sativus were first documented in the Mediterranean Crete Island (Hogan Citation2007). Some Crocus species are called ‘autumn crocus’ as they flower in autumn, i.e., September–October.
Crocus mathewii Kerndorff, the plant under study, also known as ‘Dream Dancer’ (), was discovered in 1994 by Helmut Kerndorff and Erich Pasche (Kerndorff Citation1988) in south Turkey. Its distribution is restricted to a few regions of Taurus Mountain. It grows during long summer days at dry mountain slopes from 400 to 1100 m altitudes. It can be characterized by its corm and whitish flowers with a dark purple zone in the throat (). C. mathewii was named in the honour of Brian Mathew who was famous as the ‘Crocus guru’ (Grey-Wilson & Mathew Citation1981). He proposed the properties for the taxonomic classification of Crocus (Petersen et al. Citation2008). There was confusion regarding how to differentiate between C. mathewii and C. asumaniae (Mathew & Beytop Citation1976). However, taxonomists consider them different species due to their different habitats.
C. mathewii is an infant plant to the scientific community. A careful literature survey does not provide any phytochemical investigation or biological activities of this plant. One can expect biological importance of C. mathewii after studying its vastly studied sister species. For example, the water:methanol (50:50, v/v) extract of C. sativus have been reported to possess better antioxidant activities; even higher than that of tomatoes and carrots (Papandreou et al. Citation2006). On the contrary, Ramadan et al. (Citation2012) has reported weak antioxidant activity of the ethanol extract of the same plant, although they concluded the extract as non-toxic. Sariri et al. (Citation2011) studied the antioxidant and antityrosinase activities of extract obtained from the methanol extract of C. sativus flowers. They reported the extract as 30% antityrosinase inhibitor, although they stated the extract as antioxidant on the basis of total phenolics present in the extract. Karimi et al. (Citation2010) studied free radical scavenging and ferric reducing activities of the C. sativus stigma and found notable activities, i.e., 68.2% and 78.9%, at a concentration of 300 μg/mL. This plant has also been reported to have antibacterial (Parray et al. Citation2014) and inhibitory activity on amyloid-β-aggregation (Papandreou et al. Citation2006).
Based on the medicinal properties of neighbour species, we decided to explore scientific windows on C. mathewii. Herein, we report the first scientific work performed on this species that includes the antioxidant, anticholinesterase and tyrosinase inhibitory activities as well as the GC–MS analysis.
Materials and methods
Crocus mathewii was collected from Fethiye-Babadag Mountains in Mugla Province of Turkey during October 2014. The plant was characterized by Kenan Akbaş (MSc) and Ömer Varol (PhD); working at the herbarium of the Biology Department of Mugla University. A specimen (voucher number K.A 625) was submitted at the stated herbarium. About 20 g of the plant was dried by wrapping it in a filter paper for 60 days – unexposed to light.
Extraction and fractionation
Plant material was divided into two parts: corm and aerial parts including flowers. Each part was crushed into small pieces (approx. 10 g of each) and transferred into 250 mL methanol. Extracts were obtained after 3 days and this process was repeated until the solvent became colourless. The crude extract was evaporated to dryness and dissolved in distilled water. The aqueous solution was fractionated into hexane, ethyl acetate, methanol and water.
Determination of antioxidant activity
All antioxidant activities were performed according to the standard literature procedures with slight modifications (Öztürk et al. Citation2011). Total antioxidant activity was evaluated using β-carotene–linoleic acid test (Marco Citation1968). Free radical scavenging activity was determined spectrophotometrically by the DPPH radical assay (Blois Citation1958). The spectrophotometric analysis of ABTS·+ scavenging activity was determined according to the literature method (Re et al. Citation1999). Superoxide anion radical scavenging activity was performed according to Liu’s method (Liu et al. Citation1997). CUPRAC antioxidant activity was performed according to Apak’s procedure (Apak et al. Citation2004). The extract was also tested for metal chelating activity on Fe2+ spectrophotometrically (Decker & Welch Citation1990). α-Tocopherol and BHA were used as standards in β-carotene–linoleic acid, DPPH·, ABTS·+, and CUPRAC assays. EDTA was used as a standard in metal chelating assay.
Determination of anticholinesterase activity
Acetylcholinesterase and butyrylcholinesterase inhibitory activities were measured by the spectrophotometric method developed by Ellman et al. (Citation1961).
Tyrosinase activities
Tyrosinase enzyme inhibitory activity was measured according to Khatib at al. (Citation2005) procedure with slightly modified Hearing (Citation1987) spectroscopic method. Mushroom tyrosinase enzyme was used with l-DOPA as a substrate, while kojic acid was used as a standard tyrosinase inhibitor. Kojic acid was used as a standard.
Fatty acid compositions
Esterification of acids
Crocus mathewii (20 g) crude material was dried, grind to powder and extracted with 250 mL of hexane (6 × 24 h) at room temperature. Crude hexane extract (100 mg) was vacuum filtered and dissolved in 2 mL of 0.5 mol L−1 sodium hydroxide solution. This mixture was heated on a water bath (below 50 °C). Two millilitres of BF3–MeOH complex was introduced to the reaction mixture and boiled for 3 min. After cooling, the mixture was diluted with saturated sodium chloride solution (25 mL). The synthesized esters were extracted with n-hexane, washed with potassium bicarbonate solution (4 mL, 2%) and dried over anhydrous Na2SO4. Solvent was evaporated to obtain methyl esters (Gören et al. Citation2006).
Gas chromatography
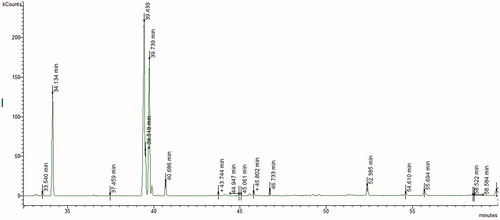
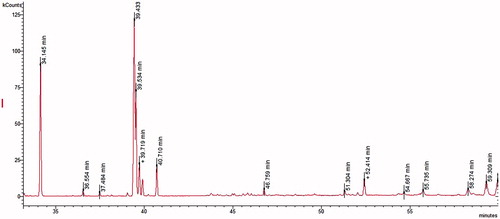
GC parameters were set according to Topcu et al. (Citation2013). Fatty acids were analyzed according to the procedure of Tel et al. (Citation2013). GC chromatograms of the hexane fractions, obtained from corm and aerial parts of C. mathewii, are given in and , respectively. Gas chromatography–mass spectrometry
All instrumental parameters to determine fatty acids methyl esters in the samples were set according to the Tel et al. (Citation2013).
Determination of IC50 values
The results obtained from biological activities are expressed as 50% inhibition concentration (IC50). The sample concentration, which provides 50% biological activity (IC50), was calculated from the graph of % activity versus sample concentration.
Statistical analysis
Antioxidant and tyrosinase inhibitory activity data () are the averages of triplicate analyses. Data were recorded as mean ± standard error of mean (SEM). Significant differences between means were determined by Student’s t-test, while p values <0.05 were regarded as significant.
Table 1. β-Carotene–linoleic acid, DPPH·, ABTS·β, CUPRAC and metal chelating antioxidant, and tyrosinase activities of the extracts obtained from Crocus mathewii.Table Footnotea
Results and discussion
Hexane, ethyl acetate, methanol and water fractions were obtained from both corm and aerial parts of C. mathewii. Separately, 5 g of plant material (combined corm and aerial parts) was boiled in water for 30 min to obtain water boiled fraction; that was used to prepare silver nanoparticles in a separate study. After nanoparticle separation through centrifuge, the remaining fraction was termed as SM1 that was extracted with hexane that yielded another nonpolar fraction SM2.
As a whole, 10 obtained extracts were subjected to antioxidant activities, i.e., β-carotene–linoleic acid, DPPH·, ABTS·+, CUPRAC and metal chelating activities ().
Ethyl acetate fraction of corm and aerial parts showed excellent IC50 values in DPPH and ABTS assays; even the values were higher than the standards α-tocopherol and BHA. In DPPH, the aerial ethyl acetate fraction showed IC50 value of 36.21 ± 0.76 mg/L, while the corm reflected 33.87 ± 0.02 mg/L. This bioactivity is higher than both α-tocopherol (IC50 116.25 ± 1.97 mg/L) and BHA (IC50 57.31 ± 0.25 mg/L). Assimopoulou et al. found higher DPPH radical scavenging activities in the methanol extract of C. sativus and assigned the activity to crocin – a component found in the stated plant (Assimopoulou et al. Citation2005). Chen et al. (Citation2008) has also found higher DPPH activities in ethanol extract of C. sativus and found 107 mg/g of α-tocopherol equivalent. However, Karimi et al. (Citation2010) has claimed that C. sativus collected from Khorasan province of Iran reflected higher DPPH activities; even higher than reported by Assimopoulou et al. (Citation2005) and Chen et al. (Citation2008).
In ABTS assay of ethyl acetate fraction, aerial parts produced IC50 value of 33.01 ± 0.79 mg/L, while the corm provided 27.87 ± 0.33 mg/L. These values were higher than α-tocopherol (52.64 ± 0.37 mg/L) but slightly lower than BHA, i.e., 19.86 ± 2.73 mg/L (). Serrano-Díaz et al. (Citation2012) also observed ABTS scavenging activities in C. sativus when they were studying the antioxidant activities of flowers and stigmas. Keyhani et al. (Citation2007) studied the corm of C. sativus for several days and found better ABTS activity when the root elongated for 6 days.
Among the all tested fractions, both corm and aerial ethyl acetate fractions showed the highest CUPRAC activity (IC50 mg/L: aerial 47.15 ± 2.09, corm 46.70 ± 2.97), though the values were less than the standard α-tocopherol (IC50 0.85 ± 0.02 mg/L) and EDTA (). In metal chelating assay, ethyl acetate fraction of corm did not show any bioactivity, while the aerial part fraction showed lower activity (IC50 591.71 ± 142.10 mg/L) than EDTA (IC50 3.47 ± 0.35 mg/L). Sariri et al. (Citation2011) used ascorbic acid to measure reducing power of C. sativus extract and found 193.91 ± 1.22 of IC50 (ascorbic acid IC50: 15.69 ± 0.08) that was a bit higher than our C. mathewii.
Methanol extract of C. mathewii also showed quite noticeable antioxidant activities. The aerial parts showed higher activity than α-tocopherol in the DPPH assay (IC50 85.56 ± 11.51 mg/L), while it also reflected ABTS assay value very close to the standards (IC50 72.90 ± 3.66 mg/L). On the other hand, this fraction did not show any metal chelating assay activity, the aerial part fraction showed bioactivity that should interest the reader (IC50 145.50 ± 2.12 mg/L) ().
As a whole, hexane, water and methanol fraction of corm showed lower antioxidant activities. It is also quite interesting that the boiled fractions (SM1 and SM2) showed the highest metal chelating activity as compared to the other fractions (). SM2 showed higher metal chelating activity (IC50 107.05 ± 8.89 mg/L) as compared to SM1 (IC50 168.20 ± 11.33 mg/L).
Table 2. The fatty acid composition (%) of aerial part and corm of Crocus mathewii.
Hexane, ethyl acetate, methanol and water extracts obtained from corm and aerial parts were subjected to acetyl- and butylcholinesterase activities. All the extracts showed very less activity against both the enzymes.
Tyrosinase is a multifunctional copper-containing oxidase enzyme that catalyses the first two steps in mammalian melanogenesis. It initiates the enzymatic browning reactions in harvested fruits. Hyperpigmentation is undesirable in human skin similar to the enzymatic browning of fruits. Researchers are in a quest to seek new potent tyrosinase inhibitors that could be safely used in foods and cosmetics (Chang Citation2009).
A kaempferol isolated from C. sativus has been reported to have tyrosinase inhibitory activity (ID50 67 μg/mL, 0.23 mM) (Kubo & Kinst-Hori Citation1999). In this study, hexane extracts of both corm and aerial parts of C. mathewii showed slight inhibition of tyrosinase activities (). Aerial parts showed slight higher inhibition (IC50 1658.69 ± 26.30 mg/L) as compared to the hexane fraction of the corm (IC50 2239.37 ± 44.08 mg/L). However, both activities were lower than the standard kojic acid (IC50 14.07 ± 1.68 mg/L). The remaining fractions did not show any noticeable inhibition of tyrosinase activity. Sariri et al. (Citation2011) found tyrosinase inhibitory activity with an IC50 value 9132.55 ± 278.72 (ascorbic acid IC50: 229.68 ± 1.06) in C. sativus that is less than the value observed in C. mathewii in the current study.
GC–MS analysis of the aerial and corm of C. mathewii showed 18 fatty acids; among these, 13 were observed in the aerial parts while 16 in the corm extract (). There are previous reports on the sister species of C. mathewii that shows more or less similar fatty acids. Yayli et al. (Citation2001) reported 22 fatty acids from Crocus vallicola. Both aerial parts and corm of C. mathewii showed less total saturation (39.6% and 26.8%, respectively) as compared to the total unsaturation (60.4% and 73.2%). In contrast, C. vallicola showed more total saturation than unsaturation (Yayli et al. Citation2001). Saturation/unsaturation ratio in aerial and corm extracts was found as 0.6 and 0.4, respectively. Linoleic acid/oleic acid ratio was found as 9.6 and 1.8 in aerial parts and corm, respectively. Among the fatty acids, Z-linoleic acid was observed as the most abundant in both aerial (35.7%) and corm (38.8%) extracts of C. mathewii.
A previous study on Crocus pelistericus showed palmitic (57.4%) and linolenic (42.6%) acids as the major fatty acids (Jankuloski et al. Citation2014). On the contrary, we found 53.9% linoleic acid (Z-linoleic 35.7%, E-linoleic 16.2%) in aerial parts while 43.9% in corm (Z-linoleic 38.8%, E-linolenic acid 5.1%). In addition, 22.2% and 18.0% palmitic acid was observed in the aerial and corm of C. mathewii, respectively.
Other fatty acids observed in C. mathewii are Z-9-palmitoleic acid (C16:1, Δ9), 9-methlyhexadecanoic acid (C17:0), margaric acid (C17:0), oleic acid (C18:1, Δ9), elaidic acid (C18:1), steraic acid (C18:0), elaidic acid (C18:1), stearic acid (C18:0), arachidonic acid (20:4), gondoic acid (C20:1), arachidic acid (C20:0), behenic acid (C22:0), tricosenoic acid (C23:1), tricosanoic acid (C23:0), tetracosenoic acid (C24:1), tetracosanoic acid (C24:0) ().
Acknowledgements
We are thankful to the Kenan Akbaş and Ömer Varol for help in plant characterization and the labs at the Department of Medicinal and Aromatic plants, Köyceğiz Vocational School Mugla University for technical support (BAP-15/029).
Declaration of interest
The authors declare no conflicts of interest.
References
- Apak R, Güçlü K, Ozyürek M, Karademir SE. 2004. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J Agric Food Chem. 52:7970–7981.
- Assimopoulou AN, Sinakos Z, Papageorgiou VP. 2005. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother Res. 19:997–1000.
- Blois M. 1958. Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200.
- Bremer B, Bremer K, Chase MW, Fay MF, Reveal JL, Soltis DE, Soltis PS, Stevens PF, et al. 2009. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc. 161:105–121.
- Chang T-S. 2009. An updated review of tyrosinase inhibitors. Int J Mol Sci. 10:2440–2475.
- Chen Y, Zhang H, Tian X, Zhao C, Cai L, Liu Y, Jia L, Yin HX, Chen C. 2008. Antioxidant potential of crocins and ethanol extracts of Gardenia jasminoides ELLIS and Crocus sativus L.: a relationship investigation between antioxidant activity and crocin contents. Food Chem. 109:484–492.
- Decker EA, Welch B. 1990. Role of ferritin as a lipid oxidation catalyst in muscle food. J Agric Food Chem. 38:674–677.
- Ellman GL, Courtney KD, Andres V, Featherstone RM. 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 7:88–95.
- Goldblatt P. 1990. Phylogeny and classification of Iridaceae. Ann Missouri Botanical Garden. 77:607–627.
- Gören AC, Kiliç T, Dirmenci T, Bilsel G. 2006. Chemotaxonomic evaluation of Turkish species of Salvia: fatty acid compositions of seed oils. Biochem Sys Ecol. 34:160–164.
- Grey-Wilson C, Mathew B. 1981. Bulbs, the bulbous plants of Europe and their allies. London, England: Collins.
- Hearing VJ. 1987. Metabolism of aromatic amino acids and amines. Methods Enzymol. 142:154–165.
- Hogan CM. 2007. Knossos fieldnotes. The modern antiquarian; [cited 2015 Sept 20]. Available from: http://www.themodernantiquarian.com/site/10854/knossos.html.
- Jankuloski Z, Arapcheska M, Hajrulai-Musliu Z. 2014. Fatty acid content in stigmas of Crocus pelistericus. Food and Agriculture Cost Action FA1101 ‘SaffronOmics’ 2014 Annual Conference, Wageningen, The Netherlands.
- Karimi E, Oskoueian E, Hendra R, Jaafar HZ. 2010. Evaluation of Crocus sativus L. stigma phenolic and flavonoid compounds and its antioxidant activity. Molecules 15:6244–6256.
- Kerndorff H. 1988. Observations of Crocus (Iridaceae) in Jorden with special reference to Crocus moabiticus. Herberita 44:33–53.
- Keyhani J, Keyhani E, Arzi L. 2007. Alterations in lignin peroxidase and ascorbate peroxidase activities in Crocus sativus L. corms exposed to copper. II International Symposium on Saffron Biology and Technology. Acta Hortic. 739:427–434.
- Khatib S, Nerya O, Musa R, Shmuel M, Tamir S, Vaya J. 2005. Chalcones as potent tyrosinase inhibitors: the importance of a 2,4-substituted resorcinol moiety. Bioorg Med Chem. 13:433–441.
- Kubo I, Kinst-Hori I. 1999. Flavonols from saffron flower: tyrosinase inhibitory activity and inhibition mechanism. J Agric Food Chem, 47:4121–4125.
- Liu F, Ooi VE, Chang ST. 1997. Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci. 60:763–771.
- Marco GJ. 1968. A rapid method for evaluation of antioxidants. J Am Oil Chem Soc. 45:594–598.
- Mathew B, Beytop T. 1976. Notes from the royal botanic gardens, Edinburgh. In: Some observations on Turkish crocus. Vol. 35. Edinburgh, United Kingdom: H. M. Stationery Off. p. 61–7.
- Öztürk M, Duru ME, Kivrak S, Mercan-Doğan N, Türkoglu A, Özler MA. 2011. In vitro antioxidant, anticholinesterase and antimicrobial activity studies on three Agaricus species with fatty acid compositions and iron contents: a comparative study on the three most edible mushrooms. Food Chem Toxicol. 49:1353–1360.
- Papandreou MA, Kanakis CD, Polissiou MG, Efthimiopoulos S, Cordopatis P, Margarity M, Lamari FN. 2006. Inhibitory activity on amyloid-beta aggregation and antioxidant properties of Crocus sativus stigmas extract and its crocin constituents. J Agric Food Chem. 54:8762–8768.
- Parray JA, Kamili AN, Hamid R, Reshib ZA, Qadric RA. 2014. Antibacterial and antioxidant activity of methanol extracts of Crocus sativus L. c.v. Kashmirianus. Front Life Sci. 8:40–46.
- Petersen G, Seberg O, Thorsøe S, Jørgensen T, Mathew B. 2008. A phylogeny of the genus Crocus (Iridaceae) based on sequence data from five plastid regions. Taxon. 57:487–499.
- Ramadan A, Soliman G, Mahmoud SS, Nofalc SM, Abdel-Rahman RF. 2012. Evaluation of the safety and antioxidant activities of Crocus sativus and propolis ethanolic extracts. J Saud Chem Soc. 16:13–21.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 26:1231–1237.
- Rudall P. 1994. Anatomy and systematics of Iridaceae. Bot J Linn Soc. 114:1–21.
- Sariri R, Sabbaghzadeh R, Poumohamad F. 2011. In vitro antioxidant and anti-tyrosinase activity of methanol extracts from Crocus sativus flowers. Pharmacol Online 3:1–11.
- Serrano-Díaz J, Sánchez AM, Maggi L, Martínez-Tomé M, García-Diz L, Murcia MA, Alonso GL. 2012. Increasing the applications of Crocus sativus flowers as natural antioxidants. J Food Sci. 77:C1162–C1168.
- Tel G, Öztürk M, Duru ME, Doğan B, Harmandar M. 2013. Fatty acid composition, antioxidant, anticholinesterase and tyrosinase inhibitory activities of four Serratula species from Anatolia. Rec Nat Prod. 7:86–95.
- Topcu G, Ozturk M, Kusman T, Demirkoz AB, Kolak U, Ulubelen A. 2013. Terpenoids, essential oil composition, fatty acid profile, and biological activities of Anatolian Salvia fruticosa Mill. Turk J Chem. 37:619–632.
- Wani BA, Mohiddin FA. 2009. Micropropagation of genus Crocus – a review. Afr J Agric Res. 4:1545–1548.
- Yayli N, Kiran Z, Seymen H, Genc H, Kücükislamoğlu M. 2001. Characterization of lipids and fatty acid methyl ester contents in leaves and roots of Crocus vallicola. Turk J Chem. 25:391–396.