Abstract
Context Myrtle, Myrtus communis L. (Myrtaceae), is a medicinal plant well known for its richness in phenolic compounds and its beneficial effects for the treatment of gastrointestinal disorders.
Objective In the present work, the protective effect of the myrtle berry seed aqueous extract (MBSAE) against esophageal reflux (ER)-induced damage in esophagus mucosa as well as the mechanisms implicated was determined.
Materials and methods In this respect, adult male Wistar rats were used and divided into seven groups: Control, ER, ER + various doses of MBSAE, ER + famotidine or ER + gallic acid. The ER was induced and animals were per orally (p.o.) treated with MBSAE or reference molecules during 6 h. The phytochemical screening was determined using colourimetric analysis.
Results MBSAE is rich in total polyphenols and anthocyanins and exhibited an important in vitro antioxidant activity. In vivo, we firstly found that ER led to marked macroscopic and histopathological changes in esophagus. The results showed, also, that the ER was accompanied by a state of oxidative stress as assessed by an increase of lipid peroxidation, a decrease of the sulphhydryl groups and glutathione levels, as well as antioxidant enzyme activities depletion. MBSAE abrogated all morphological, histopathological and biochemical alterations. We showed also that ER increased esophageal calcium, hydrogen peroxide (H2O2) and free iron levels while MBSAE treatment protected against intracellular mediators deregulation.
Conclusion Our data suggest that MBSAE exerted a potential protective effect against ER-induced damage in rat esophagus, at least in part, due to its antioxidant properties.
Introduction
Gastroesophageal reflux (GER) is a multifactorial disease, and is one of the most frequent complaints in centres of paediatrics and paediatric gastroenterology (Orenstein et al. Citation1999; Vandenplas et al. Citation2009; Lightdale & Gremse Citation2013). Some pathological conditions in the lower esophagus, including erosions, stenosis, ulcer or metaplastic epithelium are considered as complications of chronic reflux esophagitis (Ismail-Beigi et al. Citation1970; Kobayashi & Kasugai Citation1974). However, it has been generally believed that the reflux of gastric contents causes the ulceration and destruction of the normal squamous epithelium of esophagus (Hirschowitz Citation1991). The main etiological factors related to GER are lower esophageal sphincter dysfunction, decreased esophageal clearance capacity, esophageal mucosal barrier dysfunction and esophageal visceral hypersensitivity (Hassall Citation2005). However, gastric acid also plays a major role in the pathogenesis of reflux esophagitis (Hunt Citation1999). Indeed, antisecretory drugs, such as histamine H2 receptor antagonists and proton pump inhibitors, have been shown to be effective against acid reflux esophagitis in experimental animals and humans (Meuwissen & Klinkenberg-Knol Citation1988; Inatomi et al. Citation1991).
The mechanisms underlying the esophageal reflux (ER) have not yet been fully elucidated (Kawahara et al. Citation2007). There is evidence that oxidative stress and lipid peroxidation play important roles in the gastro-esophageal diseases (Singh et al. Citation2012). In this respect, numerous reports have demonstrated that esophageal lesions are closely related to the increased reactive oxygen species (ROS) levels, which lead to lipid peroxidation in the membranes by the oxidation of unsaturated fatty acids (Aiyer et al. Citation2011; Singh et al. Citation2012). Therefore, endogenous antioxidant defense systems, including antioxidant enzymes, contribute to the prevention of the toxic ROS effects (Singh et al. Citation2012).
Plant extracts, known for their richness in phenolic compounds and antioxidant ability, are mainly used by researchers to produce new drugs and they have promising results in the treatment of GER (Kawahara et al. Citation2007; Mahattanadul et al. Citation2011).
Myrtle [Myrtus communis L., (Myrtaceae), var. italica], a dark-blue fruited morph, with dark-blue fruits when completely ripe (Messaoud & Boussaid Citation2011), is naturally distributed in the Mediterranean regions including Tunisia (Pottier-Alapetite Citation1979). It is one of the most widely used and well-documented medicinal plants in the world (Clark Citation1996; Gortzi et al. Citation2008; Messaoud et al. Citation2012). The use of myrtle berry seed extract as medicinal preparations has a long tradition in a variety of countries including Tunisia (Aidi-Wannes et al. Citation2011; Messaoud & Boussaid Citation2011). However, this species is traditionally known for its beneficial effects in the treatment of gastrointestinal disorders (Clark Citation1996; Sumbul et al. Citation2010). Myrtle berries are characterised by a high antioxidant capacity, and they contain many bioactive molecules such as polyphenols, anthocyanins, proanthocyanidins, phenolic acids, flavonoids and polyunsaturated fatty acids (Messaoud & Boussaid Citation2011). Consequently, myrtle berry extract exhibits many beneficial health effects such as neuro-protective (Tumen et al. Citation2012), antimicrobial (Alem et al. Citation2008) and antidiabetic (Ziyyat et al. Citation1997) activities.
Hence, in the present study, we evaluate the putative protective effect of myrtle berry seed aqueous extract (MBSAE) against experimental ER in rat as well as the mechanisms involved.
Materials and methods
Chemicals
5,5-Dithio bis(2-nitrobenzoic acid) (DTNB), trichloroacetic acid (TCA), acetylcholine iodide, S-butyrylcholine, butylhydroxytoluene (BHT), 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis[3-ethylbenzthiazoline-6-sulphonic acid] (ABTS), methanol, ether, bovine serum albumin (BSA), orthophosphoric acid and NaCl were purchased from Sigma Chemical Co. (Sigma-Aldrich GmbH, Steinheim, Germany).
Plant collection
Myrtle berry seeds were collected in October 2013 from the region of Nefza (North-West of Tunisia) and identified by Mrs Mouhiba Ben-Naceur, professor of taxonomy in the Faculty of Sciences of Tunis, Tunisia. The voucher specimens (No. MY01) have been deposited in the herbarium of the Higher Institute of Biotechnology of Beja, Tunisia and in the Department of Biological Sciences, Faculty of Science of Bizerte, Tunisia.
Plant extract preparation
The myrtle berry seeds were dried in an incubator at 50 °C for 72 h and powdered in an electric blender (MOULINEX Ovatio 2, FR). The powder was then dissolved in double distilled water (10%, weight/volume) and incubated at room temperature for 24 h in a shaking bath. The sample was centrifuged at 10 000 g for 10 min and the supernatant was lyophilised, fractionated into aliquots and stored at −80 °C until use.
Total polyphenols determination
Total phenolic content of the myrtle berry seed extract was determined by the colourimetric Folin-Cieucalteu method (Haseeb & John Citation2006). The extract (500 μL) was added to 10 mL of water and 0.5 mL of Folin-Cieucalteu reagent. After 5 min, 8 mL of 7.5% sodium carbonate solution was added. The reaction mixture was stored in the dark for 2 h and its optical density was measured at 765 nm using a UV-visible spectrophotometer (Beckman DU 640B). Gallic acid was applied as standard, and values were expressed as mg of gallic acid equivalent (mg GAE/g DM).
Total anthocyanins determination
The total anthocyanins content of the myrtle berry seed extract was measured using the differential pH method (Lee et al. Citation2005). The absorbance of anthocyanins at 510 and 700 nm in different pH buffers (pH 1.0 and 4.5) was measured. Absorbance values were converted to total μg of cyanidin 3-glucoside per g of dry matter (DM) in seeds using the molar extinction coefficient of 26 900. Values were expressed as μg of cyanidin 3-glucoside equivalent (μg CGE/g DM).
Free radical-scavenging activities on ABTS
The antioxidant capacities of the MBSAE were evaluated using the ABTS method (Siddhuraju Citation2006). ABTS·+ was produced by reacting 7 mM ABTS aqueous solution with 2.4 mM potassium persulphate in the dark for 12–16 h at room temperature. Prior to assay, this solution was diluted in ethanol (about 1/89 v/v) and equilibrated at 30 °C to give an absorbance at 734 nm of 0.700 ± 0.02 (A0). The diluted extract (1 mL) was mixed with 3 mL of ABTS˙+ solution and incubated at 30 °C for 60 min and the absorbance was measured at 734 nm (A1). ABTS radical scavenging activity (RSA), expressed as a percentage, was estimated using the following formula: (1−A1/A0) × 100).
Hydrogen peroxide scavenging assay
The hydrogen peroxide scavenging activities were determined according to Gülçin et al. (Citation2005). Indeed, a solution of hydrogen peroxide (40 mM) was prepared in phosphate buffer saline (pH 7.4) and 0.6 mL was added to croissant concentrations of MBSAE (50–500 μg/mL). Absorbance was determined after 10 min against a blank solution containing phosphate buffer without hydrogen peroxide and the percentage scavenging of hydrogen peroxide by extract was calculated.
Animals and treatment
Healthy adult male Wistar rats (n = 70) with an average weight of 210 ± 15 g were purchased from the Pasteur Institute of Tunis. Wistar rats are used in accordance with the local ethics committee of Tunis University for the uses and the care of animals in accordance with the NIH recommendations. They were provided with standard food (standard pellet diet – Badr Utique-TN) and water ad libitum. Animals were maintained in an experimental house under controlled temperature (22 ± 2 °C) with a 12 h light–dark cycle. The rats were divided into seven groups of 10 animals each. ER was induced to all used animals except group 1. Under light ether anaesthesia, rats were laparotomised to ligate the pylorus and the junction between the forestomach and the corpus (Nakamura et al. Citation1982) and the rats were then deprived of food and water.
However, Groups 1 and 2 served as controls and had per orally (p.o.) a physiological solution. Groups 3, 4, and 5 were treated, orally, with various doses of MBSAE (25, 50 and 100 mg/kg, b.w., p.o.), while Groups 6 and 7 received famotidine and gallic acid at 20 and 50 mg/kg, b.w., p.o., respectively.
Six hours after the double ligation, animals were autopsied; their gastro-esophageal portion of the digestive tract was rapidly excised, rapidly macroscopically examined and homogenised in phosphate buffer saline. After centrifugation at 10 000g for 10 min at 4 °C, supernatant was used for biochemical determination of protein, free iron, calcium, H2O2, SH-groups, reduced glutathione (GSH) and MDA levels as well as antioxidant enzyme activities. However, blood was likewise collected in heparinised tubes. After centrifugation at 3000 g during 15 min, plasma was treated for PSA determination.
Determination of esophagitis severity
The severity of esophagitis was macroscopically scored, using an ulcer index. The following scale was used: 0, no injury; 1, erosion of mucosal epithelium; 2, the length of hemorrhagic ulcer area <20 mm; 3, the length of hemorrhagic ulcer area 20–30 mm; 4, the length of hemorrhagic ulcer area 30–40 mm; 5, the length of hemorrhagic ulcer area >40 mm or perforation (Mahattanadul et al. Citation2011).
Preparation of the rat acid esophagitis reflux model
ER was elicited by the modified method of Nakamura et al. (Citation1982). However, the rats were laparotomised under light ether anaesthesia to ligate the duodenum with silk thread at the anal side of the major duodeni papilla. In addition, the limiting ridge (transitional region between the fore stomach and corpus of stomach) was also ligated leading to the reflux of gastric juice and duodenal bile juice into the esophagus. The control group of rats underwent a control operation with no ligation. The abdomen was closed with suture and the animals were placed in their cages without food and water.
Determination of pH, titrable acidity and volume of gastric juice
Six hours after extract administration, the stomachs were opened along the greater curvature and the gastric contents were collected and centrifuged for 10 min at 2000 g. The total volume of the gastric content was expressed as mL/100 g body weight (Shay et al. Citation1947). The supernatant (1 mL) was diluted to 10 mL with distilled water and the total acid content was determined by titrating with sodium hydroxide (0.01 N) using phenolphthalein as an indicator and is expressed as mEq/L/100 g (Li et al. Citation2002). The pH values were measured using pH meter.
Histopathological analysis
Immediately, after macroscopic examination, small pieces of esophagus were harvested and washed with ice-cold saline. Tissue fragments were then fixed in a 10% neutral buffered formalin solution, embedded in paraffin and used for histopathological examination. Thick sections (5 μm) were cut, deparaffinised, hydrated and stained with haematoxylin and eosin for histological examination.
Lipid peroxidation measurement
Esophageal lipid peroxidation was determined by malondialdehyde (MDA) measurement according to the double heating method of Draper and Hadley (Citation1990). Aliquots from esophageal tissue homogenates were mixed with BHT–TCA solution containing 1% BHT (w/v) dissolved in 20% TCA (w/v) and centrifuged at 1000 g for 5 min at 4 °C. Supernatant was blended with solution containing (0.5 N HCl 120 mM TBA in 26 mM of Tris), and then heated at 80 °C for 10 min. After cooling, the absorbance of the resulting chromophore was determined at 532 nm. MDA levels were determined by using an extinction coefficient for MDA–TBA complex of 1.56 × 105 M−1 cm−1.
Plasma scavenging activities
The free radical scavenging activities of plasma were measured using the DPPH radical method according to Brand-Williams et al. (Citation1995). In this respect, 100 μL of plasma sample was added to 2 mL of 2,2-diphenyl-1-picrylhydrazyl (DPPH) in methanol solution (100 mM). After incubation at 37 °C for 30 min, 1 mL of chloroform was added and the solution was centrifuged at 3000g for 10 min. The absorbance of clear supernatant was then determined at 517 nm using spectrophotometer (Beckman DU 640B). DPPH solution was used as a control and the plasma scavenging activities (PSAs), expressed in percentage, was calculated according to the following equation (Alimi et al. Citation2012):
Non-enzymatic antioxidant levels
Sulphhydryl groups (–SH) determination
The total concentration of thiol groups (–SH) was performed according to Ellman's method (Ellman Citation1959). Aliquots from esophageal tissue were mixed with 800 μL of 0.25 M phosphate buffer (pH 8.2) and 100 μL of 20 mM EDTA, and the optical density was measured at 412 nm (A1). After adding 100 μL of 10 mM DTNB, the reaction mixture was incubated at 37 °C during 15 min and a new value (A2) was determined. The thiol groups concentration was calculated from A2 to A1 subtraction using a molar extinction coefficient of 13.6 × 103 M−1×cm−1. The results were expressed as nmol of thiol groups per mg of protein.
Reduced GSH determination
GSH was estimated in esophageal tissue by the method of Sedlak and Lindsay (Citation1968). Five hundred microlitres of tissue homogenate prepared in 20 mM EDTA (pH 4.7) was mixed with 400 μL of cold distilled water and 100 μL of 50% TCA. The samples were shaken using vortex mixer and centrifuged at 1200 g during 15 min. Two hundred microlitres of supernatant were mixed with 400 μL of 400 mM Tris–buffer (pH 8.9) and 10 μL of 10 mM DTNB. The absorbance was read at 412 nm against blank tube without homogenate.
Antioxidant enzyme activities assays
Superoxide dismutase activity assay
The activity of superoxide dismutase (SOD) was determined using modified epinephrine assays (Misra & Fridovich Citation1972). At alkaline pH, superoxide anion causes the autoxidation of epinephrine to adenochrome; while competing with this reaction, SOD decreased the adenochrome formation. One unit of SOD is defined as the amount of the extract that inhibits the rate of adenochrome formation by 50%. Enzyme extract was added to 2 mL reaction mixture containing 10 μL of bovine catalase (CAT, 0.4 U/μL), 20 μL of epinephrine (5 mg/mL) and 62.5 mM of sodium carbonate/bicarbonate buffer (pH 10.2). Changes in absorbance were recorded at 480 nm.
CAT activity assay
The activity of CAT was assessed by measuring the initial rate of H2O2 disappearance at 240 nm (Aebi Citation1984). The reaction mixture contained 33 mm H2O2 in 50 mm phosphate buffer (pH 7.0) and the activity of CAT was calculated using the extinction coefficient of 40 mM–1 cm−1 for H2O2.
GPx activity assay
The activity of glutathione peroxidase (GPx) was quantified following the procedure of Flohé and Gunzler (Citation1984). However, 1 mL of reaction mixture containing 0.2 mL of esophageal supernatant, 0.2 mL of phosphate buffer (0.1 M pH 7.4), 0.2 mL of GSH (4 mM) and 0.4 mL of H2O2 (5 mM) was incubated at 37 °C for 1 min and the reaction was stopped by the addition of 0.5 mL TCA (5%, w/v). After centrifugation at 1500g for 5 min, aliquot (0.2 mL) from supernatant was combined with 0.5 mL of phosphate buffer (0.1 M pH 7.4) and 0.5 mL DTNB (10 mM). The absorbance was read at 412 nm and the activity of GPx was expressed as nmol of GSH consumed/min/mg protein.
H2O2 determination
The esophageal H2O2 level was performed according to Dingeon et al. (Citation1975). The hydrogen peroxide reacts with p-hydroxybenzoic acid and 4-aminoantipyrine in the presence of peroxidase leading to the formation of quinoneimine that has a pink colour detected at 505 nm.
Iron measurement
The esophageal tissue non-heme iron was measured colourimetrically using ferrozine as described by Leardi et al. (Citation1998). Indeed, the iron dissociated from transferrin–iron complex by a solution of guanidine acetate was reduced by ascorbic acid and reacted with ferrozine leading to the formation of pink complex measured at 562 nm.
Calcium determination
The esophageal tissue calcium level was performed using a colourimetric method according to Stren and Lewis (Citation1957). However, at alkaline medium, calcium reacted with cresolphtalein and lead to a coloured complex measurable at 570 nm.
Protein determination
Protein concentration was determined according to Hartree (Citation1972) as is a slight change of the Lowry method. Serum albumin was used as a standard.
Statistical analysis
The data were analysed by unpaired Student's t-test and were expressed as means ± standard error of the mean (SEM). The data are representative of 10 independent experiments. All statistical tests were two-tailed, and a p value of 0.05 or less was considered significant.
Results
Phenolic contents and in vitro antioxidant activities of MBSAE
The phytochemical studies firstly showed that MBSAE exhibits high levels of total polyphenols (147.56 ± 1.97 mg GAE/g DM) and total anthocyanins (5.01 ± 0.21 μg CGE/g DM). The free radical scavenging capacity of MBSAE was estimated with ABTS and hydrogen peroxide systems. As shown in , the radical-scavenging activity of MBSAE and gallic acid increased significantly. The effective concentration 50 (EC50) calculated from the graphs is, respectively, 380.96 and 184.34 μg/mL for hydrogen peroxide and ABTS scavenging activities. These values are not very far from those of gallic acid (EC50 = 324.31 and 73.34 μg/mL, respectively) used as reference molecule ().
Figure 1. In vitro antioxidant and scavenging effects of MBSAE and gallic acid (GA) against ABTS radical (A) and hydrogen peroxide (B).
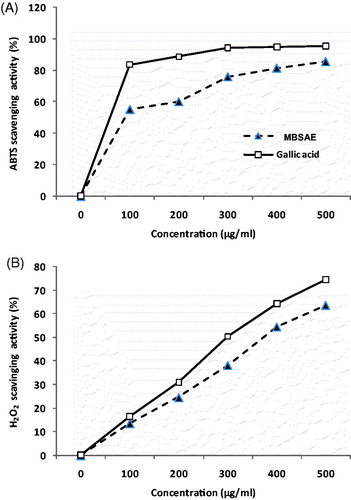
Table 1. Total polyphenols and anthocyanins contents and EC50 values of the ABTS radical as well as hydrogen peroxide of the MBSAE and gallic acid.
Effect of MBSAE on esophagitis lesions index
We firstly showed in the present study that the ligation of the pylorus and stomach for 6 h caused hemorrhagic lesions in the esophagus mucosa (). However, intragastric administration of various doses of MBSAE (25, 50 and 100 mg/kg, b.w., p.o.) significantly reduced in a dose-dependent manner the severity of esophageal lesions. The administration of famotidine or gallic acid, used as references molecules, also protected against ER-induced lesions in esophagus mucosa.
Table 2. Effect of MBSAE, famotidine (FAM) and gallic acid (GA) on ER-induced esophageal mucosa lesions.
Histopathological evaluation of esophageal damages
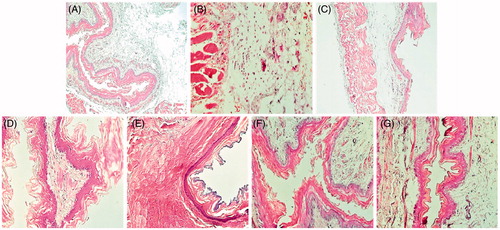
The histological observation of esophageal section showed a comparatively extensive congestion of esophageal and a small alteration of the mucosal part. MBSAE, famotidine and gallic acid administration significantly protected against ER-induced alterations in esophageal mucosa ().
Figure 2. Esophageal histology showing the protective effects of MBSAE, famotidine (FAM) and gallic acid (GA) on ER-induced histological changes in esophagus. Animals were treated with various doses of MBSAE (25, 50 and 100 mg/kg, p.o.), FAM (20 mg/kg, b.w., p.o.), GA (50 mg/kg, p.o.) or vehicle (H2O) during 6 h after ER induction. (A) Control; (B) ER; (C, D and E) ER + MBSAE (25, 50 and 100 mg/kg, p.o., respectively; (F) ER + FAM (20 mg/kg, b.w., p.o.); (G) ER + GA (50 mg/kg, p.o.).
Evaluation of pH, titratable acidity and gastric juice volume
The ER significantly decreased the pH values with an increasing volume and a titratable acidity of gastric juice. MBSAE treatment restored, significantly, the pH, the gastric juice volume and the titratable acidity in a dose-dependent manner (). However, famotidine, a standard antiulcerogenic molecule and gallic acid also protected against the disorder in the secretory profile.
Table 3. Effect of MBSAE, famotidine (FAM) and gallic acid (GA) on ER-induced changes in pH, tirable acidity and volume of gastric juice.
Effect of MBSAE on lipid peroxides in ER model
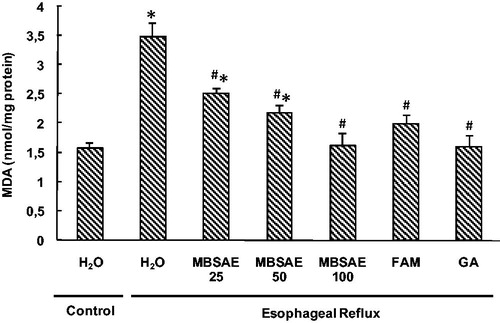
To investigate the implication of oxidative stress in the antiulcerogenic effect of MBSAE, esophageal mucosa was firstly assessed for lipid peroxidation (). As expected, the MDA levels in esophageal tissue were significantly higher in ER group. However, this increase was significant and dose-dependently attenuated by MBSAE, famotidine and gallic acid administrations.
Figure 3. Effect of MBSAE, famotidine (FAM) and gallic acid (GA) on ER-induced changes in esophageal MDA levels. Animals were treated with various doses of MBSAE (25, 50 and 100 mg/kg, p.o.), FAM (20 mg/kg, b.w., p.o.), GA (50 mg/kg, p.o.) or vehicle (H2O) during 6 h after ER induction. The data are expressed as mean ± SEM (n = 10). *p < 0.05 compared to the control group and #p < 0.05 compared to the ER group.
Effect of MBSAE on PSAs
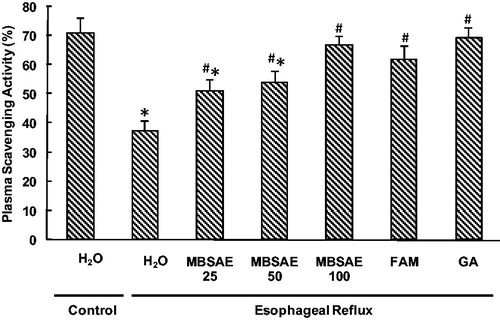
The percentages of PSAs are shown in . The ER per se was accompanied by the decrease of PSAs. However, animals treated with MBSAE showed significant and dose-dependent increases in PSAs compared to ER group. Similar effects were observed for gallic acid and famotidine used as reference molecules.
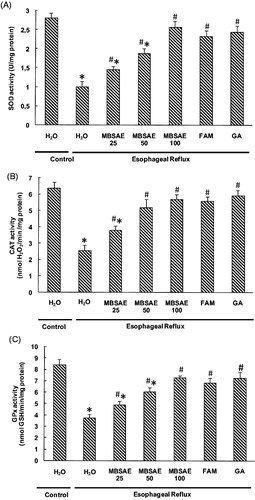
Figure 4. Effect of MBSAE, famotidine (FAM) and gallic acid (GA) on ER-induced changes in esophageal antioxidant enzymes SOD (A), CAT (B) and GPx (C). Animals were treated with various doses of MBSAE (25, 50 and 100 mg/kg, p.o.), FAM (20 mg/kg, b.w., p.o.), GA (50 mg/kg, p.o.) or vehicle (H2O) during 6 h after ER induction. The data are expressed as mean ± SEM (n = 10). *p < 0.05 compared to the control group and #p < 0.05 compared to the ER group.
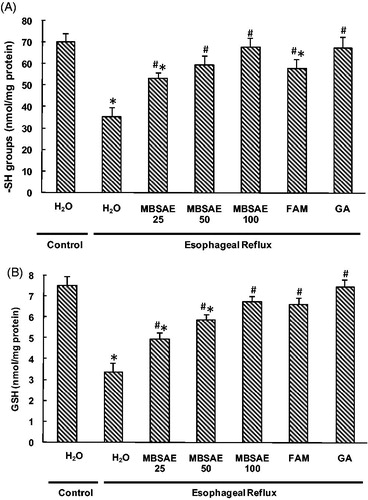
Effect of MBSAE on non-enzymatic antioxidant levels
In the present study, the effects of ER and MBSAE on esophageal non-enzymatic antioxidants levels were investigated and the results are presented in . As expected, the thiol groups and reduced GSH levels decreased significantly in ER rats compared to the control group. MBSAE (25, 50 and 100 mg/kg, b.w., p.o.) treatments improved significantly and dose-dependently the thiols groups and GSH levels. Similarly, famotidine and gallic acid administration increased the non-enzymatic antioxidants as recorded for the control levels.
Figure 5. Effect of MBSAE, famotidine (FAM) and gallic acid (GA) on ER-induced changes in esophageal non-enzymatic antioxidants levels: thiol groups (A) and reduced glutathione (B). Animals were treated with various doses of MBSAE (25, 50 and 100 mg/kg, p.o.), FAM (20 mg/kg, b.w., p.o.), GA (50 mg/kg, p.o.) or vehicle (H2O) during 6 h after ER induction. The data are expressed as mean ± SEM (n = 10). *p < 0.05 compared to the control group and #p < 0.05 compared to the ER group.
Effect of MBSAE on antioxidant enzyme activities depletion
We also studied the variation of antioxidant enzyme activities in esophagus. As shown in , ER drastically decreased the antioxidant enzyme activities such as SOD (A), CAT (B) and GPx (C). MBSAE treatment significantly reversed all ER-induced antioxidant enzymes depletion in a dose-dependent manner. Similar effects were observed for gallic acid and famotidine used as reference molecules.
Figure 6. Effect of MBSAE, famotidine (FAM) and gallic acid (GA) on ER-induced changes in PSA. Animals were treated with various doses of MBSAE (25, 50 and 100 mg/kg, p.o.), FAM (20 mg/kg, b.w., p.o.), GA (50 mg/kg, p.o.) or vehicle (H2O) during 6 h after ER induction. The data are expressed as mean ± SEM (n = 10). *p < 0.05 compared to the control group and #p < 0.05 compared to the ER group.
Effect of MBSAE on free iron H2O2 and ionisable calcium
We further looked at the effect of ER and MBSAE on intracellular mediators as hydrogen peroxide, free iron and calcium levels in esophageal tissue. As shown in , the ER increased, significantly, the levels of free iron, H2O2 and calcium. MBSAE, famotidine and gallic acid administrations protected, significantly, against ER-induced intracellular mediators disturbances in a dose-dependent manner.
Table 4. Effect of MBSAE, famotidine (FAM) and gallic acid (GA) on ER-induced changes in hydrogen peroxide, free iron and calcium levels.
Discussion
The phytochemical analysis of the MBSAE revealed the presence of total polyphenols and anthocyanins in copious amounts. Indeed, several studies conducted on the chemical composition of the seeds showed the richness of this part of plant in biologically active compounds such as phenolic compounds and polyunsaturated fatty acids with some discrepancies (Aidi-Wannes et al. Citation2011; Messaoud & Boussaid Citation2011; Benariba et al. Citation2013). These molecules attributed to MBSAE a powerful antioxidant activities, which is determined by the low EC50 value, which is defined as the concentration of the antioxidant needed to scavenge 50% of the free radicals. In this respect, our results showed a great capacity for trapping ABTS and hydrogen peroxide with lower EC50 values (184.34 and 380.96 μg/mL, respectively) but lesser than that of gallic acid (73.34 and 324.31 μg/mL respectively), used as an antioxidant reference molecule. However, due to its antioxidant properties, the myrtle berries seeds have been shown to be effective in many pharmacological and physiological situations (Aidi-Wannes et al., Citation2011; Messaoud & Boussaid Citation2011).
In vivo, we firstly showed that ER was accompanied by a decrease in pH and an increase in titratable acidity, which indicates that the used experimental ER model has been successfully established. These results are in line with previous reports (Mahattanadul et al. Citation2011; Nagahama et al. Citation2012). In this experimental model, the gastric acid secreted by parietal cells and pepsin activity in the stomach, but not intestinal alkaline content, are the most important pathogenic factors of ER (Miner Citation2006; Pawlik et al. Citation2011). However, the duodenal content such as bile, pancreatic and intestinal juice, including cholic acid, trypsin and hemolytic lecithin are also implicated in the esophageal mucosa alteration (Burnat et al. Citation2010).
MBSAE, famotidine and gallic acid administrations prevent, significantly, against pH and titratable acidity deregulation in a dose-dependent manner. Indeed, it is well known that many medicinal plants have been used in the treatment of esophageal damage by reducing gastric acidity such as Panax quinquefolium (Singh et al. Citation2012) and Morinda citrifolia (Mahattanadul et al. Citation2011).
In the present study, we also showed that ER clearly induced morphological and histopatological changes in the esophageal mucosa. Accordingly, MBSAE administration has been shown to reduce the morphological and histopathological signs of ER, by reducing the lesion index, the congestions as well as the alterations of the cell barrier in the esophageal mucosa. These results are in accordance with previous reports using others medicinal plants such as Rikkunshito, a traditional Japanese medicine (Miwa et al. Citation2010), and Morinda citrifolia (Mahattanadul et al. Citation2011).
Several pathophysiological mechanisms have been proposed to play a role in the ER, which is the most important of which is the redox imbalance status of the esophageal tissue (Oh et al. Citation2001; Chen et al. Citation2002). Within the same lines, we have shown in the present investigation that ER increased MDA levels, used as index of lipid peroxidation, and decreased the antioxidant enzyme activities of SOD, CAT and GPx. ER also induced a decrease of sulphhydrils groups and reduced GSH levels as well as PSA, which is an indicator of the generation of free radicals in tissues (Benzie & Strain Citation1996; Alimi et al. Citation2012). More importantly, MBSAE, famotidine or gallic acid administration prevent, significantly, against all changes induced by ER of gastric contents in a dose-dependent manner and return the values similar to those found in non-affected animals at the highest dose. However, it is generally accepted that ROS-mediated peroxidation of lipid structures in the tissues leading to an extensive subcellular damage and plays a major role in the pathogenesis of gastrointestinal disorders (Halliwell et al. Citation1992).
The administration of various free radical scavengers prevents damage in the esophageal mucosa and blocks free radicals derived from oxygen and/or stimulates antioxidant enzyme activities. However, various endogeneous and exogeneous scavengers are selectively used to block oxygen-derived free radicals. The superoxide anion ![]() is rapidly disproportionated by superoxide dismutase, hydrogen peroxide by CAT, hypochlorous acid and hydroxyl radicals by allopurinol (Lee et al. Citation2001). In this context, a number of previous studies have demonstrated that myrtle berry seed extracts present a good amount of antioxidant molecules (Aidi-Wannes et al. Citation2011; Messaoud & Boussaid Citation2011). However, our previous phytochemical study of MBSAE, using HPLC-PDA-ESI-MS/MS analysis, allowed us to identify 18 phenolic compounds, distributed in three major groups; hydroxybenzoic acid derivatives, anthocyanins derivatives and flavonols derivatives (Jabri et al. Citation2015). Therefore, owing mainly to their antioxidant and anti-inflammatory properties (Li et al. Citation2008), these molecules are known to exhibit many beneficial health effects such as cardio-protective, neuro-protective and anticancer activities (Cao & Prior Citation1999; Zafra-Stone et al. Citation2007).
is rapidly disproportionated by superoxide dismutase, hydrogen peroxide by CAT, hypochlorous acid and hydroxyl radicals by allopurinol (Lee et al. Citation2001). In this context, a number of previous studies have demonstrated that myrtle berry seed extracts present a good amount of antioxidant molecules (Aidi-Wannes et al. Citation2011; Messaoud & Boussaid Citation2011). However, our previous phytochemical study of MBSAE, using HPLC-PDA-ESI-MS/MS analysis, allowed us to identify 18 phenolic compounds, distributed in three major groups; hydroxybenzoic acid derivatives, anthocyanins derivatives and flavonols derivatives (Jabri et al. Citation2015). Therefore, owing mainly to their antioxidant and anti-inflammatory properties (Li et al. Citation2008), these molecules are known to exhibit many beneficial health effects such as cardio-protective, neuro-protective and anticancer activities (Cao & Prior Citation1999; Zafra-Stone et al. Citation2007).
By the end of the present study, our data also showed that the reflux of gastric contents into the esophagus compartment, significantly, increased the free iron, ionisable calcium and hydrogen peroxide levels. Both iron deficiency and iron excess can lead to cellular dysfunction, and maintaining normal iron homeostasis is therefore crucial (Andrews Citation1999). Indeed iron accumulation catalysed hydroxyl radical-mediated oxidative injury via its participation in the Fenton pathway. However, it is tempting to speculate that MBSAE is capable of chelating free iron and scavenging H2O2. The same mechanism was proposed for other plant extracts rich in phenolic compounds such as carob pod (Souli et al. Citation2015) as well as grape seeds and skin extract (Charradi et al. Citation2011). Similarly, oxidative stress status may cause an increase of calcium level in the cytoplasm of various cells (Joseph et al. Citation1997; Wang & Joseph Citation2000), this increase may be due to both the release of calcium from internal cellular organelles such as the endoplasmic or sarcoplasmic reticulum, or the import of extracellular spaces through the plasma membrane. We showed in this study that MBSAE treatment protects against calcium deregulation in ER group and restores homeostasis. However, it is tempting to speculate that MBSAE exerts in part its beneficial effect by chelating free iron and scavenging H2O2 leading to calcium homeostasis as recently described grape seeds and chamomile extracts (HamLaoui-Gasmi et al. Citation2011; Sebai et al. Citation2015).
Conclusion
We demonstrated, in the present work, that MBSAE prevents against ER-induced injury in esophageal mucosa owing in part to its antioxidant and free radical scavenging properties as well as an opposite effect on some intracellular mediators such as free iron, hydrogen peroxide and calcium. Importantly, these data confirm the use of this plant in the Tunisian traditional folk medicine for the treatment of gastrointestinal pathologies.
Declaration of interest
The authors alone are responsible for the content of this paper. Financial support of the Tunisian Ministry of Higher Education and Scientific Research is gratefully acknowledged.
References
- Aebi H. 1984. Catalase in vitro. Meth Enzymol. 105:121–126.
- Aidi-Wannes W, Mhamdi B, Sriti J, Marzouk B. 2011. Glycerolipid and fatty acid distribution in pericarp, seed and whole fruit oils of Myrtus communis var. italica. Ind Crops Prod. 31:77–83.
- Aiyer HS, Li Y, Liu QH, Reuter N, Martin RC. 2011. Dietary freeze-dried black raspberry's effect on cellular antioxidant status during reflux-induced esophagitis in rats. Nutrition 27:182–187.
- Alem G, Mekonnen Y, Tiruneh M, Mulu A. 2008. In vitro antibacterial activity of crude preparation of myrtle (Myrtus communis) on common human pathogens. Ethiop Med J. 46:63–69.
- Alimi H, Hfaeidh N, Bouoni Z, Sakly M, Ben Rhouma K. 2012. Protective effect of Opuntia ficus indica f. inermis prickly pear juice upon ethanol-induced damages in rat erythrocytes. Alcohol 46:235–243.
- Andrews NC. 1999. Disorders of iron metabolism. N Engl J Med. 34:1986–1995.
- Benariba N, Djaziri R, Bellakhdar W, Belkacem N, Kadiata M, Malaisse WJ, Sener A. 2013. Phytochemical screening and free radical scavenging activity of Citrullus colocynthis seeds extracts. Asian Pac J Trop Biomed. 3:35–40.
- Benzie IFF, Strain JJ. 1996. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 239:70–76.
- Brand-Williams W, Cuvelier ME, Berset C. 1995. Use of a free radical method to evaluate antioxidant activity. Lebenson Wiss Technol. 28:25–30.
- Burnat G, Majka J, Konturek PC. 2010. Bile acids are multifunctional modulators of the Barrett's carcinogenesis. J Physiol Pharmacol. 61:185–192.
- Cao G, Prior RL. 1999. Anthocyanins are detected in human plasma after oral administration of an elderberry extract. Clin Chem. 45:574–576.
- Charradi K, Sebai H, Elkahoui S, Ben Hassine F, Limam F, Aouani E. 2011. Grape seed extract alleviates high-fat diet-induced obesity and heart dysfunction by preventing cardiac siderosis. Cardiovasc Toxicol. 11:28–37.
- Chen X, Ding YW, Yang Gy, Bondoc F, Lee MJ, Yang CS. 2002. Oxidative damage in an esophageal adenocarcinoma model with rats. Carcinogenesis 21:257–263.
- Clark AM. 1996. Natural products as a resource for new drugs. Pharm Res. 13:1133–1144.
- Dingeon B, Ferry JP, Roullet A. 1975. Automatic assay of blood sugar by Trinder's method. Ann Biol Clin. 33:3–13.
- Draper HH, Hadley M. 1990. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 186:421–431.
- Ellman GL. 1959. Tissue sulfhydryl groups. Arch Biochem Biophys. 82:70–77.
- Flohé L, Gunzler WA. 1984. Assays of glutathione peroxidase. Methods Enzymol. 105:14–121.
- Gortzi O, Lalas S, Chinou I, Tsaknis J. 2008. Reevaluation of bioactivity and antioxidant activity of Myrtus communis extract before and after encapsulation in liposomes. Eur Food Res Technol. 226:583–590.
- Gülçin I, Alici HA, Cesur M. 2005. Determination of in vitro antioxidant and radical scavenging activities of propofol. Chem Pharm Bull. 53:281–285.
- Halliwell B, Gutteridge JM, Cross CE. 1992. Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med. 119:598–620.
- Hamlaoui-Gasmi S, Limam N, Mokni M, Limam F, Amri M, Aouani E, Marzouki L. 2011. Grape seed extract mitigates garlic-induced oxidative stress in rat spleen and plasma. J Med Plants Res. 5:6076–6084.
- Hartree EF. 1972. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 48:422–427.
- Haseeb N, John S, Gauri, Yukio K. 2006. Extraction of polyphenols from grape seeds and concentration by ultrafiltration. Sep Purif Technol. 48:176–181.
- Hassall E. 2005. Decisions in diagnosing and managing chronic gastroesophageal reflux disease in children. J Pediatr. 146:3–12.
- Hirschowitz BIA. 1991. A critical analysis, with appropriate controls, of gastric acid and pepsin secretion in clinical esophagitis. Gastroenterology 101:1149–1158.
- Hunt RH. 1999. Importance of pH control in the management of GERD. Arch Intern Med. 159:649–657.
- Inatomi N, Nagaya H, Takami K, Shino A, Satoh H. 1991. Effects of a proton pump inhibitor, AG-1749 (lansoprazole), on reflux esophagitis and experimental ulcers in rats. Jpn J Pharmacol. 55:437–451.
- Ismail-Beigi F, Horton PF, Pope CE. 1970. Histological consequences of gastroesophageal reflux in man. Gastroenterology 58:163–174.
- Jabri MA, Rtibi K, Tounsi H, Hosni K, Souli A, El-Benna J, Marzouki L, Sakly M, Sebai H. 2015. Myrtle berries seeds aqueous extract inhibits in vitro human neutrophils myeloperoxidase and attenuates acetic acid-induced ulcerative colitis in rat. Rsc Adv. 5:64865–64877.
- Joseph JA, Strain JG, Jimenez ND. 1997. Oxidant injury in PC12 cells – a possible model of calcium “dysregulation” in aging: I. Selectivity of protection against oxidative stress. J Neurochem. 69:1252–1258.
- Kawahara H, Kubota A, Hasegawa T, Phdoongsombut N, Ratanasuwon P, Kasiwong S. 2007. Effects of rikkunshito on the clinical symptoms and esophageal acid exposure in children with symptomatic gastroesophageal reflux. Pediatr Surg Int. 23:1001–1005.
- Kobayashi S, Kasugai T. 1974. Endoscopic and biopsy criteria for the diagnosis of esophagitis with a fiberoptic esophagoscope. Am J Dig Dis. 19:345–352.
- Leardi A, Caraglia M, Selleri C, Pepe S, Pizzi C, Notaro R, Fabbrocini A, De Lorenzo S, Musicò M, Abbruzzese A, et al. 1998. Desferioxamine increases iron depletion and apoptosis induced by ara-C of human myeloid leukaemic cells. Br J Haematol. 102:746–752.
- Lee J, Durst RW, Wrolstad RE. 2005. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the ph differential method: collaborative study. J AOAC Int. 88:1269–1278.
- Lee JS, Oh TY, Ahn BO, Cho H, Kim WB, Kim YB, Surh YJ, Kim HJ, Hahm KB. 2001. Involvement of oxidative stress in experimentally induced reflux esophagitis and Barrett’s esophagus: clue for the chemoprevention of esophageal carcinoma by antioxidants. Mutat Res. 480481:189–200.
- Li XL, Cai YQ, Qin H, Wu YJ. 2008. Therapeutic effect and mechanism of proanthocyanidins from grape seeds in rats with TNBS-induced ulcerative colitis. Can J Physiol Pharmacol. 86:841–849.
- Li YM, Li ZS, Zhou XP, Xie XF, Zhan XB, Tu ZX, Peng GY, Fang DC. 2002. Effect of acid inhibitor on the function and ultra structure of gastric parietal cells in rats under stress. Jiefanjun Yixue Zazhi 27:1078–1080.
- Lightdale JR, Gremse DA. 2013. Gastroesophageal reflux: management guidance for the pediatrician. Pediatrics 131:1684–1695.
- Mahattanadul S, Ridtitid W, Nima S, Phdoongsombut N, Ratanasuwon P, Kasiwong S. 2011. Effects of Morinda citrifolia aqueous fruit extract and its biomarker scopoletin on reflux esophagitis and gastric ulcer in rats. J Ethnopharmacol. 134:243–250.
- Messaoud C, Boussaid M. 2011. Myrtus communis berry color morphs: a comparative analysis of essential oils, fatty acids, phenolic compounds, and antioxidant activities. Chem Biodivers. 8:300–310.
- Messaoud C, Laabidi A, Boussaid M. 2012. Myrtus communis L. infusions: the effect of infusion time on phytochemical composition, antioxidant and antimicrobial activities. J Food Sci. 77:941–947.
- Meuwissen SGM, Klinkenberg-Knol EC. 1988. Treatment of reflux oesophagitis with H2-receptor antagonists. Scand J Gastroenterol Suppl. 146:201–213.
- Miner PB. 2006. Physiologic and clinical effects of proton pump inhibitors on non-acidic and acidic gastrooesophageal reflux. Aliment Pharmacol Ther. 23:25–32.
- Misra HP, Fridovich I. 1972. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 247:3170–3175.
- Miwa H, Koseki J, Oshima T, Kondo T, Tomita T, Watari J, Matsumoto T, Hattori T, Kubota K, Iizuka S. 2010. Rikkunshito, a traditional Japanese medicine, may relieve abdominal symptoms in rats with experimental esophagitis by improving the barrier function of epithelial cells in esophageal mucosa. J Gastroenterol. 45:478–487.
- Nagahama K, Nishio H, Yamato M, Takeuchi K. 2012. Orally administered l-arginine and glycine are highly effective against acid reflux esophagitis in rats. Med Sci Monit. 18:9–15.
- Nakamura K, Osawa Y, Furuta Y, Miyazaki H. 1982. Effects of sodium polyacrylate (PANa) on acute esophagitis by gastric juice in rats. Jpn J Pharmacol. 32:445–456.
- Oh TY, Lee JS, Ahn BO, Cho H, Kim WB, Kim YB, Surh YJ, Cho SW, Hahm KB. 2001. Oxidative damages are critical in pathogenesis of reflux esophagitis: implication of antioxidants in its treatment. Free Radic Biol Med. 30:905–915.
- Orenstein SR, Izadnia F, Khan S. 1999. Gastroesophageal reflux disease in children. Gastroenterol Clin North Am. 28:947–969.
- Pawlik M, Pajdo R, Kwiecien S, Ptak-Belowska A, Sliwowski Z, Mazurkiewicz-Janik M, Konturek SJ, Pawlik WW, Brzozowski T. 2011. Nitric oxide (no)-releasing aspirin exhibits a potent esophagoprotection in experimental model of acute reflux esophagitis. Role of nitric oxide and proinflammatory cytokines. J Physiol Pharmacol. 62:75–86.
- Pottier-Alapetite G. 1979. Flore de la Tunisie. Angiospermes, Dicotylédones Dialypé-tales. Tunis: Imprimerie officielle de la république Tunisienne.
- Sebai H, Jabri MA, Souli A, Hosni K, Rtibi K, Tebourbi O, El-Benna J, Sakly M. 2015. Chemical composition, antioxidant properties and hepatoprotective effects of chamomile (Matricaria recutita L.) decoction extract against alcohol-induced oxidative stress in rat. Gen Physiol Biophys. 34:263–275.
- Sedlak J, Lindsay RH. 1968. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 25:192–205.
- Shay H, Komarov SA, Fels SS, Mreanze D, Gruenstein M, Siplet H. 1947. A simple method for the uniform production of gastric ulceration in the rat. Gastroenterology 5:43–61.
- Siddhuraju P. 2006. The antioxidant activity and free radical-scavenging capacity of phenolics of raw and dry heated moth bean (Vigna aconitifolia) (Jacq.) Marechal seed extracts. Food Chem. 99:149–157.
- Singh P, Singh N, Sengupta S, Palit G. 2012. Ameliorative effects of Panax quinquefolium on experimentally induced reflux oesophagitis in rats. Indian J Med Res. 135:407–413.
- Souli A, Sebai H, Chehimi LRtibi K, Tounsi H, Boubaker S, Sakly M, El-Benna J, Amri M. 2015. Hepatoprotective effect of carob against acute ethanol-induced oxidative stress in rat. Toxicol Ind Health 31:802–810.
- Stren J, Lewis WH. 1957. The colorimetric estimation of calcium in serum with ocresolphthalein complexone. Clin Chim Acta 2:576–580.
- Sumbul S, Ahmad MA, Asif M, Saud I, Akhtar M. 2010. Evaluation of Myrtus communis Linn. berries (common myrtle) in experimental ulcer models in rats. Hum Exp Toxicol. 29:935–944.
- Tumen I, Senol FS, Orhan IE. 2012. Inhibitory potential of the leaves and berries of Myrtus communis L. (myrtle) against enzymes linked to neurodegenerative diseases and their antioxidant actions. Int J Food Sci Nutr. 63:387–392.
- Vandenplas Y, Rudolph CD, Di Lorenzo C, Hassall E, Liptak G, Mazur L, Sondheimer J, Staiano A. 2009. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 49:498–547.
- Wang H, Joseph JA. 2000. Mechanisms of hydrogen peroxide-induced calcium dysregulation in PC12 cells. Free Radic Biol Med. 28:1222–1231.
- Zafra-Stone S, Yasmin T, Bagchi M, Chatterjee A, Vinson JA, Bagchi D. 2007. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol Nutr Food Res. 51:675–683.
- Ziyyat A, Legssyer A, Mekhfi H, Dassouli A, Serhrouchni M, Benjelloun W. 1997. Phytotherapy of hypertension and diabetes in oriental Morocco. J Ethnopharmacol. 58:45–54.
