Abstract
Context Ficus recemosa Linn. (Moraceae) has been reported as a natural folk medicine with diverse pathological activities such as antioxidant, antidiabetic, renoprotective and cardioprotective.
Objective The present study evaluates the preventive effect of standardised ethanol extract of F. racemosa stem bark (EEFSB) on diabetic cardiomyopathy (DC) and diabetic nephropathy (DN).
Materials and methods Animals were rendered diabetic by one time administration of STZ (45 mg kg−1, i.v.) and, after 7 d, diabetic rats were randomised into four groups of eight rats each. EEFSB (200 and 400 mg kg−1) was administered to diabetic rats once daily for 8 weeks. Furthermore, the presence of phytochemicals was evaluated by HPTLC.
Results Treatment with EEFSB markedly restores the blood glucose and lipid level (p < 0.001), also reduced creatinine kinase (p < 0.001), lactate dehydrogenase (p < 0.001), C-reactive protein (p < 0.001), creatinine (p < 0.001), blood urea nitrogen (p < 0.001), collagen (p < 0.05) and albumin (p < 0.001) levels. Reduced level of sodium (p < 0.001), creatinine (p < 0.001), albumin (p < 0.001) and malondialdehyde (p < 0.01) in heart and kidney tissue along with enhanced activities of superoxide dismutase (p < 0.001) and reduced glutathione (p < 0.001). Moreover, left ventricular hypertrophic index and cardiac hypertrophic index were markedly reduced by EEFSB treatment.
Conclusion The findings of this study provided strong scientific evidence for the traditional use of F. racemosa and postulate protective effects against diabetes and its complications such as DC and DN.
Introduction
Diabetes mellitus (DM), a major growing public health disorder, is characterised by hyperglycaemia arising as a consequence of a relative or absolute deficiency of insulin secretion and/or resistance to insulin actions or both (American Diabetes Association Citation2013). It has been documented that the prevalence of diabetes was near to 171 million people in 2000 and is expected to increase up to 366 million people worldwide by 2030 (Wild et al. Citation2004). In particular, data suggest that approximately 40.9 million Indian people are affected by diabetes and these numbers are expected to be 69.9 million by 2030, thus, India will become “Diabetes Capital of the World” (Mohan et al. Citation2007). It has been suggested that diabetes affects major organ systems such as the heart, kidney and liver. In particular, the risk of developing heart failure in patients with diabetes increased 2- to 5-fold in men and women, respectively (Gilbert et al. Citation2006). Approximately, 30% of patients with type 1 diabetes develop diabetic nephropathy (DN) and 25–30% of patients with type 2 diabetes are prone to develop overt DN (Galkina & Ley Citation2006). Both diabetic cardiomyopathy and nephropathy are the most common complications and the major cause of mortality in diabetic patients. Inadequate insulin secretion and/or insulin resistance leads to abnormalities in carbohydrate, fat and protein metabolism (American Diabetes Association Citation2013). Although the mechanism of occurrence of diabetic cardiomyopathy and nephropathy is unknown, conditions such as hyperglycaemia, hyperlipidaemia and oxidative stress are believed to play an important role in the development of diabetic cardiomyopathy and nephropathy (Cai & Kang Citation2001).
Many therapeutic regimens are being used clinically such as antihyperglycaemics and antihypertensives along with lipid lowering agents to treat diabetes and its complications. However, development and progression of both nephropathy and cardiomyopathy in patients with diabetes remains a major concern for clinicians (Li et al. Citation2012). Moreover, limited tolerability and side effects confine the effectiveness of currently available antidiabetic agents. Therefore, the identification of newer pharmacological approaches to prevent, treat and cure this metabolic disorder and associated complications is of crucial importance. Natural products and/or synthetic analogues of a natural lead show utmost importance in the treatment of diabetes-associated complications. Since the dawn of history, Indian traditional practitioners have solely relied on different plant products for the treatment of diabetes and associated complications.
Ficus racemosa Linn. (Moraceae) is native to the Indian subcontinent and one of the most widely used medicinal herbs to treat diabetes (Ahmed & Urooj Citation2010a; Mollik et al. Citation2010). Ficus racemosa is well investigated for its antidiabetic (Veerapur et al. Citation2012), antioxidant (Manian et al. Citation2008), antihyperlipidaemic (Sophia & Manoharan Citation2008), antilipidperoxidative (Krishnamoorthi et al. Citation2007), cardioprotective (Ahmed & Urooj Citation2012) and renoprotective efficacy (Veerapur et al. Citation2011). Although F. racemosa has a diverse group of pharmacological actions, it has never been evaluated for its effects against diabetic complications. It seems a viable approach to search for novel remedies for any ailments from safe and effective plant sources. In the light of above background, the present study was designed to investigate the potential effect of F. racemosa in the prevention of diabetes and its associated complications.
Materials and methods
Chemicals
Streptozotocin (STZ), lupeol and thiobarbituric acid (TBA) were purchased from Sigma (St. Louis, MO). All other chemicals were of analytical grade and purchased from following companies Merck (Mumbai, India), Sisco (Mumbai, India) and Qualigens (Mumbai, India).
Plant material
Stem bark of F. racemosa was collected from Mahuva (21.0833°N, 71.8000°E) region of Bhavnagar district in Gujarat, India, in November 2011 and shade-dried. The plant materials were identified and authenticated by Dr. H. B. Singh, Chief Scientist and Head of Department of Raw Materials Herbarium and Museum, National Institute of Science Communication and Information Resources, New Delhi, India. Voucher specimen (No. SU/DPS/HERB/47) was submitted in the Department of Pharmaceutical Sciences, Saurashtra University, Rajkot, Gujarat, India, for future reference.
Animals and diet
Three-month-old male Wistar albino rats (170–200 g body weight) were obtained from central animal house, Department of Pharmaceutical Sciences, Saurashtra University, Rajkot, Gujarat, India. Animals were housed as five rats per cage under well-controlled conditions of temperature (25 ± 1 °C), humidity (55 ± 5%) and 12 h light-dark cycle (Lights on 07:30–19:30 h). Animals had free access to standard pellet diet (Amrut, Pranav Agro Industries Ltd, Vadodara, Gujarat, India) and water given ad libitum. The study was approved by the Institutional Animal Ethics Committee (Protocol approval no. SU/DPS/IAES/2012/1217) as per the guidance of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forest, Animal Welfare Division, Government of India, New Delhi.
Preparation of ethanol extract of F. racemosa stem bark (EEFSB)
The dried stem bark of F. racemosa (100 g) was coarsely powdered and passed through sieve (#60). Pulverised stem bark of F. racemosa was then defatted with petroleum ether at 60 °C for 2–3 h in a Soxhlet apparatus. Resulting residue was dried and extracted further with 95% ethanol (Nikhil et al. Citation2009). Subsequently, extract was filtered, concentrated on a rotary evaporator at 40–45 °C under negative pressure (<150 mbar) and lyophilised. A greenish-black powdered material was obtained (∼18 g) and stored in desiccators until further use. The dose of the EEFSB was selected on the bases of the literature (Veerapur et al. Citation2012).
Phytochemical screening
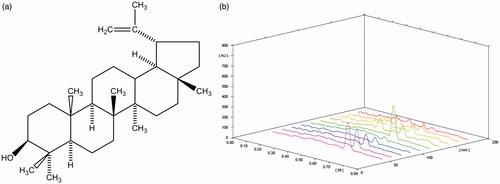
To investigate the presence of major chemical constituents such as alkaloids, glycosides, terpenoids, flavanoids, tannins and coumarins, we performed preliminary phytochemical screening of EEFSB using standard procedures and tests (Evans Citation2002). To evaluate the presence of lupeol in EEFSB, high-performance thin layer chromatography (HPTLC) was performed.
HPTLC was performed using 20 mm × 10 mm aluminium-backed plate coated with silica gel 60F254 (Merck KGaA, Darmstadt, Germany). Standard solution of lupeol and test solution of EEFSB were applied to the plates as bands of 6.0 mm wide, 14.5 mm apart and 10.00 mm distance from bottom of the plate using CAMAG (Muttenz, Switzerland) Linomat 5 equipped with 100 μL Hamilton syringe (Hamilton Company, Reno, NV). Plate was developed up to 80 mm at ambient temperature, using n-hexane:ethyl acetate (80:20 v/v) as a mobile phase in CAMAG twin trough chamber 20 × 10 cm, previously saturated with a mobile phase for 10 min. After development, plates were air dried and derivatised with p-anisaldehyde sulphuric acid reagent and incubated at 103 °C for 5 min. Plates were densitometrically scanned at 345 nm using CAMAG TLC Scanner (CAMAG, Muttenz, Switzerland) equipped with a D2 lamp and WINCAT software (CAMAG, Muttenz, Switzerland).
Induction of experimental diabetes
Diabetes was induced by single intravenous injection of STZ (45 mg kg−1), dissolved in cold citrate buffer, pH 4.5. Control animals were received only citrate buffer. After 7 d of STZ injection, animals which showed urinary glucose (Diastix, Bayer HealthCare, Thane, India) 300 mg/dL or greater were considered as diabetic and included in the present study (Zafar et al. Citation2009).
Experimental design
A total of 32 rats were used and divided randomly into four groups of eight animals each in the present study. The treatment schedule was as follows:
Group 1: Normal untreated rats
Group 2: Disease control rats
Group 3: Diabetic rats treated with EEFSB (200 mg kg−1)
Group 4: Diabetic rats treated with EEFSB (400 mg kg−1)
Animals were administered EEFSB by oral route for 8 weeks and fasting blood glucose levels were checked at the end of the experiment. Animals were kept individually in metabolic cage for urine collection over the period of 24 h to study the urinary markers after completion of experiment. Blood samples were collected in plain tubes from anaesthetised animals via retro-orbital plexus and serum was separated out for the estimation of biochemical parameters. Finally, rats were sacrificed under deep ether anaesthesia after an overnight fasting, kidneys and heart were dissected out immediately. Subsequently, isolated organs were washed in ice-cold saline, patted dry, weighed and subjected for further analysis.
Biochemical analysis
Serum parameters
Serum was prepared according to the method described by Fischer et al. (Citation1982). Fasting glucose level was estimated by the glucose oxidase–peroxidase method (Trinder Citation1969). Serum blood urea nitrogen (BUN) was estimated by Urease–GLDH methodology (Kassirer Citation1971) and albumin was estimated using the method described by Doumas et al. (Citation1971).
Lipid profile
Total cholesterol (T-C), HDL-cholesterol (HDL-C), triglyceride (TG), and LDL-cholesterol (LDL-C) were analysed as per the instruction of the manufacturer using commercial kits (Agappe Diagnostics Ltd., Kochi, India) in semi-autoanalyser (Rapid Diagnostic Pvt. Ltd., Delhi, India).
Cardiac biomarkers
Creatinine kinase (CK), lactate dehydrogenase (LDH), and C-reactive protein (CRP) were analyzed using commercial kits (Agappe Diagnostics Ltd., Kochi, India) in semi-autoanalyser.
Hypertrophic index
Left ventricular and cardiac hypertrophic index were measured according to the method described by Patel and Goyal (Citation2011) with slight modification. Briefly, the left ventricular and cardiac hypertrophic indexes were calculated by taking the ratio of weight of left ventricle and whole heart to femur length, respectively.
Urine parameter
Urinary albumin, creatinine and sodium (Na) were estimated using commercially available biochemical kits (Agappe Diagnostics Ltd., Kochi, India).
Tissue parameters
Kidney and heart homogenate of left ventricle (LV) were analysed for the estimation of total protein (Lowry et al. Citation1951), malondialdehyde (MDA) (Ohkawa et al. Citation1979), reduced glutathione (GSH) (Moron et al. Citation1979), superoxide dismutase (SOD) (Misra & Fridovich Citation1972), and collagen (Drobnik et al. Citation2009).
Histopathology
The abdomen and the thoracic cage were cut open to isolate kidneys and heart, respectively, from each animal. Isolated kidneys and heart were cleaned off from extraneous tissue and preserved in 10% neutral formalin for further analysis. One of the isolated kidney and LV of heart was then embedded in paraffin wax using conventional methods and cut into 5 μm thick sections, stained with haematoxylin and eosin (H & E) dye and finally mounted in diphenyl xylene. Sections were observed under microscope (Leica DM 2000 trinocular microscope, Leica Inc., San Diego, CA) for histopathological changes in kidney and LV of heart. A minimum of 10 fields were examined for each slide and assigned for the severity of changes using scores on a scale of none (−), mild (+), moderate (++) and severe damage (+++) (Ozgur et al. Citation2009).
Statistical analysis
The results were expressed as mean ± SEM. The statistical significance was assessed using one-way analysis of variance (ANOVA) followed by post hoc Tukey’s range test using Graph Pad Prism 5.0 (Graph Pad Software, San Diego, CA). A p-value of <0.05 was considered as statistically significant at 95% confidence level.
Results
Phytochemical screening
Phytochemical analysis of EEFSB confirmed the presence of alkaloids, glycosides, saponins, tannins and flavonoids. HPTLC chromatogram () shows the presence of phenolic compound lupeol (9.045 ± 0.074 μg/mg dried extract) in EEFSB.
Serum parameters
Effect of EEFSB on serum glucose level in diabetic rats
STZ-treated rats exhibited significantly (p < 0.001) increased levels of serum glucose as compared with control rats. Treatment with EEFSB (200 and 400 mg kg−1) showed significant (p < 0.001) reduction in glucose levels as compared with diabetic control rats ().
Table 1. Estimation of serum parameters Effect of EEFSB on serum parameters.
Effect of EEFSB in serum TG, T-C, LDL-C and HDL-C
Serum TG, T-C and LDL-C levels were significantly (p < 0.001) increased, whereas HDL-C was reduced (p < 0.001) as compared with the control group. Treatment with EEFSB (200 and 400 mg kg−1) showed significant reduction (p < 0.001) in serum TG, and LDL-C levels, whereas significantly (p < 0.001) increased HDL-C level as compared with diabetic control rats ().
Effect of EEFSB on serum CK, LDH and CRP level
In diabetic control rats, the levels of CK, LDH and CRP were significantly (p < 0.001) increased as compared with control rats (). EEFSB-treated groups (200 and 400 mg kg−1) showed considerably decreased levels of serum CK, LDH and CRP when compared with diabetic control rats.
Effect of EEFSB on serum creatinine, BUN and albumin levels
As shown in , a significant (p < 0.001) increase of serum creatinine, BUN and albumin levels was observed in STZ-treated rats as compared with control rats. Interestingly, EEFSB treatment, at both the doses 200 and 400 mg kg−1, exhibited marked reduction in serum creatinine, BUN and albumin levels.
Tissue parameters
Effect of EEFSB on MDA, SOD, GSH and collagen levels in heart
As depicted in , the diabetic control group showed significantly (p < 0.001) increased levels of MDA and collagen, whereas SOD and GSH levels were significantly (p < 0.001) decreased as compared with control animals. Administration of EEFSB (200 and 400 mg kg−1) for 60 d markedly reduced the MDA and collagen levels as well as significantly (p < 0.001) heightened the activities of endogenous antioxidants such as SOD and GSH as compared with diabetic control animals. Collagen was significantly increased in the heart of diabetic animals (p < 0.001) compared with control animals, whereas significantly decreased in EEFSB (400 mg kg−1)-treated animals (p < 0.05).
Table 2. Effect of EEFSB on tissue and urine parameters.
Effect of EEFSB on cardiac hypertrophy
As shown in , a significant (p < 0.001) increase in the left ventricular hypertrophic index and the cardiac hypertrophic index was observed in diabetic rats. EEFSB treatment significantly (p < 0.001) reduced the left ventricular hypertrophy and cardiac hypertrophy as compared with diabetic control rats.
Table 3. Estimation of hypertrophic index.
Effect of EEFSB on MDA, SOD and GSH markers in kidney tissue
As an index of oxidative stress, levels of MDA, SOD and GSH were assessed. The findings showed significant increase in MDA levels (p < 0.05) and reduction in activities of endogenous protective mechanisms, i.e. SOD (p < 0.001) and GSH (p < 0.01) in diabetic control rats. EEFSB treatment at both the doses significantly (p < 0.001) reduced MDA levels and restored SOD and GSH activities in experimental animals when compared with the diabetic group ().
Urine markers
Effect of EEFSB on urinary sodium, creatinine, and albumin levels
STZ-treated rats showed significant (p < 0.001) increase in urinary sodium, creatinine and albumin levels as compared with control rats. Treatment with EEFSB (200 and 400 mg kg−1) daily for 60 d considerably decreased urinary sodium, creatinine and albumin levels in STZ-induced diabetic rats ().
Histopathology
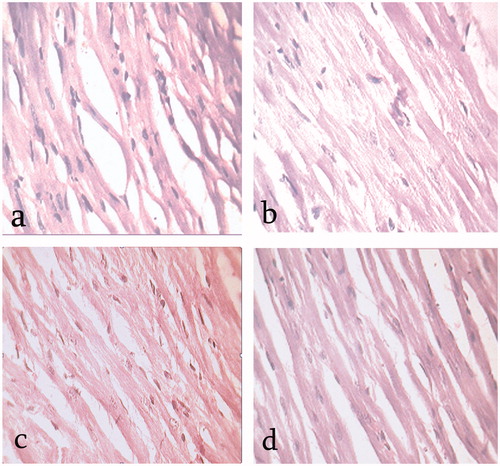
Heart
Histopathological study on the left ventricle of rat heart from each studied group was performed in order to evaluate any abnormalities in vasculature. The diabetic control group showed increased extra cellular metric and disarray of fibre (). Interestingly, EEFSB-treated (200 and 400 mg kg−1) rats showed reduced extracellular matrix and disarray of fibre (), respectively. There were no signs of abnormality in the normal control group ().
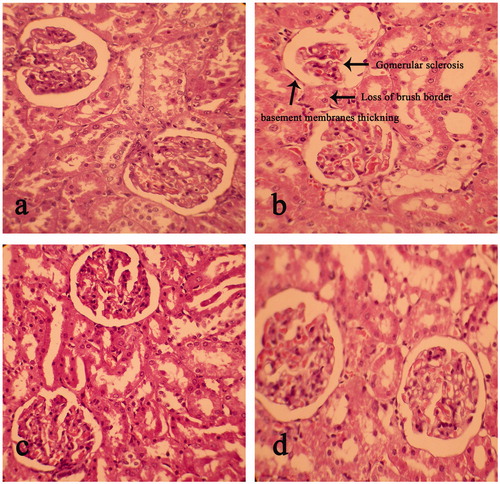
Kidney
The light microscopic observations of kidney histoarchitecture showed no abnormal features in the control group (). The diabetic control group showed signs of abnormalities in histoarchitecture of kidney tissue such as renal lesions including marked glomerular sclerosis, loss of brush border, adhesion to Bowman’s capsule as well as thickening of tubular and glomerular capillary basement membranes (). EEFSB-treated rats showed less evident histopathological abnormalities such as normal tubule, intact Bowman’s capsule and markedly reduced glomerular sclerosis in kidney tissues as revealed in .
Discussion
STZ-induced diabetes is a well-documented model of experimental diabetes and its complications. It has been widely stated that oxidative stress generated due to free radicals is involved in diabetes development by pancreatic cell destruction (Lenzen Citation2008) and manifest secondary complications in diabetic animals (Cai & Kang Citation2001; Ramkumar et al. Citation2011).
Ficus racemosa stem bark contains lupeol as a major active constituent (Khan & Sultana Citation2005). Phytochemical analysis and HPTLC profiling revealed the presence of super antioxidant lupeol as one of the active compounds in EEFSB. Lupeol, a strong natural antioxidant, decreases the peroxidation and has been documented to possess anti-diabetic, cardio-protective and reno-protective activities (Siddique & Saleem Citation2011). In harmony with this, the findings of the present study showed improved cardio-protection and reno-protection in EEFSB-treated rats. At least in part, these protective effects of EEFSB could be attributed to antioxidant activity of lupeol.
In this study, 8 weeks of treatment with 200 and 400 mg kg−1 EEFSB to diabetic rats resulted in significant reduction of fasting blood glucose level, which clearly explains the antidiabetic activity of this plant and confirmed the traditional indications (Ahmed & Urooj Citation2010b).
Free fatty acids (FFA) coming from various indigenous or exogenous sources are the principle energy substrate for heart. A significant increase in adipose tissue lipolysis produces elevated plasma levels of FFA in diabetic rats (Rodrigues et al. Citation1998). Abnormal lipid metabolism results into cardiomyopathy, where free fatty acid uptake by myocardium is indirectly proportional to myocardial dysfunction. Correction of lipid abnormalities may improve heart function in STZ-induced diabetic rats and the strategy which can counteract metabolic derangements may help to improve cardiomyopathy (Patel & Goyal Citation2011). In the present study, it was found that the oral administration of EEFSB decreases both plasma glucose levels and elevated plasma concentration of TG, T-CHO, LDL-C and improves plasma concentration of HDL-C compared with the diabetic control group, which may be a major contributing factor which slows down the progression of cardiomyopathy in diabetic rats.
Further, serum CK, CRP and LDH levels were also reported to increase in diabetic patients, and may serve as a marker for cardiovascular risk and cardiac muscular damage (Huang et al. Citation2006). Myocardial injury disintegrates contractile elements and sarcoplasmic reticulum of the cardiac myocytes thereby these enzymes leak out from cellular compartment into the plasma (Pasupathi et al. Citation2009). In context to this, STZ-induced diabetes and metabolic derangements produced detrimental elevation in CK, CRP and LDH levels in the diabetic control group, whereas these alterations were diminished in EEFSB-treated rats in a dose-dependent manner suggesting cardio-protective effect of the plant. This effect might be attributed to free radical scavenging properties of EEFSB and thereby reducing oxidative stress. Several studies reported that the collagen and protein accumulate within the interstitium leading to anatomical and physiological changes in heart (Rodrigues et al. Citation1998; Poornima et al. Citation2006). This accumulation of collagen and protein content in heart may produce cardiac stiffness and fibrosis, resulting in cardiac dysfunction (Patel & Goyal Citation2011). EEFSB significantly reduced the concentration of collagen and protein and thus, protected the heart from stiffness and fibrosis. The accumulation of collagen and protein in interstitium resulted in hypertrophy of heart which was calculated by LV weight-to-femur length ratio and heart weight-to-femur length ratio (Balakumar & Singh Citation2006). Both the number of LV hypertrophic index and cardiac hypertrophic index were significantly increased in diabetic rats compared with control rats and EEFSB showed significant reduction in both the index compared with diabetic rats.
The another life-threatening secondary complication of diabetes is nephropathy and is characterised by decreased serum albumin and significantly increased urine albumin level due to abnormal kidney function in diabetic patients (Don & Kaysen Citation2010). Albumin levels in urine serves as a marker to determine both the diagnosis of diabetic nephropathy and its progression in patients with type 2 diabetes. Persistent albuminuria signifies progressive kidney disease characterised by a relentless decline in kidney function, ultimately leading to end-stage renal disease (Parving et al. Citation2001). In the present study, EEFSB showed significant improvement in serum albumin and reduced excretion of urine albumin, which suggested improvement in the metabolism and kidney functions in diabetic rats as compared with control rats. Shahid et al. (Citation2005) reported that increased levels of BUN and creatinine in diabetes associated with hypertension are the main characteristics for the development of DN. EEFSB significantly reduced the concentration of both BUN and creatinine in plasma as well as urine, thus revealed the protective effect of EEFSB on both kidney and heart in diabetic condition.
Oxidative stress contributes the pathogenesis of DN and DC through overproduction of ROS and reduction of antioxidant enzyme activities. An imbalance between the production of ROS and antioxidants is believed to be involved in diabetes-induced renal failure (Cai Citation2006; Sayed Citation2012). In the present study, increased renal MDA, whereas decreased GSH and SOD activities were found in diabetic animals compared with the control group. Interestingly, treatment of diabetic animals with EEFSB (400 mg kg−1) significantly improved these parameters as compared with lower dose of EEFSB (200 mg kg−1). These results support antioxidant activity of EEFSB (Paarakh Citation2009), which plays a crucial role in the defence against oxygen free radicals. Further, the histopathological studies are well correlated with the above observations.
Conclusion
In conclusion, F. racemosa bark showed potent blood glucose lowering and antioxidative activity against STZ-induced diabetes and these beneficial activities may contribute to its cardio- and reno-protective properties against diabetic cardiomyopathy and nephropathy. The pronounced effect was observed at a dose of 400 mg kg−1 of EEFSB. Further studies are warranted to identify and isolate principle component of EEFSB such as lupeol and to evaluate molecular mechanism(s) for observed activity against diabetic complications.
Declaration of interest
The authors declare that they have no conflicts of interest concerning this article. The authors wish to acknowledge the financial support provided by Indian Council of Medical Research (ICMR), New Delhi (File no. 59/21/2013/BMS/TRM; IRIS no. 2012-27230).
References
- Ahmed F, Urooj A. 2010a. Traditional uses, medicinal properties, and phytopharmacology of Ficus racemosa: a review. Pharm Biol. 48:672–681.
- Ahmed F, Urooj A. 2010b. In vitro studies on the hypoglycemic potential of Ficus racemosa stem bark. J Sci Food Agric. 90:397–401.
- Ahmed F, Urooj A. 2012. Cardioprotective activity of standardized extract of Ficus racemosa stem bark against doxorubicin-induced toxicity. Pharm Biol. 50:468–473.
- American Diabetes Association. 2013. Diagnosis and classification of diabetes mellitus. Diabetes Care 36: S67–S74.
- Balakumar P, Singh M. 2006. The possible role of caspase-3 in pathological and physiological cardiac hypertrophy in rats. Basic Clin Pharmacol Toxicol. 99:418–424.
- Cai L. 2006. Suppression of nitrative damage by metallothionein in diabetic heart contributes to the prevention of cardiomyopathy. Free Radic Biol Med. 41:851–861.
- Cai L, Kang YJ. 2001. Oxidative stress and diabetic cardiomyopathy: a brief review. Cardiovasc Toxicol. 1:181–193.
- Don BR, Kaysen G. 2010. Serum albumin concentration and chronic kidney disease. US Nephrol. 5:20–27.
- Doumas BT, Watson WA, Biggs HG. 1971. Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta. 31:87–96.
- Drobnik J, Ciosek J, Slotwinska D, Stempniak B, Zukowska D, Marczynski A, Tosik D, Bartel H, Dabrowski R, Szczepanowska A. 2009. Experimental hypothyroidism increases content of collagen and glycosaminoglycans in the heart. J Physiol Pharmacol. 60:57–62.
- Evans WC. 2002. Trease and Evans' pharmacognosy. London: WB Saunders Harcourt Publishers Ltd.
- Fischer GW, Hunter KW, Wilson SR. 1982. Modified human immune serum globulin for intravenous administration: in vitro opsonic activity and in vivo protection against group B streptococcal disease in suckling rats. Acta Paediatr Scand. 71:639–644.
- Galkina E, Ley K. 2006. Leukocyte recruitment and vascular injury in diabetic nephropathy. J Am Soc Nephrol. 17:368–377.
- Gilbert RE, Connelly K, Kelly DJ, Pollock CA, Krum H. 2006. Heart failure and nephropathy: catastrophic and interrelated complications of diabetes. Clin J Am Soc Nephrol. 1:193–208.
- Huang EJ, Kuo WW, Chen YJ, Chen TH, Chang MH, Lu MC, Tzang BS, Hsu HH, Huang CY, Lee SD. 2006. Homocysteine and other biochemical parameters in Type 2 diabetes mellitus with different diabetic duration or diabetic retinopathy. Clin Chim Acta. 366:293–298.
- Kassirer JP. 1971. Clinical evaluation of kidney function–glomerular function. N Engl J Med. 285:385–389.
- Khan N, Sultana S. 2005. Chemomodulatory effect of Ficus racemosa extract against chemically induced renal carcinogenesis and oxidative damage response in Wistar rats. Life Sci. 77:1194–1210.
- Krishnamoorthi V, Divianathan S, Subramanian B, Manoharan S. 2007. Antihyperglycemic and antilipidperoxidative effects of Ficus racemosa (Linn.) bark extracts in alloxan induced diabetic rats. J Med Sci. 7:330–338.
- Lenzen S. 2008. Oxidative stress: the vulnerable beta-cell. Biochem Soc Trans. 36:343–347.
- Li B, Liu S, Miao L, Cai L. 2012. Prevention of diabetic complications by activation of Nrf2: diabetic cardiomyopathy and nephropathy. Exp Diabetes Res. 2012:216512.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem. 193:265–275.
- Manian R, Anusuya N, Siddhuraju P, Manian S. 2008. The antioxidant activity and free radical scavenging potential of two different solvent extracts of Camellia sinensis (L.) O. Kuntz, Ficus bengalensis L. and Ficus racemosa L. Food Chem. 107:1000–1007.
- Misra HP, Fridovich I. 1972. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 247:3170–3175.
- Mohan V, Sudha V, Radhika G, Radha V, Rema M, Deepa R. 2007. Gene-environment interactions and the diabetes epidemic in India. Tai ES, Gillies PJ, editors. 1st ILSI International Conference on Nutrigenomics: Nutrigenomics - Opportunities in Asia. Forum of nutrition. Vol. 60. Singapore: Karger. p. 118–26.
- Mollik MAH, Hossan MS, Paul AK, Taufiq-Ur-Rahman M, Jahan R, Rahmatullah M. 2010. A comparative analysis of medicinal plants used by folk medicinal healers in three districts of Bangladesh and inquiry as to mode of selection of medicinal plants. Ethnobot Res Appl. 8:195–218.
- Moron MS, Depierre JW, Mannervik B. 1979. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 582:67–78.
- Nikhil KS, Yatindra K, Seema P, Thakur RN, Sudhir SG. 2009. Antidiabetic potential of alcoholic and aqueous extracts of Ficus racemosa Linn. bark in normal and alloxan induced diabetic rats. Int J Pharm Sci Drug Res. 1:24–27.
- Ohkawa H, Ohishi N, Yagi K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 95:351–358.
- Ozgur O, Akalin PP, Nuri B, Fatih H. 2009. Pathological changes in the acute phase of streptozotocin-induced diabetic rats. Bull Vet Inst Pulawy. 53:783–790.
- Paarakh PM. 2009. Ficus racemosa Linn. – an overview. Nat Prod Radiac. 8:84–90.
- Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P. 2001. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 345:870–878.
- Pasupathi P, Rao YY, Farook J, Saravanan G, Bakthavathsalam G. 2009. Oxidative stress and cardiac biomarkers in patients with acute myocardial infarction. Eur J Sci Res. 27:275–285.
- Patel SS, Goyal RK. 2011. Prevention of diabetes-induced myocardial dysfunction in rats using the juice of the Emblica officinalis fruit. Exp Clin Cardiol. 16:87–91.
- Poornima IG, Parikh P, Shannon RP. 2006. Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res. 98:596–605.
- Ramkumar KM, Vanitha P, Uma C, Suganya N, Bhakkiyalakshmi E, Sujatha J. 2011. Antidiabetic activity of alcoholic stem extract of Gymnema montanum in streptozotocin-induced diabetic rats. Food Chem Toxicol. 49:3390–3394.
- Rodrigues B, Cam MC, McNeill JH. 1998. Metabolic disturbances in diabetic cardiomyopathy. Mol Cell Biochem. 180:53–57.
- Sayed AA. 2012. Ferulsinaic acid modulates SOD, GSH, and antioxidant enzymes in diabetic kidney. Evid Based Complement Alternat Med. 2012:580104.
- Shahid SM, Jawed M, Mahboob T. 2005. Ionic and allied variations in normotensive and hypertensive diabetic patients. J Pak Med Assoc. 55:153–158.
- Siddique HR, Saleem M. 2011. Beneficial health effects of lupeol triterpene: a review of preclinical studies. Life Sci. 88:285–293.
- Sophia D, Manoharan S. 2008. Hypolipidemic activities of Ficus racemosa Linn. bark in alloxan induced diabetic rats. Afr J Tradit Complement Altern Med. 4:279–288.
- Trinder P. 1969. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol. 22:158–161.
- Veerapur VP, Prabhakar KR, Thippeswamy BS, Bansal P, Srinivasan KK, Unnikrishnan MK. 2012. Antidiabetic effect of Ficus racemosa Linn. stem bark in high-fat diet and low-dose streptozotocin-induced type 2 diabetic rats: a mechanistic study. Food Chem. 132:186–193.
- Veerapur VP, Thippeswamy BS, Prabhakar KR, Nagakannan P, Shivasharan BD, Bansal P, Sneha SD, Mishra B, Priyadarsini KI, Unnikrishnan MK. 2011. Antioxidant and renoprotective activities of Ficus racemosa Linn. stem bark: bioactivity guided fractionation study. Biomed Prev Nutr. 1:273–281.
- Wild S, Roglic G, Green A, Sicree R, King H. 2004. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053.
- Zafar M, Naqvi S, Ahmed M, Kaimkhani ZA. 2009. Altered kidney morphology and enzymes in streptozotocin induced diabetic rats. Int J Morphol. 27:783–790.