Abstract
Context Worldwide ethnobotanical research has shown the importance of home gardens as sources of medicinal plants. These resources are worthy of further study in the Argentinean Atlantic Forest due to the richness of medicinal flora and their importance for local people.
Objective We studied richness, composition, cultural importance and medicinal uses of plants in home gardens of rural, semirural and urban areas in the Iguazú Department (Misiones, Argentina). Our hypothesis claims that people living in different environments have a similar array of medicinal plants in their gardens and they use them in a similar way.
Materials and methods The analysis was based on 76 interviews and plant inventories of home gardens. During guided walks in gardens, voucher specimens were collected. To analyse composition, Simpson similarity index was applied and a new index was proposed to measure culturally salient species.
Results All the environments had similar species composition with species differing in less than 30% of them. The most culturally salient taxa were Mentha spicata L. (Lamiaceae), in rural, Artemisia absinthium L. (Asteraceae), in semirural, and Aloe maculata All. (Xanthorrhoeaceae), in urban areas. The body systems treated with medicinal plants were similar across study sites.
Discussion The results suggest a “core repertoire” of medicinal plants and a widespread exchange of plants among local population. The cultural importance index informs us about plant adaptability, based on the efficacy and the versatility of medicinal resources.
Conclusion In this changing context where mobility and migrations constitute everyday life, medicinal plants in home gardens are part of local healthcare sovereignty.
Introduction
Home gardens are one of the oldest forms of land use, as old as shifting cultivation systems according to some authors. Kumar and Nair (Citation2004) made an important summary of the state of knowledge of the home gardens in tropical environments, and state that these places include a multi-story combination of trees and crops intensively managed, and which accomplishes different uses for family needs. These systems usually have a very important role in subsistence and sovereignty for people who manage them, since they are culturally accurate source of food, medicine and other relevant resources (Montagnini Citation2006).
Home gardens are acknowledged for their role in the maintenance of local biodiversity (Kumar & Nair Citation2004; Pulido et al. Citation2008) by high levels of diversity, which they house, mainly in the humid tropics (Fernandez & Nair Citation1986; Altieri & Merrick Citation1987; Montagnini Citation2006). The multipurpose character of home gardens is very frequent, as are multi-strata combinations, which in time, result in an evolving landscape, where each home garden represents a dynamic system of constantly evolving practices rooted in the knowledge of natural environment and oral traditions of different cultures (Altieri & Toledo Citation2011).
Home gardens also provide a wide range of ecosystem services, which differ from those supplied by other types of agroecosystems (Calvet-Mir et al. Citation2012). Taking this into account, Perfecto and Vandermeer (Citation2008) proposed to include home gardens into the land sharing strategy for biodiversity conservation.
Pochettino et al. (Citation2012) have analysed these multipurpose strategies, including diversity in species, varieties and uses for Argentinean home gardens. These authors compare home gardens from a traditional context and urban-pluricultural ones, concluding that home gardens vary in materiality and spatiality, and represent a dynamic place for experimentation, as well as reservoirs of plant varieties with different degree of association with human beings.
Home gardens importance as a source of medicinal plants
Many ethnobotanical studies have demonstrated that more plants are used for medicinal purposes than for any other objective in most of the rural societies around our globe (Bennett & Prance Citation2000). The growing attention paid by ethnobotanists to urban areas in the last 20 years has clearly contributed to the acknowledgement of the importance of medicinal plants for urban dwellers, too. These plants are purchased in health stores, markets and ethnic shops, as well as cultivated in home gardens, urban allotments and, in some cases, also foraged on free scraps of land (Corlett et al. Citation2003; Pieroni & Vandebroek Citation2007; Almada Citation2010; Hurrell Citation2014).
Many pieces of research have shown the importance of home gardens as a source of medicinal plants. However, this pattern of using household space for the cultivation of medicinal species is not uniform among ethnic groups inhabiting different environments of the globe. For instance, in central Europe, researchers have observed a varied approach towards medicinal plant management in both rural and urban gardens. In Tyrol mountain region in Austria, Vogl-Lukasser and Vogl (Citation2004) registered 79 species used as medicines in Alpine rural home gardens. Kołodziejska-Degórska (Citation2008) highlight the same important role of medicinal plants grown in home gardens among Polish migrants living in Romanian Carpathian region. In contrast, in Poland, there is no such tradition to cultivate medicinal plants. Recent studies in Polish rural home gardens reported a very limited number of medicinal species cultivated in such gardens (Bach & Bałdysiak Citation2008; Dzięciołowska & Latkowska Citation2009).
Ethnobotanical studies on home gardens in the Spanish Catalan mountain region had a pioneering character within the whole of Europe. In their seminal work, Agelet et al. (Citation2000) claimed that the structure of home gardens in the Catalan region had not changed since medieval times. They found that, apart from food production, home gardens were oriented towards the cultivation and the management of medicinal plants. However, Agelet et al. (Citation2000) and other scholars conducting their research in the American northern hemisphere have observed a decreasing number and role of medicinal plants in home gardens, especially from the 1950s onward (Rico-Gray et al. Citation1990; Caballero Citation1992).
Researchers working in South America find a different situation. Medicinal plants grown in home gardens and around the house in this part of the world are widely employed in home phytotherapy by mestizos and Indigenous people living in rural areas (Finerman & Sackett Citation2003). For some Indigenous groups, medicinal plants now have a greater importance than in the past, due to social changes. For example, Heckler (Citation2007), in her study of the role of phytotherapy among the Piaroa from Venezuela, describes the cultural change in this Amazonian group, in which the role of shamanic cures has decreased in favour of biomedicine and general symptom-specific treatment. In this new medical context, the Piaroa have shown a greater acceptance of medicinal plants, which they obtained from the neighbouring mestizo group. The great majority of these resources represent exotic species (Heckler Citation2007).
In the Atlantic Forest, the most advanced studies dedicated to the role of medicinal plants in home gardens have been carried out in Brazil (Eichemberg et al. Citation2009) and partly in Paraguay (Soria & Basualdo Citation2005) among the mestizo population. There is still a lack of similar studies focused on the composition, richness, cultural importance and medicinal uses of plants grown in home gardens among the inhabitants of Misiones, Argentina, which forms a part of the Atlantic Forest of the upper Paraná basin.
The importance of medicinal plants in the Misiones province and Atlantic Forest ecoregion
The importance of medicinal plants in Misiones is worth studying for two basic reasons: the richness of the medicinal flora found in this region and the relevance of medicinal botanical resources for Indigenous and mestizo people, as well as for European immigrants living in rural parts of Misiones. Amat and Yajía (Citation1998) counted 282 species of medicinal plants occurring there. Moreau (Citation2006) added 30 species grown in the forest to the list, in the north-eastern region of the province among the multiethnic population. Keller (Citation2008) counted nearly 400 species applied in ethnomedicine by the Mbya Guarani people. Kujawska and Hilgert (Citation2014) presented a list of 129 botanical species applied in home phytotherapy by Polish migrants and their descendants in northern Misiones. Therefore, more than 10% of the total flora of Misiones, which accounts for 3000 species, are used in the complementary medicine by different ethnic groups (Keller & Romero Citation2006; Moreau Citation2006; Keller Citation2008; Kujawska et al. Citation2012; Kujawska & Hilgert Citation2014). Although the published material shows that medicinal plants form an integral part of everyday life for the local population and are the base for home therapies, we still know very little about how these groups manage medicinal plants. Studies of other parts of the Atlantic forest ecoregion point to similar tendencies and highlight the role of medicinal plants in the home phytotherapy of mestizo people from Brazil (Caiçaras) (Begossi et al. Citation2002; Giraldi & Hanazaki Citation2010; Bolson et al. Citation2015) and Paraguay (Soria & Basualdo Citation2005; Moreno Citation2007), the majority of whom are small farmers. The latter author wrote to this respect “one of the cultural characteristics …[that] is most remarkable about Paraguayans of all ages and social conditions – the daily consumption of medicinal plants. These plants provide health care, nutrients, refreshment and savings on bus fare and visits to doctors” (Moreno Citation2007).
External and internal migrations and their influence on plant knowledge, cultivation and exchange
In 2007, the global urban population was, for the first time in history, bigger than the rural population (United Nations Population Fund Citation2007). Migrations from rural to urban areas have an important impact on forests and rural environments (Izquierdo et al. Citation2008; Padoch et al. Citation2008; Lee et al. Citation2012) resulting in different landscapes, due to political and economic strategies combined with sociocultural factors (Jordan et al. Citation2009). As Pieroni and Vandebroek (Citation2007) observed, when people migrate, they do not come to a new place empty handed. They bring knowledge about the use of certain species along with them and, if circumstances permit, also seeds and seedlings to propagate in the new place (Nazarea Citation2005). Other researchers also suggested that during the migratory process people tend to recreate culturally salient species (Corlett et al. Citation2003; Nesheim et al. Citation2006), and preserve part of their native herbal landscape (Sõukand & Kalle Citation2010).
Misiones exhibits the same tendencies observed in the neighbouring Brazil (Padoch et al. Citation2008), Paraguay (Palau Citation1998), and generally worldwide, i.e., the constant and steady flow of rural populations towards urban centres (Izquierdo et al. Citation2008). Nevertheless, in Misiones, the change in the rural population varies between the province departments. Between 1991 and 2001, the rural population decreased in 10 departments, while it increased in seven departments. The department of Iguazú, our study area, has gained urban population in the period of the last three decades. Although the urban area in Misiones increased over the decades, it occupied only 1% of the province by 2006 (Izquierdo et al. Citation2008).
Some researchers have proposed that rural home gardens have different purposes, goals and usages from urban ones, which results in different garden compositions (Mosina et al. Citation2014). However, in our study area, and taking medicinal species into account, we suggest that this differentiation will not be observed, due to the constant interaction between people from rural and urban environments, and the general importance of medicinal species for people of the Misiones province. To examine this statement, we put forward the following objectives and hypotheses. Our objectives were to study the (1) richness, (2) composition, (3) cultural importance, and (4) medicinal uses of plant species in rural, semirural and urban areas in the Iguazú Department of Misiones province, Argentina. For all these objectives, we postulate the leading hypothesis which claims that rural, semirural and urban home gardens in northern Misiones share their richness, composition and culturally important medicinal species, as well as the medicinal uses ascribed to them. Therefore, we expect to find high levels of similarity between these three environments.
Materials and methods
Study area and its inhabitants
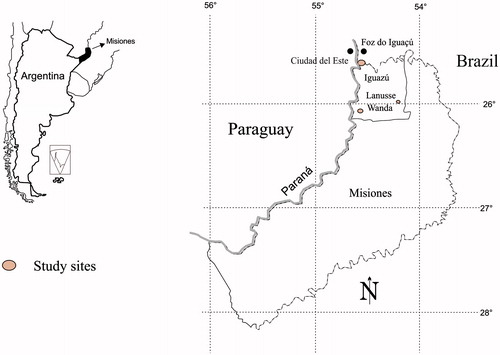
The research took place in the Department of Iguazú, the northwestern part of the province of Misiones, bordered in the north and west by Brazil and Paraguay, respectively. Misiones is one of the smallest Argentinean provinces, but at the same time, it is the home to great plant diversity (Placi & Di Bitetti Citation2006). This region is also very complex in socio-cultural terms. Up to 1767, it was part of the “theocratic empire” of the Jesuit missions, who gave the province its modern name “Misiones”. With the expulsion of the Jesuits in 1767, the indigenous Guarani people practically abandoned the region of present-day Misiones. Throughout the nineteenth century, this was an area used for logging, yerba mate [Ilex paraguariensis A.St.Hil. (Aquifoliaceae)] extraction and livestock pasturing in the south. Misiones has a longstanding migrant history stretching back over a century. Pathways for immigrant settlements were created first to rural areas, and later to urban centres. In 1897, the first European immigrants arrived in the south of Misiones. They were of Polish and Ukrainian origin (Bartolomé Citation1982). The process of populating the province with European peasant families continued until the 1940s, varying in character but the settlement basically relied on an ethnic pattern (Ferrero Citation2005). The northern part of Misiones began to be populated by European migrant families in the 1920s and 1930s (Kraustofl, Citation2011). Since the beginning of the twentieth century, northern Misiones has also received a steady flow of Paraguayan and Brazilian migrants. Most of the Paraguayan migrants have been criollos, of mixed European and Indigenous origin, who spoke Guarani as their first language. At the same time, Brazilians who settled in Misiones were both of European and criollo origin (Zamudio et al. Citation2010). The organised migration of European people stopped in the 1940s, while the flow of criollos from neighbouring countries has persisted until now. The main economic activity in the Iguazú Department is tourism, as well as forestry, agriculture and, to a lesser extent, cattle breeding. Forestry is based on monoculture plantations of exotic species for the paper and timber industries (Schiavoni Citation1998). The main cash crop of the department is yerba mate (Ilex paraguariensis); and manioc [Manihot esculenta Crantz (Euphorbiaceae)] and maize [Zea mays L. (Poaceae)] are the staples.
Our research was conducted in two towns – Puerto Iguazú and Wanda – both with semirural areas on the outskirts; and in one rural location – Colonia Gobernador J. J. Lanusse (hereinafter, Lanusse for short) (). Puerto Iguazú has 32 038 inhabitants, according to the National Census INDEC (Citation2001). It is expected that this number has increased, as it is a city which attracts people who are looking for work (Barreto Citation2002). Wanda has a population of 12 779 (INDEC Citation2001). There is no official information about the number of dwellers in Lanusse, but according to unofficial estimates, there are 150 inhabitants living there. Both Wanda and Lanusse were founded as rural colonies in 1936, and they were populated mainly with Polish migrants and to a lesser extent with Ukrainian, Belarusian and Czech families. Wanda transformed into a municipal town in the 1960s, while Lanusse has conserved its rural character. Today all three locations are inhabited by Argentinian criollos, descendants of European migrants, Brazilian and Paraguayan migrants, and in the case of Iguazú and Lanusse, inhabitants are also Indigenous Mbya Guarani people, resulting in a multicultural environment.
There is no formal market of medicinal plants in the area, but exchange of these resources is frequent within informal circuits. In this part of Misiones, limited access to medicinal plants from the market makes the local populations from both rural and urban areas more reliant on cultivated, protected and gathered plant species than on purchased plants. The freshly cut plants obtained from other people are incorporated directly into home medicine, or are dried and stored, while seeds and seedlings are planted in home gardens. The transplantation of medicinal plants from other habitats to home gardens is frequent too (Moreau Citation2006; Keller Citation2008; Hilgert et al. Citation2014).
Interviewing methods
Study participants were recruited from criollos and descendants of Polish migrants, while three interviewees were directly of Polish origin. Altogether, we interviewed 76 study participants in the period between 2009 and 2013, from whom we obtained written and oral informed consent, according to their literacy level. Forty of these study participants came from the urban centres of Puerto Iguazú and Wanda (23 and 18, respectively); 20 interviewees lived in semirural areas mainly on the outskirts of Puerto Iguazú and Wanda (7 and 13, respectively), while 15 informants were from Lanusse and the rural areas surrounding Wanda (Puerto Wanda and Esperanza Centro). In total, we collected information from 61 women and 15 men, distributed as follows: (1) urban: 35 women, six men, (2) semirural: 14 women, six men, and (3) rural: 11 women and four men.
The interviews were based on medicinal plant inventories carried out in 76 home gardens. We collected the information this way to understand the current practices related to medicinal plant cultivation in home gardens, the corresponding knowledge and uses. This method, accompanied by detailed information about informant sampling, allows conducting diachronic/return research with a long-time perspective.
During guided walks in gardens, we collected voucher specimens of all the species that could not be identified on the spot or were common cultivars. The voucher specimens were identified by the authors (V. F. and M. K.) and stored in the herbaria of Instituto de Biología Subtropical in Puerto Iguazú and in Instituto de Botánica del Nordeste en Corrientes, Argentina. Plant scientific name and author names were verified using the Plant List (http://www.theplantlist.org/).
Data analysis
The richness of medicinal species was measured as the total number of species registered in the home gardens of each area. The mean of medicinal species per garden per area was estimated for each locality. The minimum and the maximum in each locality were taken into account in order to demonstrate the variation between home gardens. The similarity was determined according to the Simpson similarity index: I = Nc/N1, where Nc is the number of taxa shared by two localities and N1 is the number of taxa of the less diverse locality. Since Simpson similarity index allows us to compare two settings at the time, the index determination was made for each possible comparison. That means rural with semirural comparison, rural with urban comparison and semirural with urban comparison.
In order to measure culturally salient species in the three study areas: rural, semirural and urban, we chose to combine two indices: relative importance index (RI) proposed by Bennett and Prance (Citation2000) and relative frequency of citation index (RFC), according to Tardío and Pardo de Santayana (Citation2008). Data on medicinal uses were ordered according to the pharmacological properties attributed to each taxon and to the specific body system treated (Bennett & Prance Citation2000). The relative importance index was designed to measure medicinal plant versatility and takes into account two factors: the relative number of body systems (RelBS) treated with a given plant taxon and the relative number of pharmacological properties (RelPH) ascribed to this species. The frequency of citations for a given species is defined as the number of informants who mentioned a useful species in each study place: rural, semirural and urban. The relative frequency of citations (RFC) is obtained by dividing the frequency of citations by the total number of participants in the survey (Tardío & Pardo de Santayana Citation2008). In our case, we calculated RFC for each study area. Therefore, the formula for our lineal index of cultural importance (CI) is as follows: [(RelPH + RelBS)/2 + RFC]/2 × 100. The maximum value that a species can obtain within this index is 100. In , plants with scores from 58 above are listed, achieved within these indices (RI, RFC and CI). Fifty-eight was considered the threshold score in this analysis.
We also performed a qualitative analysis of the medicinal uses ascribed to plants in the three study areas, in order to see if people in these places use medicinal plants for similar or different ailments and illnesses. Medicinal uses were also compared between the environments with the Shannon–Wiener Index and a t-test according to Hutcheson (Moreno Citation2001).
Results
General findings
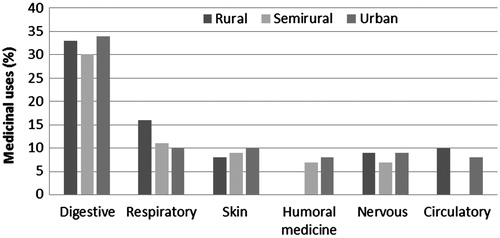
All home gardens were multipurpose, fulfilling the productive, recreational and aesthetic needs of local people in the three study areas. These home gardens had an array of medicinal plants, which grew between other plants that served different functions. presents general data on the medicinal plants used in the three environments. The richness of these plants is greater in rural and semirural areas. The mean number of medicinal species per garden is similar for rural and semirural environments, and less than half that of urban environments. There is a wide range of variations between the garden with the least and the greatest number of medicinal plants. Rural and semirural areas shared the same minimum (five species) and a maximum of 38 and 35, respectively. Urban gardens had a minimum of one and maximum of 19 medicinal taxa. The number of medicinal uses ascribed to plants is also higher in rural and semirural areas; however, the number of medicinal uses per species and the number of body systems treated with medicinal plants are similar among the three study settings.
Table 1. Medicinal plants and their uses in the home gardens of three study areas: rural, semirural and urban.
Plant composition analysis
The Simpson similarity index showed that all the environments had more than 70% of shared species (). This level of similarity indicates that the studied gardens, despite environmental differences, are quite homogeneous (similar array of medicinal plants is preserved). Similarity is ascendant, from rural compared with urban areas, to rural with semirural areas, and the highest similarity is between semirural and urban areas.
Table 2. Composition analysis, number of shared and exclusive species from each environment and similarity index between them (%).
Table 3. The most important species in the three areas, according to three indices: RI, RFC and CI.
The home garden composition includes plants from the native forest and native ruderal species, as well as cultivated plants introduced from other environments and ruderal species associated with anthropic areas all around the world. In rural and urban environments, the number of introduced medicinal species is larger than the number of native ones. Only semirural areas had a higher number of native species in comparison with exotic ones.
We find more native species exclusive to rural and semirural environments than to urban areas. In fact, all the native species from urban areas are shared with semirural environments. Therefore, the studied home gardens stock medicinal plants, which are related either to cosmopolitan knowledge or to tradition. For example, among the introduced exclusive species in the rural area there are Allium cepa L. (Amaryllidaceae) “cebolla”, Beta vulgaris L. (Amaranthaceae) “remolacha”, Brugmansia suaveolens (Humb. & Bonpl. ex Willd.) Bercht. & J. Presl (Solanaceae) “floripón”, Lactuca sativa L. (Asteraceae) “lechuga”, Malva parviflora L. (Malvaceae) “malva”, Melia azedarach L. (Meliaceae) “paraíso” and Tanacetum vulgare L. (Asteraceae) “catinga de mulata”. The native species: Bauhinia microstachya (Raddi) J.F. Macbr. (Fabaceae) “escalera de mono”, Campomanesia guazumifolia (Cambess.) O. Berg (Myrtaceae) “siete capotes”, Eugenia pyriformis Cambess. (Myrtaceae) “uvajay”, Jacaranda micrantha Cham. (Bignoniaceae) “caroba”, Lippia brasiliensis (Link) T.R.S. Silva (Verbenaceae) “yateí caá”, Panicum tricholaenoides Steud. (Poaceae) “cola de caballo”, Persicaria punctata (Elliott) Small (Polygonaceae) “caá tai”, Plinia peruviana (Poir.) Govaerts (Myrtaceae) “jabuticaba” and Sambucus australis Cham. & Schltdl. (Adoxaceae) “sauco” are all exclusive to rural environments.
Semirural environments have less exclusive medicinal species than rural ones. In this case, we found four introduced species: Artemisia vulgaris L. (Asteraceae) “artemisa”, Borago officinalis L. (Boraginaceae) “borraja”, Calendula officinalis L. (Asteraceae) “caléndula” and Pimpinella anisum L. (Apiaceae) “anís”, and eight native species: Achyrocline flaccida (Weinm.) DC., (Asteraceae) “marcela”, Achyrocline satureioides (Lam.) DC. (Asteraceae) “marcela”, Lippia turbinata Griseb. (Verbenaceae) “poleo”, Parietaria debilis G.Forst. (Urticaceae) “caá piky”, Rollinia salicifolia Schltdl.(Annonaceae) “araticú”, Smilax cognata Kunth. (Smilacaceae) “zarzaparrilla”, Solanum sisymbriifolium Lam. (Solanaceae) “espina colorada” and Tabernaemontana catharinensis A.DC. (Apocynaceae) “horquetero”. Finally, in urban home gardens, the exclusive medicinal species observed were all introduced: Cinnamomum camphora (L.) J. Presl. (Lauraceae) “alcanfor”, Eucalyptus cf. saligna (Myrtaceae) “eucalipto”, Eucalyptus globulus Labill (Myrtaceae) “eucalipto”, Ocimum basilicum L. (Lamiaceae) “albahaca”, Rosa spp. (Rosaceae) “rosa” and Tradescantia pallida (Rose) D.R. Hunt (Commelinaceae) “penicilina”.
The most salient medicinal plants
Among the most versatile species, which cure the highest number of ailments and have the greatest number of pharmacological actions, only Mentha spicata, “menta” was present in all three study settings. Semirural and urban areas also shared Matricaria chamomilla L. (Asteraceae) “manzanilla” as the most versatile taxon. The remaining species mentioned in each study area were different. In the rural area, the most versatile species – apart from mint –were Citrus and Allium species – both introduced, and one native – Sida cordifolia L. (Malvaceae) “malva blanca”. In the semirural areas, the most versatile species were two introduced species, Matricaria chamomilla and Artemisia absinthium L. (Asteraceae) “ajenjo”, and one native species belonging to Plantago genus “llantén”. In the urban area, the most versatile species were introduced species too, but partly different from those in semirural areas. Here the most versatile taxa were Aloe maculata All. (Xanthorrhoeaceae) “aloe hoja ancha”, Cymbopogon citratus (DC.) Stapf (Poaceae) “cedrón” and Zingiber officinale Roscoe (Zingiberaceae) “jengibre”, and only one native botanical: Piper mikanianum (Kunth) Steud (Piperaceae) “pariparoba” ().
When we merged the three study areas together, Aloe maculata scored the highest number of citations (29), followed by Allophylus edulis (A. St.-Hil., A. Juss. & Cambess.) Radlk., (Sapindaceae) “cocú”, and Artemisia absinthium, which achieved the same number of citations (25). In the three study settings, we observed slightly greater similarities in the most frequently cited species than in references to the most versatile species. Artemisia absinthium and Eugenia uniflora L., (Myrtaceae) “pitanga” were two of the most frequent species in all areas. Aloe maculata and Allophylus edulis were among the most frequently cited species in semirural and urban areas, and the native Lippia alba (Mill.) N.E. Br. ex Britton & P. Wilson (Verbenaceae) “salvia” was also frequently mentioned in both rural and urban areas. Nonetheless, each study area conserved its particularities. Matricaria chamomilla was cited as one of the plants with the highest frequency only in the rural area, while Aloysia polystachya (Griseb.) Moldenke (Verbenaceae) “burrito” was among the most cited species only in the semirural area, and Aloe arborescens Mill. (Xanthorrhoeaceae) “aloe fino” gained importance only in the urban setting.
When we combined the above-mentioned values, the relative importance (plant versatility) and the relative frequency of citations, we obtained the most culturally prominent species in each study area (). Mentha spicata was the most culturally salient specie in the rural area, Artemisia absinthium in the semirural area and Aloe maculata in the urban area. All these species are introduced in northern Misiones. However, M. spicata was mentioned as one of the most culturally important species in all study areas, and Aloe maculata was shared between the semirural and the urban area. Other culturally important species were particular and unique to each of the study settings.
Medicinal uses
In total, 17 body systems were treated with medicinal plants in the three study areas. The number of body systems treated in each study area was 14 for rural, 17 for semirural and 15 for urban. These values are very similar across study sites and show that, in all these places, the number of body systems treated reached saturation point. Digestive and respiratory ailments prevailed, followed by skin and nervous system, in different orders of importance. In both rural and urban areas, interviewees included circulatory illnesses in the group of most important ailments, and in semirural and urban areas, study participants considered humoral medicine syndromes as the most relevant health condition ().
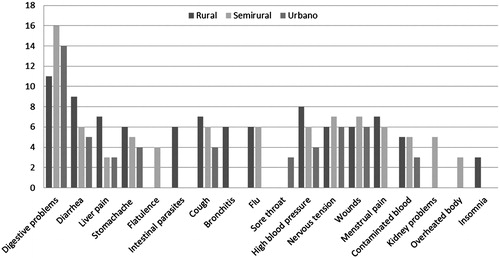
When we look closer, we find that, in rural areas, digestive problems are more diversified than in other places, as shown by the number of medicinal plants used to treat conditions such as digestive problems, diarrhoea, liver pain, stomachache and intestinal parasites (). As for respiratory system ailments, the greatest number of species was used to treat coughs in all places. In rural areas, bronchitis was also among the most important respiratory illnesses, while in semirural areas, flu was the most important one and it stood for the number of plant species used to cure it. There was quite a large degree of uniformity in all places for skin problems – the most salient ailments were wounds and problems of the nervous system – in all study sites, nervous tension was noted and treated with the largest list of botanical species.
When we compared the uses of the medicinal plants among the environments performing the Shannon–Wiener index, we observed that rural and semirural gardens were similar. There were, however, significant differences between rural and urban home gardens (t = 4.9 >t0.05(2)596) and between semirural and urban ones (t = 4.7 > t0.05(2)127).
Discussion
How much do rural and semirural home gardens actually share with urban ones with respect to medicinal plants?
As reflected by the species richness, people in Misiones cultivate and manage more medicinal species in rural and semirural areas. Nonetheless, general composition in the three study areas is strikingly similar, and the singularities are expressed in the exclusive species of each area. The high levels of similarity suggest a widespread habit of plant exchange among local population, which leads to an important flow of genetic material between zones. Among the exotic species mint, chamomile and aloe stand out, a fact that could be traced back to their historic presence in South America for at least four centuries (Bennett & Prance Citation2000). For this reason, these plants form integral part of local criollo people herbal remedies, too. In addition, these three species have been prominent medicinal resources in folk pharmacopoeias of the Iberian Peninsula (Pardo-de-Santayana et al. Citation2005), as well as in Eastern European regions (Paluch Citation1991; Kujawska & Hilgert Citation2014). In Misiones, these plants constitute legacy elements of European migrant culture and they are also part of a “home building” strategy (Jordan et al. Citation2009) and claiming of space in an unfamiliar environment. Therefore, their use is constantly verified in both intra and inter-cultural contexts.
The high levels of similarity among the environments in a pluricultural context suggest that knowledge about medicinal species is linked to traditions, as proposed by Hurrell (Citation2014). The concept of “linked to traditions” was proposed for urban areas, but in our opinion, it can be expanded to rural and semirural environments as well. We also suggest that there is a “core repertoire” of medicinal plants, in line with the proposal of Ellen and Platten (Citation2011), in the Iguazú Department, but each area conserves its particularities, too.
The presence of shared species in the three study areas could also be related to certain elements of rurality, especially to agriculture. Izquierdo et al. (Citation2008) suggest that although there has been rural–urban migration in Misiones, “the productive lands containing agriculture and plantations have not been abandoned”. This fact may have enhanced the flow of plant material between rural and urban zones in Misiones. Those elements are preserved in cities and are important to understand how urban space is used and occupied as set forth by Almada (Citation2010). This author also proposed two hypotheses to substantiate the reason for conservation practices of culturally important species, although space is minimal in the urban environment. The first suggests that elements of rurality are persistent in urban areas and they resist despite these modes of peasant lifestyle that are marginalised in urban context. Second, to cultivate, is to refer to a lifestyle considered “good and pure”, or based on solid values, a lifestyle that has historically been associated with the countryside by urban dwellers (Almada Citation2010). In our case, the semirural and urban areas take elements of the two hypotheses, since in the discourse of the interviewees, the values of medicinal plants are associated with their past life as farmers and the value that plants have for their family that still live in the countryside.
According to Garland and Chakraborty (Citation2007), ethnic minorities living in rural areas, which have strong bonding social capital, tend to be exclusive and intolerant of “others”. However in our study area, we observe a different situation, more characteristic of groups exhibiting bridging social capital, as proposed by Onyx and Bullen (Citation2000). This suggests that the multiethnic society of the northern Misiones, composed mainly of mestizos and European descendants, not only retains elements of its legacy culture, inscribing them in the landscape of Misiones but also explores knowledge and practices “gained” from local indigenous people by using native species in home phytotherapy.
What can we learn from the cultural indices of medicinal plant importance?
Many researchers have underlined the advantages of quantitative ethnobotanical indices as valuable tools that are adequate for the comparison of knowledge of natural resources between different environments and different ethnic groups (Philips & Gentry Citation1993; Ladio & Lozada Citation2003; Sousa Araújo et al. Citation2012; Mathur & Sundaramoorthy Citation2013). Moreover, cultural importance indices in ethnobotanical studies have potential as relevant indicators for in situ conservation, on the species level, of medicinal plants for the benefit of local people (Lambert et al. Citation1997). The identification of species perceived as culturally important that are used in home phytotherapy is crucial to further recommendations for national, provincial and local ecological agencies and NGOs.
There are a few ways of measuring the cultural importance of plants. But we were unable to use them given the characteristics of our data. For example, the Philips and Gentry (Citation1993) index is based on the use value of species. Nonetheless, this index is more adequate for return field research, or when information on plant use is obtained during numerous sessions with the same pool of informants. Another way of measuring the cultural importance of plant species is by analysing free lists (Weller & Romney Citation1988). This index takes into account the “salience or psychological prominence of plants listed by respondents” (Quinlan et al. Citation2002), and therefore, it is often called the cultural salience index (Smith Citation1993). This index combines frequency of mention with order of mention – which we lacked in our inventories. Therefore, we combined two indices: frequency of mention with plant versatility. Bennett and Prance (Citation2000) proposed the measure of medicinal plant importance based on the versatility (the number of body systems treated and the number of pharmacological properties), which they performed in their study of exotic species used in native and folk pharmacopoeias of the northern part of South America. Why did we not simply compare these to indices (frequency of citations and plant versatility)? During data analysis, we observed that some of the species were very versatile but poorly cited and others were widely cited but had very few medicinal properties. Therefore, we decided that combining these two factors – frequency of citations, which indicates informant consensus and plant efficacy, and plant versatility, which informs us about plant adaptability, would produce a list of the most culturally relevant (salient) species present in home gardens in the three study areas.
Although our research demonstrated a moderate preference for exotic species among the study population (especially in the urban setting), which could be interpreted as working against native plant biodiversity conservation, this should not be taken strictly. Taking into account statements by Pochettino et al. (Citation2012) that highlight the home garden as the preferred area for experimentation and innovation, it is not rare to find exotic species there and not so much in other conservative areas such as secondary forest patches, where people also gather medicinal species. The dynamism and the historical background of northern Misiones is another reason that sums the presence of exotic resources.
Do people in rural, semirural and urban areas share knowledge about the medicinal uses of plants growing in their home gardens?
All the plant species discussed here are home remedies, which are employed to treat mainly minor health conditions traditionally cured at home, such as flu, the common cold, slow digestion, diarrhoea, wounds and agitation. The predominance of digestive, respiratory and skin problems is striking; however, it is confirmed by more general studies dedicated to medicinal plant use among mestizo and European diasporas in Misiones (Keller & Romero Citation2006; Moreau Citation2006; Zamudio et al. Citation2010; Kujawska & Hilgert Citation2014); and, viewed more broadly, in the Atlantic Forest ecoregion (Begossi et al. Citation2002; Eichemberg et al. Citation2009).
Apart from the health problems, uniformly cited by our study participants and traditionally treated with plants in Misiones, we registered a considerable number of medicinal botanicals used to treat the so-called “modern complaints” within circulatory system disorders, namely hypertension and high levels of cholesterol. There is a high degree of consensus and homogeneity in the use of both native and exotic species in the treatment of “modern circulatory ailments” in the three study areas. Our results suggest that exotic species are more appreciated in the treatment of these ailments. This may indicate that these particular exotic species are perceived as very efficient in the treatment of circulatory problems, and, therefore, their popularity expands the boundary of rural and urban areas. This example evokes a possible explanation for the gradual abandonment of native species in the rural–urban gradient/spectrum – the culturally perceived efficacy of new exotic plants apt for easy propagation and cultivation, which could eventually replace native ones.
Overall, the qualitative analysis of the most cited ailments and plant resources employed in their treatment indicates that these three areas have a lot in common, and people living in rural, semirural and urban centres share grosso modo the knowledge of medicinal plants and their uses. However, each of these areas conserves its particularities, expressed as preferences towards certain plants, their availability (access to seeds, seedlings, proximity of other environments that plants can be transplanted from, quality of the seed bank/soil), as well as access to knowledge about them. Therefore, the quantitative scrutiny of the diversity of medicinal plant uses suggests that the semirural area plays the role of a “bridge” between rural and urban areas. This analysis demonstrated that rural and semirural home gardens exhibited similarities in this respect, although there were statistical differences between urban and rural areas and semirural and urban areas, too.
Conclusions
The body systems treated in local home medicine are very similar in all three study settings, but partly the species used are different across the sites. We observed that home gardens are multifunctional spaces, where medicinal species are shared between two – or three – environments, and with exclusive botanicals in each area. However, the home gardens, which exhibit more richness of medicinal resources and preserve more similar purposes, are located in rural and semirural areas.
In this changing and dynamic context, where mobility and migrations are part of everyday life in the area, medicinal plants in home gardens are components of the strategy to preserve health care sovereignty for local people. Following the hypothesis of Padoch et al. (Citation2008), “urban ward migrants in Amazonia are not really absent from rural zones; they remain members of households with livelihood activities in both rural and urban environments”, we consider that the persistence of the same species in different areas is related to this flow of people and their genetic resources.
Given the conclusions of the present research, we consider that some relevant aspects for future studies on regional herbal medicine are, along with the gradient of rurality: (1) the flow of trade in plants and knowledge associated with them among different areas, (2) the issue of whether there are significant differences in the cast of medicinal species of major cultural importance, (3) identification of ways and places of obtaining these resources.
Funding information
Research was financed by funds from Polish Ministry of Science and Higher Education, National Science Center [2013/09/N/HS3/02226], Consejo Nacional de Investigaciones Científicas y Técnicas, PIA 10103 BIRF 7520 AR.
Acknowledgements
Our special thanks to the study participants from Puerto Iguazú, Wanda and Lanusse, who generously shared their knowledge. We are also grateful to Washington Soares Ferreira Júnior for providing us with some useful references and giving us prompts in choosing the adequate indices of cultural importance, and to Guillermo Gil for his help to estimate the home garden medicinal plants diversities.
Disclosure statement
The authors report that they have no conflict of interests regarding the publication of this paper.
References
- Agelet A, Bonet MA, Vallèa J. 2000. Home gardens and their role as a main source of medicinal plants in mountain regions of Catalonia (Iberian Peninsula). Econ Bot. 54:295–309.
- Almada DE. 2010. Sociobiodiversidade urbana: Por uma etnoecologia das ciudades. In: Atanazio da Silva V, Santos de Almeida A, de Albuquerque UP, editors. Etnobiologia e etnoecologia. Pessoas & natureza na América Latina. Pernambuco, Brazil: Nupeea. p. 37–64.
- Altieri MA, Merrick LC. 1987. In situ conservation of crop genetic resources through maintenance of traditional farming systems. Econ Bot. 41:86–96.
- Altieri M, Toledo VM. 2011. The agroecological revolution in Latin America: rescuing nature, ensuring food sovereignty and empowering peasants. J Peasant Stud. 38:587–612.
- Amat G, Yajía ME. 1998. Plantas vasculares utilizadas en la fitoterapia popular de la Provincia de Misiones, Argentina. In: Amat AG, editor. Farmacobotánica y farmacognosia en Argentina (1980–1998). La Plata, Argentina: Ediciones Científicas Americanas. p. 119–152.
- Bach A, Bałdysiak D. 2008. Ogrody zagród góralskich–tradycja i współczesność. Zeszyty Problemów Postępów Nauk Rolniczych. 525:21–26.
- Barreto M. 2002. El proceso de urbanización del Nordeste Argentino a finales del siglo XX; [cited 25 Nov 2014]. Available from: http://www1.unne.edu.ar/cyt/2002/01-Sociales/S-011.pdf.
- Bartolomé LJ. 1982. Colonias y colonizadores en Misiones. Posadas, Argentina: Facultad de Humanidades y Ciencias Sociales, Universidad de Misiones.
- Begossi A, Hanazaki N, Tamashiro JK. 2002. Medicinal plants in the Atlantic forest (Brazil): knowledge, use and conservation. Hum Ecol. 30:281–299.
- Bennett BC, Prance GT. 2000. Introduced plants in the indigenous pharmacopeia of northern South America. Econ Bot. 54:90–102.
- Bolson M, Hefler SR, Dall′Oglio Chaves EI, Gasparotto AJ, Cardozo ELJ. 2015. Ethno-medicinal study of plants used for treatment of human ailments, with residents of the surrounding region of forest fragments of Paraná, Brazil. J Ethnopharmacol. 161:1–10.
- Caballero J. 1992. The Maya Home gardens of the Yucatan Peninsula: past, present and future. Etnoecol. 1:35–54.
- Calvet-Mir L, Gómez-Baggethun E, Reyes-García V. 2012. Beyond food production: ecosystem services provided by home gardens. A case study in Vall Fosca, Catalan Pyrenees, Northeastern Spain. Ecol Econ. 74:153–160.
- Corlett J, Dean E, Grivetti L. 2003. Hmong gardens: botanical diversity in an urban setting. Econ Bot. 57:365–379.
- Dzięciołowska M, Latkowska MJ. 2009. Rural front garden in Podlasie (Hajnówkacounty). Ann of Warsaw University of Life Sciences – SGGW. Horticult Landscapes Archit. 30:79–87.
- Eichemberg MT, Amorozo de Mello MC, Cunha de Moura L. 2009. Species composition and plant use in old urban home gardens in Rio Claro, Southeast of Brazil. Acta Bot Brasilica. 23:1057–1075.
- Ellen R, Platten S. 2011. The social life of seeds: the role of networks of relationships in the dispersal and cultural selection of plant germplasm. J Royal Anthropolog Inst (N.S.). 17:563–584.
- Fernandez ECM, Nair PKR. 1986. An evaluation of the structure and functions of tropical home gardens. Agroforest Syst. 21:279–310.
- Ferrero B. 2005. Estudio de la gestión territorial y de los recursos naturales, de la población rural del Área de Influencia de la Reserva de Biosfera Yabotí–Argentina. Buscando alternativas para un desarrollo local sustentable en torno a una Reserva de Biosfera.ProgramaManandBiosphera.Unesco.
- Finerman R, Sackett R. 2003. Using home gardens to decipher health and healing in the Andes. Med Anthropol Q. 17:459–482.
- Garland J, Chakraborty N. 2007. Protean times’?: exploring the relationships between policing, community and ‘race’ in rural England. Criminol Crim Justice. 7:347–365.
- Giraldi M, Hanazaki N. 2010. Uso e conhecimento tradicional de plantas medicinais no Sertão do Ribeirão, Florianópolis, SC, Brasil. Acta Bot Brasil. 24:395–406.
- Heckler SL. 2007. Herbalism, home gardens, and hybridization: wõthïhã medicine and cultural change. Med Anthropol Q. 21:41–63.
- Hilgert NI, Lambaré DA, Vignale ND, Stampella P, Pochettino ML. 2014. Especies naturalizadas o antropizadas? Apropiación local y la construcción de saberes sobre los frutales introducidos en época histórica en el norte de Argentina. Rev Biodiv Neotrop. 4:69–87.
- Hurrell J. 2014. Urban Ethnobotany in Argentina: theoretical advances and methodological strategies. Ethnobiol Conserv. 3:1–11.
- INDEC. 2001. Censo nacional de población, hogares y viviendas. Instituto Nacional de Estadística y Censos.
- Izquierdo AE, Angelo CD, Aide TM. 2008. Thirty years of human demography and land-use in the Atlantic Forest of Misiones, Argentina: an evaluation of the forest transition model. Ecol Soc. 13:3.
- Jordan K, Krivokapic-Skoko B, Collins J. 2009. The ethnic landscape of rural Australia: non-Anglo-Celtic immigrant communities and the built environment. J Rural Stud. 25:376–385.
- Keller HA. 2008. Etnobotánica de comunidades guaraníes de Misiones, Argentina; valoración de vegetación como fuente de recursos. Corrientes, Argentina: Tesis de doctorado, Universidad Nacional del Nordeste.
- Keller HA, Romero HF. 2006. Plantas medicinales utilizadas por campesinos del área de influencia de la Reserva de Biósfera Yabotí (Misiones, Argentina). Bonplandia. 15:125–141.
- Kołodziejska-Degórska I. 2008. Z czego uwarić herbate? Dzikie rośliny jadalne w polskich wsiach na południowej Bukowinie (Rumunia). In: Łuczaj Ł, editor. Dzikie rośliny jadalne–zapomniany potencjał przyrody, Materiały z konferencji Przemyśl–Bolestraszyce, 13 września 2007r. –Bolestraszyce: Arboretumi Zakład Fizjografii w Bolestraszycach. p. 219–226.
- Kraustofl E. 2011. Prywatna kolonizacja w Misiones: Kolonie Wanda i Lanusse, 1936–1960. Relacje zebrane wlatach 2005–2007. In: Stemplowski R, editor. Polacy, Rusini i Ukraińcy, Argentyńczycy. Osadnictwo w Misiones 1892–2009. Warszawa: BibliotekaIberyjska. p. 471–512.
- Kujawska M, Hilgert NI. 2014. Phytotherapy of Polish migrants in Misiones, Argentina: legacy and acquired plant species. J Ethnopharmacol. 153:810–830. and corrigendum 155:1629–1640.
- Kujawska M, Pardo de Santayana M. 2015. Management of medicinally useful plants by European migrants in South America. J Ethnopharmacol. 172:347–355.
- Kujawska M, Zamudio F, Hilgert NI. 2012. Honey-based mixtures used in home medicine by non-indigenous population of Misiones, Argentina. Evidence-Based Complement Altern Med, Special Issue: Ethnobiol Ethnopharmacol Latin Am Article ID 579350.
- Kumar BM, Nair PKR. 2004. The enigma of tropical home gardens. Agroforest Syst. 61:135–152.
- Ladio AH, Lozada M. 2003. Comparison of wild edible plant diversity and foraging strategies in two aboriginal communities of northwestern Patagonia. Biodivers Conserv. 12:937–951.
- Lambert J, Sirivastava J, Vietmeyer N. 1997. Medicinal plants. Rescuing a global heritage. Washington DC: World Bank Technical Paper, 355
- Lee HD, Smith C, Herbohn J, Harrison S. 2012. More than just trees: assessing reforestation success in tropical developing countries. J Rural Stud. 28:5–19.
- Mathur M, Sundaramoorthy S. 2013. Census of approaches used in quantitative Ethnobotany. Ethno Med. 7:31–58.
- Montagnini F. 2006. Home gardens of Mesoamerica: biodiversity, food security, and nutrient management. In: Kumar BM, Nair PKR, editors. Tropical home gardens: a time tested example of sustainable agroforestry. Netherlands: Springer. p. 61–84.
- Moreau D. 2006. Valorisation de l'usage des plantes medicinales dans la province Misiones (Argentina): explorationd'une alternative de conservation de la foret atlantique. Memoire pour le Diplome Universitaired’ Etudes Complementaire en Ethnobotanique appliquee, annee 2005–2006. Lille: Universite de Lille 2, Droit et Sante, Faculte des Sciences Pharmaceutiques et Biologiques.
- Moreno CE. 2001. Métodos para medir la biodiversidad. M & T-Manuales y Tesis SEA. 1:84.
- Moreno NB. 2007. The role of the medicinal plants in rural Paraguayan livelihoods reasons for extensive medicinal plant use in Paraguay. Suplemento Antropológico, Rev. Centro Estudios Antropológicos 42:1–159.
- Mosina GKE, Maroyi A, Potgieter MJ. 2014. Comparative analysis of plant use in peri-urban domestic gardens of the Limpopo Province, South Africa. J Ethnobiol Ethnomed. 10:35. doi:10.1186/1746-4269-10-35.
- Nazarea V. 2005. Heirloom seeds and their knowledge. Tucson, USA: University of Arizona Press.
- Nesheim I, Dhillion SS, Stølen KA. 2006. What happens to traditional knowledge and use of natural resources when people migrate? Hum Ecol. 34:99–131.
- Onyx J, Bullen P. 2000. Measuring social capital in five communities. J Appl Behav Sci. 36:23–42.
- Padoch C, Brondizio E, Costa S, Pinedo-Vásquez M, Sears RR, Siqueira A. 2008. Urban forest and rural cities: multi-sited households, consumption patterns, and forest resources in Amazonia. Ecol Soc. 13:2 [online].
- Palau T. 1998. La agricultura paraguaya al promediar la década de 1990: situación, conflictos y perspectivas. In: Giarracca N, Cloquell S, editors. Las agriculturas del Mercosur. Buenos Aires, Argentina: La Colmena-CLACSO.
- Paluch A. 1991. Choroby, zioła, znachorzy. Namysłów: WA & ZA.
- Pardo de Santayana M, Blanco E, Morales R. 2005. Plants known as té in Spain: an ethno-pharmaco-botanical review. J Ethnopharmacol. 98:1–19.
- Perfecto I, Vandermeer J. 2008. Biodiversity conservation in tropical agroecosystems: a new conservation paradigm. Ann N Y Acad Sci. 1134:173–200.
- Philips O, Gentry AH. 1993. The useful plants of Tambopata, Peru: I. Statistical hypothesis tests with a new quantitative technique. Econ Bot. 47:15–32.
- Pieroni A,Vandebroek I, editors. 2007. Traveling cultures and plants. The ethnobiology and ethnopharmacy of human migrations. Studies in environmental anthropology and ethnobiology, vol. 7. Oxford, New York: Berghahn Books. p. 1–283.
- Placi G, Di Bitetti M. 2006. Situación ambiental en laecorregión del Bosque Atlántico del Alto Paraná (Selva Paranaense). In: Brown A, Martinez Ortiz U, Acerbi M, Corcuera J. editors. La situación ambiental argentina. Buenos Aires, Argentina: Fundación Vida Silvestre Argentina. p. 193–210.
- Pochettino ML, Hurrell J, Lema V. 2012. Local botanical knowledge and agrobiodiversity: home gardens at rural and periurban contexts in Argentina. In: Luna Maldonado AI, editor. Horticulture. Argentina: In Tech, 105–132. OpenBooks. http://www.intechopen.com/books/horticulture/local-botanical-knowledge-and-agrobiodiversity-home gardens-at-rural-and-periurban-contexts-in-argent. Accesed on 25 November 2014.
- Pulido MT, Pagaza-Calderón EM, Martínez-Ballesté A, Maldonado-Almanza B, Saynes A, Pacheco RM. 2008. Home gardens as an alternative for sustainability: challenges and perspectives in Latin America. In: Albuquerque UP, Alves-Ramos M, editors. Current topics in ethnobotany research. Kerala: Signpost. p. 55–79.
- Quinlan MB, Quinlan RJ, Nolan JM. 2002. Ethnophysiology and herbal treatments of intestinal worms in Dominica, West Indies. J Ethnopharmacol. 80:75–83.
- Rico-Gray V, Garcia-Franco JG, Chemas A, Puch A, Sima P. 1990. Species composition, similarity, and structure of Mayan home gardens in Tixpeual and Tixcacaltuyub, Yucatan, Mexico. Econ Bot. 44:470–487.
- Schiavoni G. 1998. Colonos y ocupantes. Parentesco, reciprocidad y diferenciación social en la frontera agraria de Misiones. Posadas: Editorial Universitaria.
- Smith JJ. 1993. Using ANTHROPAC 3.5 and a spread sheet to compute a Free List Salience Index. Cult Anthropol Methods. 5:1–3.
- Soria N, Basualdo I. 2005. Medicina herbolaria de la comunidad Kavaju Kangue. SOA, Paraguay: Departamento de Caazapá, Asunción.
- Sõukand R, Kalle R. 2010. Herbal landscape: the perception of the landscape as a source of medicinal plants. Trames. 14:207–226.
- Sousa Araújo T, Almeida A, Melo J, Medeiros M, Ramos M, Silva R, Almeida C, Albuquerque U. 2012. A new technique for testing distribution of knowledge and to estimate sampling sufficiency in ethnobiology studies. J Ethnobiol Ethnomed. 8:11.
- Tardío J, Pardo de Santayana M. 2008. Cultural importance indices: a comparative analysis based on the useful wild plants of Southern Cantabria (Northern Spain). Econ Bot. 62:24–39.
- United Nations Population F. 2007. State of world population 2007: unleashing the potential of urban growth. New York, USA: UNFPA.
- Vogl-Lukasser B, Vogl C. 2004. Ethnobotanical research in home gardens of small farmers in the alpine region of Osttirol (Austria): an example for bridges built and building bridges. Ethnobot Res Appl. 2:111–137.
- Weller S, Romney AK. 1988. Systematic data collection. Newbury Park, CA: Sage Publications.
- Zamudio F, Kujawska M, Hilgert NI. 2010. Honey as medicinal and food resource. Comparison between Polish and multiethnic settlements of the Atlantic Forest, Misiones, Argentina. Open Complement Med J, Special Issue: Med Ethnobiol. 2:58–73.