Abstract
Context Dodonaea viscosa (L.) Jacq (Sapindaceae) has been used in traditional medicine as antimalarial, antidiabetic and antibacterial agent, but further investigations are needed.
Objective This study determines the antioxidant and anticholinesterase activities of six compounds (1–6) and two crystals (1A and 3A) isolated from D. viscosa, and discusses their structure–activity relationships.
Materials and methods Antioxidant activity was evaluated using six complementary tests, i.e., β-carotene-linoleic acid; DPPH•, ABTS•+, superoxide scavenging, CUPRAC and metal chelating assays. Anticholinesterase activity was performed using the Elman method.
Results Clerodane diterpenoids (1 and 2) and phenolics (3–6) – together with three crystals (1A, 3A and 7A) – were isolated from the aerial parts of D. viscosa. Compound 3A exhibited good antioxidant activity in DPPH (IC50: 27.44 ± 1.06 μM), superoxide (28.18 ± 1.35% inhibition at 100 μM) and CUPRAC (A0.5: 35.89 ± 0.09 μM) assays. Compound 5 (IC50: 11.02 ± 0.02 μM) indicated best activity in ABTS assay, and 6 (IC50: 14.30 ± 0.18 μM) in β-carotene-linoleic acid assay. Compounds 1 and 3 were also obtained in the crystal (1A and 3A) form. Both crystals showed antioxidant activity. Furthermore, crystal 3A was more active than 3 in all activity tests. Phenol 6 possessed moderate anticholinesterase activity against acetylcholinesterase and butyrylcholinesterase enzymes (IC50 values: 158.14 ± 1.65 and 111.60 ± 1.28 μM, respectively).
Discussion and conclusion This is the first report on antioxidant and anticholinesterase activities of compounds 1, 2, 5, 6, 1A and 3A, and characterisation of 7A using XRD. Furthermore, the structure–activity relationships are also discussed in detail for the first time.
Introduction
Antioxidants are common preservatives, and mostly synthetic antioxidants have been used to prevent oxidative deterioration in food and pharmaceutical industries. The use of antioxidants may slow the progress of Alzheimer’s disease (AD) and minimise neuronal degeneration (Atta-ur-Rahman & Choudhary Citation2001) by inhibiting acetylcholinesterase enzyme. Since the only valid hypothesis being accepted is the lack or deficiency of acetylcholine (Grossberg Citation2003), acetylcholinesterase inhibitors are used to treat Alzheimer's disease (Scalbert et al. Citation2005). However, most of these drugs have side effects such as liver damage and bradycardia (Dökmeci Citation2000). Synthetic antioxidants also caused liver damage and carcinogenesis in rats (Grice Citation1988; Ito et al. Citation1983). That provoked scientists to search new natural and harmless antioxidants, as well as anticholinesterase compounds.
Dodonaea viscosa (L.) Jacq (Sapindaceae) is a flowering evergreen shrub. Dodonaea is a genus of 60 species that is widely distributed in warmer parts of Australia, South Africa, North America and South Asia countries (Abdullah Citation1973). Traditionally, D. viscosa has been used against skin diseases (Pirzada et al. Citation2010), and used as an antimalarial (Clarkson et al. Citation2004), antidiabetic (Ahmad et al. Citation2002) and antibacterial agent (Ahmad et al. Citation2002; Clarkson et al. Citation2004; Choudhary et al. Citation2013).
Owing to the pharmacological significance of D. viscosa, it was subjected to phytochemical investigations. Herein, the antioxidant and anticholinesterase activities of isolated compounds as well as their structure–activity relationships are reported. To the best of our knowledge, this is the first report of the antioxidant and anticholinesterase activities of isolated compounds (1, 2, 5, 6, 1A and 3A). Crystal compound 7A was also characterised using XRD for the first time.
Materials and methods
General experimental procedure and chemicals
Bioactivity measurements were carried out on a 96-well microplate reader, SpectraMax 340PC384, Molecular Devices (Silicon Valley, CA), at the Department of Chemistry, Muğla Sıtkı Kocman University. The measurements and calculations of the activity results were evaluated using Softmax PRO v5.2 software (Molecular Devices, Silicon Valley, CA). XRD analysis parameters were as follows: data collection, cell refinement and data reduction: CrysAlis PRO (Agilent Citation2010); structure solution and refinement software: SHELXS97 (Sheldrick Citation2008); molecular graphics: X-SEED (Barbour Citation2001); cif editor: publCIF (Westrip Citation2010); refinement: on F2 full matrix least-squares.
Ethanol, n-hexane, methanol, ammonium acetate, copper (II) chloride, ferrous chloride, N-methyl-phenazoniummethylsulphate (PMS), nicotinamide adenine dinucleotide (NADH), nitrotetrazoliumblue chloride (NBT) and ethylenediaminetetraacetic acid (EDTA) were obtained from E. Merck (Darmstadt, Germany). β-Carotene, α-tocopherol, linoleic acid, polyoxyethylenesorbitanmonopalmitate (Tween-40), neocuproine, butylated hydroxyl anisole (BHA), 1,1-diphenyl-2-picrylhydrazyl (DPPH),3-(2-pyridyl)-5,6-di(2-furyl)-1,2,4-triazine-5′,5′′-disulphonic acid disodium salt (Ferene), Tris-HCl, 2,2′-azinobis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS), galantamine, acetylcholinesterase from electric eel (AChE, Type-VI-S, EC 3.1.1.7, 425.84 U/mg), butyrylcholinesterase from horse serum (BChE, EC 3.1.1.8, 11.4 U/mg), 5,5′-dithiobis (2-nitrobenzoic) acid (DTNB), acetylthiocholine iodide and butyrylthiocholine chloride were obtained from Sigma Chemical Co. (Sigma-Aldrich GmbH, Sternheim, Germany). All other chemicals and solvents were of analytical grade.
Plant material, extraction and isolation
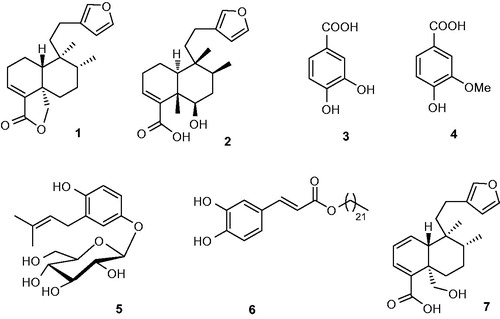
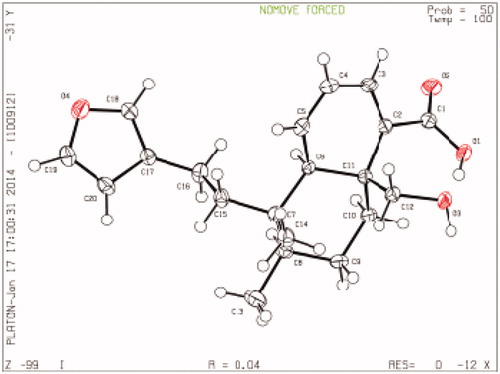
Plant collection and voucher specimen information has been published previously (Muhammad et al. Citation2012a). The plant material (20 kg, shade-dried) was ground into powder and extracted at room temperature with 35 L MeOH three times for 15 days. After removing the solvent, the extract was suspended in H2O and extracted with n-hexane, CHCl3, EtOAc and n-BuOH to yield n-hexane (116 g), CHCl3 (890 g), EtOAc (173 g) and n-BuOH (337 g) extracts, respectively. The crystals 1A and 3A, and compounds 1–6 were isolated and characterised according to previous reports (Anis et al. Citation2001; Mohammad et al. Citation2010, Citation2012b). Using NMR, MS and XRD, the purified compounds were elucidated as hautriwaic lactone (1), crystal of 1 (1A), 6β-hydroxy-15,16-epoxy-5β,8β,9β,10α-cleroda-3,13 (16),14-trien-18-oic acid (2), 3,4-dihydroxybenzoic acid (3), crystal of 3 (3A), vanillic acid (4), nebrodenside A (5) and docosyl caffeate (6). The structures of the isolated compounds (1–7) were given in . The CHCl3 fraction was subjected to MPLC [silica gel, hexane/EtOAc (1:0–0:1)] to obtain 23 fractions (Fr. A–W). Crystal of 15,16-epoxy-19-hydroxy-1,3,13-(16),14-clerodatetraen-18-oic acid (7A) (6.5 mg) was obtained from Fr. C as colourless crystals. Previously, 7A was characterised by Ortega et al. (Citation2001), although they did not obtain it in a crystal form. In this study, we obtained it in a crystal form. The stereochemistry of the 7A had been ambiguous, hence we determined it through XRD for the first time ().
Crystal data for compound 7A
C20H26O4, MW 330.28, orthorhombic, P2121, a = 6.7744 (1) Å, b = 14.0747 (2) Å, c = 9.5068 (2) Å, α = 90.00°, β = 107.457 (2)°, γ = 90.00°, V = 864.70(3) Å3, Z= 2, Mo Kα radiation, 3377 reflections, 225 parameters, μ = 0.701 mm−1, T = 100 K, R = 0.0364, Rw = 0.0992, S = 1.066. The XRD data for 7A (Deposition no. CCDC1040933) have been deposited at the Cambridge Crystallographic Data Centre ().
Determination of antioxidant activity
All activities were performed according to the standard literature procedures with slight modifications (Tel et al. Citation2012). Total antioxidant activity was evaluated using β-carotene-linoleic acid model test (Marco Citation1968). Free radical scavenging activity was determined spectrophotometrically by the DPPH assay (Blois Citation1958). The spectrophotometric analysis of ABTS•+ scavenging activity was determined according to the literature (Re et al. Citation1999). Superoxide anion radical scavenging activity was performed according to PMS-NADH-NBT method (Öztürk et al. Citation2014). CUPRAC antioxidant activity was performed according to Apak’s procedure (Apak et al. Citation2004). Metal chelating activity of the compounds on Fe2+ was performed spectrophotometrically (Decker & Welch Citation1990).
Determination of anticholinesterase activity
Acetylcholinesterase and butyrylcholinesterase inhibitory activities were measured using the spectrophotometric method developed by Ellman et al. (Citation1961) with slight modification (Tel et al. Citation2012).
Determination of IC50 and A0.5 values
The results are expressed as 50% inhibition concentration (IC50). The sample concentration that provides 50% activity (IC50) was calculated from the graph of percent inhibitory activity versus sample concentration. The sample concentration having 0.50 absorbance (A0.5) was calculated from the plot of CUPRAC absorbance against sample concentration.
Statistical analysis
The data of antioxidant and anticholinesterase activities were the averages of triplicate analyses. Data were recorded as mean ± standard error of mean (SEM). Significant differences between means were determined by Student’s t-test, and p values <0.05 were considered as significant.
Results and discussion
Antioxidant activity
The antioxidant activity of diterpenes (1, 2 and 1A) and phenolics (3–6 and 3A) isolated from Dodonaea viscosa was tested using β-carotene-linoleic acid, DPPH radical scavenging, ABTS cation radical scavenging, superoxide anion radical scavenging (O2•–), CUPRAC and metal chelating assays (). BHA, α-tocopherol and EDTA were used as positive standards. The tests were performed at different concentrations to calculate the IC50 and A0.50 values. Results were statistically significant (p < 0.05) when compared with those of controls in each test.
Table 1. Antioxidant activity of the compounds of Dodonaea viscosa by the β-carotene-linoleic acid, DPPH•, ABTS•+, O2•–, CUPRAC and metal chelating assaysTable Footnotea.
The antioxidants and the lipid peroxidation inhibitors are easily tested in the β-carotene-linoleic acid assay, where H• is transferred to the media by the antioxidant. Compound 6 exhibited the highest lipid peroxidation inhibitory activity (IC50: 14.30 ± 0.18 μM), followed by 5 (IC50: 40.30 ± 0.36 μM), 2 (IC50: 107.45 ± 1.05 μM), 3A (IC50: 108.46 ± 1.08 μM) and 3 (IC50: 147.45 ± 1.14 μM) (). Compounds 1, 1A and 4, however, showed weak inhibition activity (20.56 ± 1.09, 14.34 ± 0.40 and 5.42 ± 0.58% at 100 μM concentrations, respectively).
In DPPH•, ABTS•+ scavenging and CUPRAC assays, antioxidant transfers electron to the media. DPPH• and ABTS•+ are reduced by an electron or a radical species; thus, their absorbencies decrease at 517 and 734 nm, respectively. In CUPRAC assay, however, cupric is reduced to cuprous by an electron released by antioxidant. Then the cuprous forms a stable complex with neocuproine, which increases the absorbance at 450 nm. In DPPH• assay, 3A (IC50: 27.44 ± 1.06 μM) exhibited better activity than α-tocopherol and very close activity to those of α-tocopherol and BHA, followed by 6 (IC50: 78.54 ± 1.24 μM), 3 (IC50: 166.06 ± 1.65 μM) and 5 (IC50: 170.49 ± 1.76 μM). All other compounds exhibited weak DPPH radical scavenging activity (IC50 >200 μM). In ABTS•+ assay, 5 (IC50: 11.02 ± 0.02 μM) exhibited the highest activity, even higher than that of α-tocopherol (IC50: 17.18 ± 0.36 μM) and BHA (IC50: 13.68 ± 0.54 μM). Compound 3A (IC50: 19.28 ± 0.56 μM) also indicated better activity which was very close to that of α-tocopherol. Compounds 3 (IC50: 49.78 ± 0.19 μM) and 6 (IC50: 51.61 ± 0.74 μM) demonstrated noticeable ABTS•+ scavenging activity as well. In CUPRAC assay, however, compound 3A was the best reductant indicating 35.89 ± 0.09 μM A0.50 value. At the same conditions, the A0.50 values of BHA and α-tocopherol were 35.71 ± 1.68 and 40.48 ± 1.87 μM A0.50, respectively. Compounds 3 (A0.50: 55.78 ± 0.09 μM), 5 (A0.50: 72.22 ± 0.21 μM) and 4 (A0.50: 80.76 ± 0.08 μM) also exhibited good reducing capacity but less than those of antioxidant standards ().
The superoxide anion radical scavenging activity tests the superoxide quenching of the antioxidant. The superoxide generated by N-methyl-phenazoniummethylsulphate (PMS) from dissolved oxygen in NADH media. In this method, there is also electron transfer from antioxidant to quench the superoxide. The calculated IC50 values of compounds 1–6 were over 200 μM; thus, only the inhibition percentages at 100 μM are given (). The compounds (3A and 6) indicating good antiradical activity also exhibited higher superoxide scavenging activities than the other compounds (1–5 and 1A). Herein, compound 2, only active in β-carotene-linoleic acid assay, is interesting, and the activity can be explained by –OH at C-6 position ().
Metal chelating activity tests the secondary antioxidants that can bind the transition metals. Transition metals are the effective pro-oxidants, which accelerate the lipid oxidation by breaking down hydrogen and lipid peroxides to reactive free radicals via the Fenton Reaction (Halliwell & Gutteridge Citation1984). Iron is the typical example. Therefore, it is necessary to test the secondary antioxidants. Compounds 3 (26.55 ± 0.18%), 3A (26.56 ± 0.04%) and 6 (25.18 ± 0.84%) possessed to chelate iron at 100 μM concentration.
Anticholinesterase activity
shows the acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory activities of the compounds (1–6, 1A and 3A) where galantamine was used as the standard. Compound 6 exhibited moderate activity against AChE and BChE enzymes (IC50: 158.14 ± 1.65 and 111.60 ± 1.28 μM, respectively). Compound 1 (IC50: 250.51 ± 1.54 μM) only indicated mild BChE inhibitory activity. According to Atta-ur-Rahman and Choudhary (Citation2001), there is a correlation between antioxidant and anticholinesterase activities. However, only compound 6 followed this correlation in this study.
Table 2. Anticholinesterase activity of the compounds of Dodonaea viscosaTable Footnotea.
Structure–activity relationships
The skeleton of clerodane diterpenoids 1 and 2 is almost the same. Compound 1 possesses a lactone while 2 have two additional –OHs. Compound 1A is the crystalline form of 1. The diterpenes (1, 1A and 2) were tested for their potential antioxidant activity using six methods. Compound 2 exhibited noticeable lipid peroxidation inhibitory activity only in β-carotene-linoleic acid assay. The –OH at C-6 may be responsible for higher activity of 2 when compared with 1 or 1A ().
The origin of antioxidant activity of phenolics is due to their hydroxyl groups. The location of the –OH groups can increase or decrease the activity. Particularly, the compounds having second –OH at ortho or para position exhibit higher antioxidant activity than at meta position. Effect of the functional group is responsible for antioxidant activity in the following order: –OH> –OAc> –C = O (oxo) (Farvin and Jacobsen Citation2013). In all antioxidant assays, the phenolic compounds (3, 3A, 5 and 6) exhibited good antioxidant activity. Compound 4 was active only in CUPRAC assay.
The structures of 3 and 4 are similar to each other. The difference is the replacement of − OH at C-3 position by the − OCH3 group in 4. However, the difference causes decrease in the antioxidant activity in case of − OCH3 group.
Remarkably, all antioxidant activity tests revealed that the crystal (monohydrate) (3A) showed higher antioxidant activity than its amorphous form (3). On the other hand, anhydrous crystal 1A showed close activity to its amorphous form (1). The reason for the higher activity is the presence of water in crystallisation in 3A.
The antioxidant activity of phenolic compounds 5 and 6 is also comparable. Compound 5 has only one –OH group, and 6 has two ortho –OH groups at benzene ring. Because of these two ortho –OH groups, 6 exhibited better antioxidant activity than 5 in β-carotene, DPPH, superoxide and metal chelating assays. Interestingly, 5 exhibited better activity than 6 in ABTS and CUPRAC assays. The activity of 5 in ABTS and CUPRAC assays could be related to –OH group at C-1 position. The 3-methyl-2-butenyl group at C-2 position donates electrons to benzene ring; probably that is the reason for the better activity of 5. Additionally, it is not easy for compound 5 to approach DPPH• molecule due to its bulky 3-methyl-2-butenyl group. The structure–activity relationships showed that the position and number of –OHs can cause a tremendous effect on the biological activities.
Conclusion
Antioxidant and anticholinesterase activities of clerodane diterpenoids (1 and 2) and phenolics (5 and 6), isolated from D. viscosa, were determined for the first time. Unlike previous reports, compound 7A was obtained in a crystal form and characterised by XRD for the first time. XRD structure confirmation also diminished the ambiguity in the stereochemistry of 7A. It can be understood from the structure–activity relationship that the presence and position of –OH in the molecule is very important to give antioxidant activity. More –OH groups and its presence at ortho or para increases the biological activity, however, a meta-OH decreases. On one hand, compounds 3A and 6 exhibited higher antioxidant activity in all tests while 5 in ABTS and in β-carotene-linoleic acid assays. Interestingly, monohydrate 3A showed better antioxidant activity than amorphous 3. On the other hand, amorphous 1 exhibited close activity to that of its crystal 1A. It means that water of crystallisation can affect the antioxidant activity of a certain compound. We expect these compounds to have anticancer and enzyme inhibitory activities; similar to other antioxidants. Moreover, compound 6 was also found as a moderate inhibitor against AChE and BChE enzymes.
Funding information
Authors are thankful to The Scientific and Technological Research Council of Turkey (TUBITAK-BIDEB 2216 and 2211 programs) to provide financial support.
Acknowledgements
Authors are thankful to International Center for Chemical and Biological Sciences (ICCBS) and Chemistry Departments of Karachi and Mugla Sitki Kocman Universities for technical and instrumental support.
Disclosure statement
The authors report that they have no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abdullah P. 1973. Sapindaceae. In: Nasir E, Ali SI, editors. Flora of West Pakistan. Rawalpindi: Gordon College. pp. 39,1–3.
- Agilent Crys Alis PRO. 2010. Agilent technologies. Yarnton, Oxfordshire: England.
- Ahmad H, Ahmad A, Jan MM. 2002. The medicinal plants of Salt Range. J Biol Sci. 2:175–177.
- Anis I, Anis E, Ahmed S, Mustafa G, Malik A, Amtul Z, Atta-ur-Rahman, et al. 2001. Thrombin inhibitory constituents from Duranta repens. Helv Chim Acta. 84: 649–655.
- Apak R, Güçlü K, Özyürek M, Karademir SE. 2004. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J Agric Food Chem. 52:7970–7981.
- Atta-ur-Rahman, Choudhary MI. 2001. Bioactive natural products as a potential source of new pharmacophores. A theory of memory. Pure Appl Chem. 73:555–560.
- Barbour LJ. 2001. X-Seed – a software tool for supramolecular crystallography. J Supramol Chem. 1:189.
- Blois MS. 1958. Antioxidant determinations by the use of a stable free radical. Nature. 181:1199–1200.
- Choudhary MI, Mohammad YM, Musharraf SG, Onajobi I, Mohammad A, Anis I, Shah MR, Atta-ur-Rahman. 2013. Biotransformation of clerodane diterpenoids by Rhizopus stolonifer and antibacterial activity of resulting metabolites. Phytochemistry. 90:56–61.
- Clarkson C, Maharaj VJ, Crouch NR, Grace WM, Pillay P, Matsabisa MG, Bhagwandin N, Smith PJ, Folb PI. 2004. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J Ethnopharmacol. 92:177–191.
- Decker EA, Welch B. 1990. Role of ferritin as a lipid oxidation catalyst in muscle food. J Agric Food Chem. 38:674–677.
- Dökmeci I. 2000. Basic concepts in pharmacology. Istanbul, Turkey: Nobel Tıp Publication Press.
- Ellman GL, Courtney KD, Andres V, Featherstone RM. 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 7:88–95.
- Farvin KHS, Jacobsen J. 2013. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 138:1670.
- Grice HP. 1988. Safety evaluation of butylated hydroxyanisole from the perspective of effects on forestomach and oesophageal squamous epithelium. Food Chem Toxicol. 26:717–723.
- Grossberg GT. 2003. Cholinesterase inhibitors for the treatment of Alzheimer's disease: getting on and staying on. Curr Ther Res Clin Exp. 64:216–235.
- Halliwell B, Gutteridge JMC. 1984. Oxygen-toxicity, oxygen radicals, transition-metals and disease. Biochem J. 219:1–14.
- Ito N, Fukushima S, Haqlwara A, Shibata M, Ogiso T. 1983. Carcinogenicity of butylated hydroxyanisole in F344 rats. J Natl Cancer Inst. 70:343–352.
- Marco GJ. 1968. A rapid method for evaluation of antioxidants. J Am Oil Chem Soc. 45:594–598.
- Mohammad A, Shah MR, Anis I, McKee V, Frese JW. 2010. 7-[2-(3-Furyl) ethyl]-7,8-dimethyl-3, 5, 6, 6a, 7, 8, 9, 10-octahydro-1H-naphtho [1, 8a–c] furan-3-one. Acta Crystallogr E. 66: 2529–2529.
- Muhammad A, Anis I, Khan A, Marasini BP, Choudhary MI, Shah MR. 2012a. Biologically active C-alkylated flavonoids from Dodonaea viscosa. Arch Pharm Res. 35:431–436.
- Muhammad A, Anis I, Ali Z, Awadelkarim S, Khan A, Khalid A, Shah MR, Galal M, Khan IA, Choudhary MI. 2012b. Methylenebissantin: a rare methylene-bridged bisflavonoid from Dodonaea viscosa which inhibits Plasmodium falciparum enoyl-ACP reductase. Bioorg Med Chem Lett. 22:610–612.
- Ortega A, Garcia PE, Cardenas J, Mancera C, Marquina S, Garduno MLC, Maldonado E. 2001. Methyl dodonates, a new type of diterpenes with a modified clerodane skeleton from Dodonaea viscosa. Tetrahedron. 57:2981–2989.
- Öztürk M, Tel G, Aydogmus-Öztürk F, Duru ME. 2014. The cooking effect on two edible mushrooms in Anatolia: fatty acid composition, total bioactive compounds, antioxidant and anticholinesterase activities. Rec Nat Prod. 8:189–194.
- Pirzada AJ, Shaikh W, Usmanghani K, Mohiuddin E. 2010. Antifungal activity of Dodonaea viscosa Jacq extract on pathogenic fungi isolated from superficial skin infection. Pak J Pharm Sci. 23:337–340.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 26:1231–1237.
- Scalbert A, Manach C, Morand C, Remesy C. 2005. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 45:287–306.
- Sheldrick GM. 2008. A short history of SHELX. Acta Crystallogr A Found Crystallogr. 64:112–122.
- Tel G, Apaydın M, Duru ME, Öztürk M. 2012. Antioxidant and cholinesterase inhibition activities of three Tricholoma species with total phenolic and flavonoid contents: the edible mushrooms from Anatolia. Food Anal Methods. 5:495–504.
- Westrip S. 2010. PublCIF: software for editing, validating and formatting crystallographic information files. J Appl Crystallogr. 43:920–925.