Abstract
Context Ethnopharmacological studies have demonstrated that plants of the Combretum genus presented antidiabetic activity, including Combretum lanceolatum Pohl ex Eichler (Combretaceae).
Objective This study investigated the hepatic mechanisms of action of C. lanceolatum flowers ethanol extract (ClEtOH) related to its antihyperglycaemic effect in streptozotocin-diabetic rats.
Materials and methods Male Wistar rats were divided into normal (N) and diabetic control (DC) rats treated with vehicle (water); diabetic rats treated with 500 mg/kg metformin (DMet) or 500 mg/kg ClEtOH (DT500). After 21 d of treatment, hepatic glucose and urea production were investigated through in situ perfused liver with l-glutamine. Changes in the phosphoenolpyruvate carboxykinase (PEPCK) levels and in the activation of adenosine monophosphate-activated protein kinase (AMPK) and insulin-signalling intermediates were also investigated.
Results Similar to DMet, DT500 rats showed a reduction in the rates of hepatic production of glucose (46%) and urea (22%) in comparison with DC. This reduction was accompanied by a reduction in the PEPCK levels in liver of DT500 (28%) and DMet (43%) when compared with DC. AMPK phosphorylation levels were higher in the liver of DT500 (17%) and DMet (16%) rats. The basal AKT phosphorylation levels were increased in liver of DT500 rats, without differences in the insulin-stimulated AKT phosphorylation and in the insulin receptor levels between DC and DT500 rats.
Discussion and conclusion The antidiabetic activity of ClEtOH can be attributed, at least in part, to inhibition of hepatic gluconeogenesis, probably due to the activation of both AMPK and AKT effectors and reduction in the PEPCK levels.
Introduction
Diabetes mellitus (DM) is a chronic endocrine disorder resulting from defects in pancreas insulin production and secretion and/or insulin resistance in peripheral tissues, leading to abnormalities in carbohydrate, lipid and protein metabolism. Lifestyle changes in modern society, such as diminished physical activity and increased ingestion of high-energy foods have explained the high incidence of DM, which is reaching epidemic proportions worldwide (Hossain et al. Citation2007; van Dieren et al. Citation2010). In 2013, more than 382 million people had diabetes, with estimation to rise to 592 million worldwide by 2035 (Guariguata et al. Citation2014).
Chronic hyperglycaemia accounts for the establishment of diabetic complications, such as retinopathy, nephropathy, autonomic and peripheral neuropathies and vascular diseases, these contributing to the morbidity and mortality related to this disease (Nickerson & Dutta Citation2012). Impaired glucose uptake by adipose and skeletal muscle tissues and increased rate of hepatic gluconeogenesis appear as the main causes of hyperglycaemia in insulin-deficient and/or resistant states. Therefore, therapeutic options that restore these biochemical disturbances are beneficial to treat diabetic patients, such as metformin, a widely prescribed antidiabetic agent, which improves peripheral glucose uptake and inhibits hepatic glucose output, among other benefits (Klip & Leiter Citation1990; Viollet et al. Citation2012). It is interesting to note that metformin has derived its structure from guanidine, a compound isolated from Galega officinalis L. [Fabaceae (Leguminosae)] (Bailey & Day Citation1989). Hence, it stimulated the number of studies focusing on the search of novel medicines from natural origin to treat DM; these studies are now concerned not only in confirming the traditional knowledge about the use of medicinal plants but also in investigating the mechanisms of action that explain the antihyperglycaemic effect of herbal species.
Some studies have reported that plants from Combretum genus possess antidiabetic activity (Pannangpetch et al. Citation2008; Ojewole & Adewole Citation2009; Chika & Bello Citation2010). Among these, Combretum lanceolatum Pohl (Combretaceae), commonly known as “pombeiro-vermelho” can be cited as largely distributed in the Brazilian Pantanal. Recent studies by Dechandt et al. (Citation2013) demonstrated that the treatment of streptozotocin (STZ)-diabetic rats with the C. lanceolatum flowers ethanol extract (ClEtOH) for 21 d improved various parameters classically altered in diabetes, including a decrease in the blood and urinary glucose levels. In addition, a decrease in the urinary urea and an increase in the weight of soleus and extensor digitorum longus (EDL) muscles were also observed, suggesting that the extract could be acting, at least in part, through reduction in the hepatic gluconeogenesis. Also, the beneficial effects of the treatment with ClEtOH were very similar to that promoted by metformin. Furthermore, in vitro incubations of liver slices with ClEtOH or with metformin were able to stimulate adenosine monophosphate-activated protein kinase (AMPK) (Dechandt et al. Citation2013).
Considering that at least part of the inhibitory action of metformin on hepatic gluconeogenesis occurs through AMPK stimulation (Lochhead et al. Citation2000; Zhou et al. Citation2001), the objective of this study was to investigate the changes in the rate of gluconeogenesis and in the AMPK activation in liver of diabetic rats treated with ClEtOH, which could be related with its antihyperglycaemic effect. For this, the rate of hepatic gluconeogenesis in situ, the protein levels of phosphoenolpyruvate carboxykinase (PEPCK), key enzyme of this process and the changes in the phosphorylation levels of AMPK in liver were determined. Changes in the insulin-signalling components, insulin receptor (IR) and AKT were also investigated.
Materials and methods
Plant material and extraction preparation
Flowers of C. lanceolatum were collected in Poconé, Mato Grosso, Brazil (S 16°18′56.4″; W 056°32′21.5″; 126 m of elevation) in July 2010 and identified by Dr. Germano Guarin Neto, in Central Herbarium of UFMT, where a voucher specimen was deposited under the number 39 149. The flowers of C. lanceolatum were dried at room temperature and grounded in electric grinder; later the powder (5.960 g) was placed in maceration with ethanol (13 L at each extraction) at room temperature under occasional shaking, in seven cycles of 7 d. The mixture was filtered and the ethanol was evaporated under reduced pressure at 38 °C and the residue was dried at 40 °C to afford ClEtOH (2.350 g; 39.43%). ClEtOH was stored at 4 °C and, at the time of use, it was suspended in water and orally administered by gavage.
Animals
Male Wistar rats weighing 180–200 g (38–40 d old) were kept into individual metabolic cages, housed under environmentally controlled conditions (24 ± 1 °C) with a 12 h light/dark cycle and they had free access to water and normal lab chow diet (Purina®Labina). All experiments were performed between 08:00 and 10:00 am. The animals were managed according to the Brazilian College of Animal Experimentation (COBEA) and procedures received prior institutional approval by Committee for Ethics in Animal Experimental from UFMT (protocol no. 23108.029613/09-3).
Induction of diabetes mellitus
Experimental diabetes mellitus was induced by a single intravenous injection of streptozotocin (STZ, 40 mg/kg) dissolved in 0.01 M citrate buffer (pH 4.5), in previously 15 h fasted rats. Non-diabetic, control animals were obtained injecting vehicle (0.01 M citrate buffer). Five days after STZ administration, rats with postprandial glycaemia values of approximately 500 mg/dL were used in the experiments. Plasma glucose levels were determined by the glucose oxidase method (Bergmeyer & Bernt Citation1974) using a commercial kit (Labtest Diagnostica SA, Lagoa Santa, Brazil).
Experimental design and analysis
The animals were randomly assigned into four groups: N, non-diabetic rats (treated with vehicle, water); DC, diabetic control rats (treated with water); DMet, diabetic rats treated with 500 mg/kg metformin; DT500, diabetic rats treated with 500 mg/kg ClEtOH. The groups received vehicle (water), metformin or ClEtOH by oral gavage, once a day, for 21 d. Body weight was monitored daily. Plasma glucose levels were monitored at every 5 d (blood samples collected from the tip of the tail). At the end of the treatment, the animals were euthanised in order to collect the liver and blood samples, as well as to perform liver perfusion analysis. Liver and serum samples were stored at −80 °C until analysis.
Insulin serum levels were determined through immunoenzymatic assay (Millipore Corporation, Billerica, MA). In liver, the protein levels of PEPCK and the changes in the AMPK activation and in the insulin-signalling components (IR and AKT) were determined through Western blotting. Hepatic glucose and urea production from l-glutamine were investigated through in situ perfused liver.
Liver perfusion
Livers of rats fasted for 15 h were perfused in situ as previously described (Obici et al. Citation2008). After a 10 min pre-perfusion period, the gluconeogenic substrate l-glutamine, under saturating concentration (5 mM), was dissolved into the perfusion fluid and infused during 50 min. Samples of the effluent perfusion fluid were collected at 5 min intervals and the concentrations of glucose and urea were measured by glucose oxidase reaction (Bergmeyer & Bernt Citation1974) and urease reaction (Bernt & Bergmeyer Citation1965), respectively, using commercial kits (Labtest Diagnostica SA, Lagoa Santa, Brazil). The differences in the glucose and urea production during and before the infusion of l-glutamine were used to calculate the area-under-the-curves (AUC), expressed as μmol/g.
Western blotting analysis
Fragments of liver were homogenised in 50 mM Tris-HCl buffer (pH 7.4) at 4 °C containing 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% sodium dodecyl sulphate (SDS), 10 mM sodium pyrophosphate, 100 mM sodium fluoride, 10 mM sodium orthovanadate, 5 μg/mL aprotinin and 1 mM phenylmethylsulphonyl fluoride. The homogenates were centrifuged at 11 000 rpm for 40 min at 4 °C and the supernatants were used for analysis. Protein levels were determined using bovine serum albumin as standard (Bradford Citation1976). Samples containing 100 μg protein were prepared with sample buffer (250 mM Tris-HCl, 50% glycerol, 10% SDS, 500 mM dithiothreitol, 0.1% bromophenol blue, pH 6.8) and subjected to SDS-PAGE analysis on 10% acrylamide gels (Laemmli Citation1970). Proteins were electroblotted onto nitrocellulose membranes (Towbin et al. Citation1979). After blocking, membranes were incubated overnight at 4 °C with anti-PEPCK (1:5000, Santa Cruz, CA), anti-AMPK (1:1000, Cell Signaling Technology, Danvers, MA), anti-p-[Thr172]-AMPK (1:1000, Cell Signaling Technology, Danvers, MA) anti-IR (1:750, Santa Cruz, CA), anti-AKT (1:500, Santa Cruz, CA) and anti-p-[Ser473]-AKT (1:500, Santa Cruz, CA). Anti-histone (1:500, Santa Cruz, CA) was used as the internal control. Primary antibodies were detected by peroxidase-conjugated secondary antibodies and visualised with chemiluminescent substrate (1.25 mM luminol, 0.198 mM p-coumaric acid, 0.00915% hydrogen peroxide, 0.1 M Tris-HCl and pH 8.5). Band intensities were quantified using the ImageJ Program (Version 1.38, NIH, Bethesda, MD).
Insulin-signalling studies
To examine the changes in the insulin-signalling components in liver of each experimental group, animals were fasted 5 h before the morning of the experiment. After this period, the animals were anesthetised with ketamine/xylazine (50 mg/5 mg for each kilogram of animal) and then intraperitoneally injected with 0.9% sodium chloride (non-stimulated animals; basal values) or with 10 U insulin/kg (stimulated animals); after 10 min, liver samples were quickly removed and stored at −80 °C until analysis. The IR and AKT protein levels and the phosphorylation levels of AKT (serine-473 residue) were determined through Western blotting analysis as described above.
Statistical analysis
Data were expressed as mean ± standard error of mean (SEM). Statistical analysis was performed with GraphPad Prism 5.01 (GraphPad Software, Inc., La Jolla, CA). One-way analysis of variance (ANOVA) followed by the Newman–Keuls test was used to analyse differences among different groups. Unpaired Student’s t-test was used to compare the changes in the AKT phosphorylation levels between non-stimulated and insulin-stimulated groups. Differences were considered significant at p < 0.05.
Results
Confirmation of the antidiabetic activity of ClEtOH
At the beginning of the experiment, DC, DMet and DT500 rats exhibited similar values of plasma glucose and body weight. As expected, the initial and final postprandial glycaemia values were increased in DC rats when compared with N. After 21 d of treatment, postprandial glycaemia was decreased in DMet (39%; p < 0.05) and DT500 (31%; p < 0.05) rats. Similarly, fasting glycaemia values were also decreased in DMet (41%; p < 0.05) and DT500 (27%; p < 0.05) rats in comparison with DC ().
Table 1. Body weight and postprandial and fasting plasma glucose levels of diabetic rats non-treated (DC), treated for 21 d with 500 mg/kg metformin (DMet) or with 500 mg/kg Combretum lanceolatum flowers ethanol extract (DT500).
It was observed a minor body weight gain in DC rats when compared with N. DMet and DT500 rats had a greater weight gain; after 21 d of treatment, the body weight values were 11% (p < 0.05) and 18% (p < 0.05) increased, respectively, when compared with DC ().
These results confirm previous findings regarding the antidiabetic activity of ClEtOH (Dechandt et al. Citation2013), allowing the investigation of the hepatic mechanisms of action of the extract.
Insulin serum levels
The postprandial serum insulin levels were significantly lower in all groups of diabetic rats in comparison with N rats, evidencing the diabetogenic effect of STZ. The treatment of diabetic rats with metformin or with ClEtOH did not change the insulin serum levels when compared with DC rats (N = 2.983 ± 0.512; DC = 0.645 ± 0.154; DMet = 0.772 ± 0.144; DT500 = 0.659 ± 0.082, ng/mL, n = 5–10 animals). Therefore, the changes observed in the hepatic gluconeogenesis cannot be attributed to the ClEtOH action in stimulating the pancreatic insulin production/secretion and/or in inhibiting the insulin degradation.
Rates of hepatic gluconeogenesis and ureogenesis
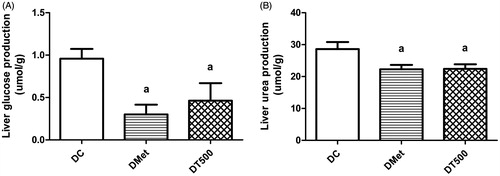
As expected, liver of DMet rats perfused with l-glutamine showed a reduction in the rates of glucose (68%; p < 0.05) and urea production (22%; p < 0.05) in comparison with DC rats (). The hepatic glucose production was 46% lower (p < 0.05) in DT500 rats when compared with DC; this reduction was accompanied by a decrease of 22% (p < 0.05) in the urea production ().
Figure 1. Hepatic glucose (A) and urea (B) production from saturating concentration of l-glutamine (5 mM) in diabetic rats non-treated (DC), treated for 21 d with 500 mg/kg metformin (DMet) or with 500 mg/kg Combretum lanceolatum flowers ethanol extract (DT500). Data represent mean ± SEM of 5–7 animals per group. ap < 0.05 versus DC (ANOVA followed by Newman–Keuls).
PEPCK protein levels
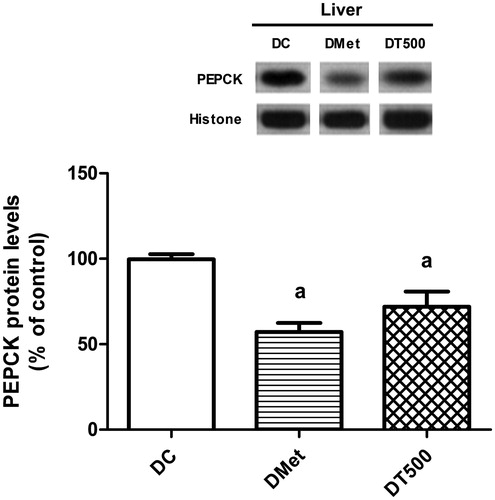
Corroborating the findings on the hepatic glucose and urea production, it was observed that the protein levels of PEPCK in liver of rats from DMet and DT500 groups were 43% (p < 0.05) and 28% (p < 0.05) decreased, respectively, in comparison with DC ().
Figure 2. Protein levels of PEPCK in liver of diabetic rats non-treated (DC), treated for 21 d with 500 mg/kg metformin (DMet) or with 500 mg/kg Combretum lanceolatum flowers ethanol extract (DT500). Data represent mean ± SEM of 6–7 animals per group. ap < 0.05 versus DC (ANOVA followed by Newman–Keuls).
Changes in the AMPK activation and in the insulin-signalling intermediates
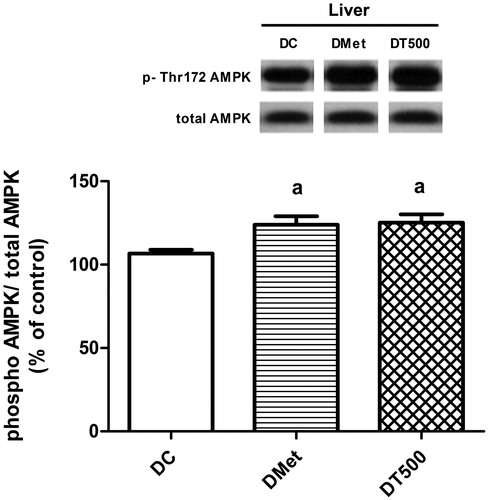
AMPK was activated in liver of DMet rats, since the AMPK phosphorylation levels were 16% higher (p < 0.05) than values from DC. In a similar magnitude, DT500 rats showed a 17% increase (p < 0.05) in the phosphorylation levels of AMPK ().
Figure 3. Phosphorylation (Thr-172 residue) levels of AMPK in liver of diabetic rats non-treated (DC), treated for 21 d with 500 mg/kg metformin (DMet) or with 500 mg/kg Combretum lanceolatum flowers ethanol extract (DT500). Data represent mean ± SEM of 13–15 animals per group. ap < 0.05 versus DC (ANOVA followed by Newman–Keuls).
The protein levels of IR were not different in liver of DT500 rats in comparison with values of N or DC rats ().
Figure 4. Insulin-signalling changes in liver of normal rats (N), diabetic rats non-treated (DC) or treated for 21 d with 500 mg/kg Combretum lanceolatum flowers ethanol extract (DT500). Protein levels of IR (A) and basal (−) or insulin-stimulated (+) phosphorylation (Ser-473 residue) levels of AKT (B). Data represent mean ± SEM of 5–7 animals per group. Comparison among basal values (ANOVA followed by Newman–Keuls): ap < 0.05 versus N(−); bp < 0.05 versus DC(−). Comparison between basal and insulin-stimulated phosphorylation (Student’s t-test): cp < 0.05 versus DC(−); dp < 0.05 versus DT500(−).
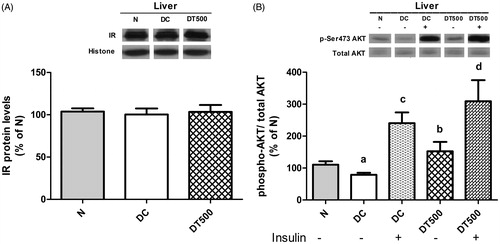
The basal AKT phosphorylation in liver of DC rats was 32% lower [p < 0.05; DC(−) versus N(−)] than N, showing the impairment in the hepatic insulin signalling, probably due to the low insulin levels as a consequence of pancreatic beta cells destruction by STZ. The basal AKT phosphorylation levels were similar between N and DT500 rats [DT500(−) versus N(−)], showing that diabetic rats treated with ClEtOH recovery, at least in part, the capacity to activate the insulin signalling. This improvement can be also noted when comparing DC and DT500 rats, since the basal AKT phosphorylation of DT500 rats was approximately 2-fold increased [p < 0.05; DT500(−) versus DC(−)] in comparison with DC (). As expected, the insulin administration increased the AKT phosphorylation in comparison with basal values, in both DC [162%; p < 0.05; DC(+) versus DC(−)] and DT500 [157%; p < 0.05; DT500(+) versus DT500(−)] rats; however, no differences were found in the insulin-stimulated AKT phosphorylation levels between DC and DT500 rats [; DT500(+) versus DC(+)].
Discussion
Previous studies demonstrated that ClEtOH reduced plasma glucose levels in STZ-diabetic rats after 21 d of treatment; the extract also reduced the urinary urea levels and the mass loss of soleus and EDL skeletal muscles (Dechandt et al. Citation2013), indicating a possible role in the inhibition of gluconeogenesis. Attempting for this, the present study showed that at least part of the antihyperglycaemic effect of ClEtOH is related to the reduction of the hepatic glucose output, since its production was reduced in liver of diabetic rats treated with ClEtOH. Furthermore, this inhibitory effect on the glucose production was accompanied by a decrease in the protein levels of PEPCK, a key enzyme of gluconeogenesis process, probably due to the activation of both AMPK and AKT effectors in liver of diabetic rats treated with ClEtOH.
It is well known that the regulation of liver gluconeogenesis involves the transcriptional control of glucose-6-phosphatase (G6Pase) and PEPCK, enzymes that catalyse committed steps of this process. In DM, the association of low insulin levels (or insulin resistance state) with increased levels of glucagon and glucocorticoids contributes to the increased expression of gluconeogenic enzymes and the high rate of glucose production and disposal (Jacobson et al. Citation2005; Rizza Citation2010). Along with the diminished inhibitory effect of insulin on gluconeogenesis, the increased glucose output is favoured by the augmented offering of substrates to the liver, mainly lactate, glycerol and alanine. Attempting to clarify the relative contribution of the different substrates for gluconeogenesis in DM, Bazotte et al. investigated the hepatic glucose output in diabetic rats (fasting or fed states) using liver perfusion with isolated substrates at saturating concentrations and at a constant flow rate (Ferraz et al. Citation1997; Akimoto et al. Citation2000). The authors found that although alanine is the most important amino acid substrate for gluconeogenesis, the glucose production in liver of diabetic rats perfused with l-alanine was unchanged in comparison with perfusions without the amino acid, in both fasted (Ferraz et al. Citation1997) and fed (Akimoto et al. Citation2000) diabetic rats. Different from l-alanine, the production of glucose and urea was much increased in liver of diabetic rats perfused with l-glutamine (Akimoto et al. Citation2000), justifying the option for this amino acid in the present study. Finally, the glucose production in liver of diabetic rats perfused with l-lactate, pyruvate or glycerol was also increased (Ferraz et al. Citation1997).
The restoration of liver PEPCK levels has been pointed out as one important target to decrease both liver glucose output and hyperglycaemia in DM. Among the pharmacological options to treat DM, it has been demonstrated that metformin suppresses hepatic glucose production through the down regulation of PEPCK expression; this action is achieved after the activation of AMPK (Lochhead et al. Citation2000; Zhou et al. Citation2001). AMPK is a serine/threonine protein kinase that controls the physiological energy dynamics in several tissues (Hardie Citation2007). In liver, AMPK inhibits glucose production reducing the PEPCK expression through inhibition of CREB-regulated transcription coactivator 2 (CRTC2) (Koo et al. Citation2005) and forkhead box O (FoxO1) (Barthel et al. Citation2002), two transcriptional factors that control the gene expression of gluconeogenic enzymes (Jitrapakdee Citation2012). Data of the present study showed that AMPK phosphorylation levels were increased in liver of diabetic rats treated with metformin (), which explains, at least in part, the decrease in the PEPCK levels () and the subsequent fall in the hepatic glucose output (). Since l-glutamine was used in the experiments as gluconeogenic substrate, the amino acid deamination (and the subsequent urea production) is an essential step prior to the disposal of alpha-ketoglutarate to gluconeogenesis process; in this way, decrease in the urea output was also observed in liver of diabetic rats treated with metformin (). Taken together, these results showed that gluconeogenesis was inhibited in diabetic rats treated with metformin explaining, at least in part, the decrease in plasma glucose levels ().
It has been widely recognised that AMPK is an important target to treat DM and other metabolic disorders (Gruzman et al. Citation2009; Viollet et al. Citation2009). Therefore, the discovery of plant preparations that exert its antidiabetic activity through AMPK activation is extremely interesting throughout the search of isolated compounds or during the development of phytotherapic formulations to be used in diabetes treatment. In this way, our data confirm that ClEtOH led to AMPK activation, since its phosphorylation levels were increased in liver of diabetic rats treated with the extract (). Quercetin, the major constituent of ClEtOH, can be mentioned as responsible for the AMPK activation; the in vitro incubation of liver slices with quercetin, ClEtOH or metformin increased the AMPK phosphorylation (Dechandt et al. Citation2013). In agreement with these findings, Sato et al. (Citation2013) also observed that the phosphorylation of AMPK was increased in liver from adult offspring rats of protein-restricted dams that received quercetin during lactation, which could be a beneficial mechanism against the long-term metabolic disturbances related to malnutrition during gestational periods. So, the AMPK activation can explain, at least in part, the decrease in the protein levels of PEPCK observed in liver of diabetic rats treated with ClEtOH ().
A decrease in both glucose () and urea () outputs by the liver of diabetic rats treated with ClEtOH was observed. It is important to highlight that both glucose and urea production values were very similar between diabetic rats treated with metformin or with ClEtOH, as well as the PEPCK levels, demonstrating that the pharmacological efficacy of ClEtOH in STZ-diabetic rats was equivalent to that of the classical antidiabetic agent. It has been reported that plants of the Combretum genus or their isolated compounds exert antidiabetic activities through PEPCK inhibition. In a study of Zhang et al. (Citation2008), it was demonstrated that the stimulation of AMPK and the subsequent PEPCK down-regulation expression in liver of diabetic db/db mice are involved in the improvement of glucose tolerance promoted by combretastatin A-4, a resveratrol structural analogue isolated from Combretum caffrum tree. Welch (Citation2010) observed that high-fat diet diabetic mice treated for 7 weeks with the n-butanol fraction from Combretum micranthum leaf extract showed a decrease in liver PEPCK expression, which could explain the antidiabetic activity (Chika & Bello Citation2010).
The inhibition of PEPCK expression and the subsequent decrease in the glucose production by gluconeogenesis can be also achieved through the activation of another kinase, named protein kinase B (PKB) or AKT. It is well established that AKT is an important insulin downstream effector involved in the hepatic gluconeogenesis inhibition; once activated by insulin, AKT phosphorylates and inhibits FoxO1 transcriptional factor, so inhibiting the PEPCK gene expression (Cheng & White Citation2011). Interestingly, our present data showed that the basal phosphorylation levels of AKT were increased in liver of diabetic rats treated with ClEtOH, when compared with non-treated diabetic rats, reaching values similar to that found in N rats (). Considering that no changes were observed neither in the abundance of IR () nor in the insulin-stimulated AKT phosphorylation () in liver of rats treated with ClEtOH, it can be suggested that the extract did not improve the insulin sensitivity. In addition, the basal AKT stimulation occurs without changes in the serum insulin levels (see Results section). Taken together, these data suggested that ClEtOH probably has compounds that, isolated or combination, present an insulin-like action, stimulating insulin-signalling intermediates. Corroborating our hypothesis, Dai et al. (Citation2013) attributed the antidiabetic effect of quercetin to its ability to stimulate AKT (and also AMPK) in skeletal muscle cells, leading to an increased glucose uptake. Finally, the stimulation of hepatic AKT could explain not only the decrease in the PEPCK levels and in gluconeogenesis but also the increase in the hepatic glycogen content previously observed in diabetic rats treated with ClEtOH (Dechandt et al. Citation2013); the stimulation of liver glycogen synthesis could be an additional mechanism of the extract that also contributes to reduce hyperglycaemia.
Conclusions
The findings of the present study suggest that the inhibition of gluconeogenesis participates as one important mechanism of action of C. lanceolatum flower extract related to its antihyperglycaemic effect, which probably depends on the activation of both AMPK and AKT effectors. Combretum lanceolatum appears to be a promising Brazilian plant to be used in phytotherapic formulations to reduce hyperglycaemia and to improve other disturbances related to diabetes mellitus.
Acknowledgements
The authors thank Air Francisco Costa and Diego Luiz Doneda for the technical assistance and to the Brazilian foundations, Programa Institutos Nacionais de Ciência e Tecnologia em Áreas Úmidas – CNPq/MCT, Centro de Pesquisas do Pantanal and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). This work is part of a dissertation presented by Juliany Torres Siqueira as a partial requirement for the Master’s degree in Chemistry at the Department of Chemistry, UFMT.
Disclosure statement
The authors report that they have no conflicts of interest.
References
- Bailey CJ, Day C. 1989. Traditional plant medicines as treatments for diabetes. Diabetes Care. 12:553–564.
- Akimoto LS, Pedrinho SR, Lopes G, Bazotte RB. 2000. Rates of gluconeogenesis in perfused liver of alloxan-diabetic fed rats. Res Commun Mol Pathol Pharmacol. 107:65–77.
- Barthel A, Schmoll D, Krüger KD, Roth RA, Joost HG. 2002. Regulation of the forkhead transcription factor FKHR (FOXO1a) by glucose starvation and AICAR, an activator of AMP-activated protein kinase. Endocrinology. 143:3183–3186.
- Bergmeyer HU, Bernt E. 1974. Determination of glucose with glucose-oxidase and peroxidase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. New York: Verlag Chemie-Academic Press. p. 1205–1215.
- Bernt E, Bergmeyer HU. 1965. Urea. In: Bergmeyer HU, editor. Methods of enzymatic analysis. New York: Academic Press. p. 401–406.
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 72:248–254.
- Cheng Z, White MF. 2011. Targeting Forkhead box O1 from the concept to metabolic diseases: lessons from mouse models. Antioxid Redox Signal. 14:649–661.
- Chika A, Bello SO. 2010. Antihyperglycaemic activity of aqueous leaf extract of Combretum micranthum (Combretaceae) in normal and alloxan-induced diabetic rats. J Ethnopharmacol. 129:34–37.
- Dai X, Ding Y, Zhang Z, Cai X, Bao L, Li Y. 2013. Quercetin but not quercitrin ameliorates tumor necrosis factor-alpha-induced insulin resistance in C2C12 skeletal muscle cells. Biol Pharm Bull. 36:788–795.
- Dechandt CR, Siqueira JT, Souza DL, Araujo LC, Silva VC, Junior Sousa-PT, Andrade CMB, Kawashita NH, Baviera AM. 2013. Combretum lanceolatum Pohl flowers extract shows antidiabetic activity through activation of AMPK by quercetin. Braz J Pharmacog. 23:291–300.
- Ferraz M, Brunaldi K, Oliveira CE, Bazotte RB. 1997. Hepatic glucose production from L-alanine is absent in perfused liver of diabetic rats. Res Commun Mol Pathol Pharmacol. 95:147–155.
- Gruzman A, Babai G, Sasson S. 2009. Adenosine monophosphate-activated protein kinase (AMPK) as a new target for antidiabetic drugs: a review on metabolic, pharmacological and chemical considerations. Rev Diabet Stud. 6:13–36.
- Hardie DG. 2007. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 8:774–785.
- Hossain P, Kawar B, El Nahas M. 2007. Obesity and diabetes in the developing world-a growing challenge. N Engl J Med. 356:213–215.
- Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. 2014. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 103:137–149.
- Jacobson PB, von Geldern TW, Ohman L, Osterland M, Wang J, Zinker B, Wilcox D, Nguyen PT, Mika A, Fung S, et al. 2005. Hepatic glucocorticoid receptor antagonism is sufficient to reduce elevated hepatic glucose output and improve glucose control in animal models of type 2 diabetes. J Pharmacol Exp Ther. 314:191–200.
- Jitrapakdee S. 2012. Transcription factors and coactivators controlling nutrient and hormonal regulation of hepatic gluconeogenesis. Int J Biochem Cell Biol. 44:33–45.
- Klip A, Leiter LA. 1990. Cellular mechanism of action of metformin. Diabetes Care. 13:696–704.
- Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, et al. 2005. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 437:1109–1111.
- Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227:680–685.
- Lochhead PA, Salt IP, Walker KS, Hardie DG, Sutherland C. 2000. 5-Aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase. Diabetes. 49:896–903.
- Nickerson HD, Dutta S. 2012. Diabetic complications: current challenges and opportunities. J Cardiovasc Transl Res. 5:375–379.
- Obici S, Lopes-Bertolini G, Curi R, Bazote RB. 2008. Liver glycogen metabolism during short-term insulin-induced hypoglycemia in fed rats. Cell Biochem Funct. 26:755–759.
- Ojewole JA, Adewole SO. 2009. Hypoglycaemic effect of mollic acid glucoside, a 1alpha-hydroxycycloartenoid saponin extractive from Combretum molle R. Br. ex G. Don (Combretaceae) leaf, in rodents. J Nat Med. 63:117–123.
- Pannangpetch P, Taejarernwiriyakul O, Kongyingyoes B. 2008. Ethanolic extract of Combretum decandrum Roxb. decreases blood glucose level and oxidative damage in streptozotocin-induced diabetic rats. Diabetes Res Clin Pract. 79:S107–108.
- Rizza RA. 2010. Pathogenesis of fasting and postprandial hyperglycemia in type 2 diabetes: implications for therapy. Diabetes. 59:2697–2707.
- Sato S, Mukai Y, Saito T. 2013. Quercetin intake during lactation modulates the AMP-activated protein kinase pathway in the livers of adult male rat offspring programmed by maternal protein restriction. J Nutr Biochem. 24:118–123.
- Towbin H, Staehelin T, Gordon J. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 76:4350–4354.
- van Dieren S, Beulens JW, van der Schouw YT, Grobbee DE, Neal B. 2010. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil. 17 Suppl 1:S3–S8.
- Viollet B, Guigas B, Leclerc J, Hebrard S, Goelzer LL, Mounier R, Andreelli F, Foretz M. 2009. AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta Physiol (Oxf). 196:81–98.
- Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. 2012. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond). 122:253–270.
- Welch CR. 2010. Chemistry and pharmacology of kinkéliba (Combretum micranthum), a West African medicinal plant. [Ph.D thesis]. The State University of New Jersey, Graduate Program in Medicinal Chemistry. Available from: http://gradworks.umi.com/33/97/3397364.html. (Accessed on 10 April 2015).
- Zhang F, Sun C, Wu J, He C, Ge X, Huang W, Zou Y, Chen X, Qi W, Zhai Q. 2008. Combretastatin A-4 activates AMP-activated protein kinase and improves glucose metabolism in db/db mice. Pharmacol Res. 57:318–323.
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al. 2001. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 108:1167–1174.
