Abstract
Context Garcinia achachairu Rusby (Clusiaceae) popularly known as ‘achachairu’ is used in folk medicine to treat rheumatism, inflammation, pain and gastric disorder.
Objective The present study investigated the chemical profile and antiproliferative effects of the methanolic extract, fractions and two xanthones, against some carcinoma cell lines in vitro.
Materials and methods The compounds were isolated and identified by chromatographic and spectroscopic methods. The extract, fractions and compounds were tested human tumour cell lines of U-251 (glioma), MCF-7 (breast), NCI/ADR-RES (ovary expressing multi-drug resistance phenotype), 786-0 (kidney), NCI-H460 (lung, non-small cells), PC-3 (prostate) and HT-29 (colon), non-tumour cell line HaCat (human keratinocytes) in doses of 0.25–250 μg mL − 1 for 48 h. The antiproliferative activity was determined by spectrophotometric quantification (at 540 nm) of the cellular protein content using sulphorhodamine B assay. The prediction of parameters involved in the molecular bioavailability was executed directly by ChemDoodle (version 5.0.1) software (iChemLabs, LLC, Somerset, NJ).
Results 3-Demethyl-2-geranyl-4-prenylbellidypholine (1) and 1,5,8-trihydroxy-4′,5′-dimethyl-2H-pyrane (2,3:3,2)-4-(3-methylbut-2-enyl) xanthone (2), gartanin (3) and stigmasterol (4) were identified on the basis of spectroscopic techniques. Compounds 1 and 2 exhibited cytocidal activity, especially against breast, prostate and kidney cell lines, with TGI values of 15.8, 4.9, 9.1 and 39.4, 44.7, 40.9 μg/mL, respectively.
Discussion and conclusion The presence of two sets of hydrophobic and hydrophilic groups in separate domains in each molecule might play a role in the mediation of tumour-specific action. Our data show that G. achachairu have potent antiproliferative action and should be considered an important source of potent anticancer compounds.
Introduction
Medicinal plants are very important for the pharmaceutical industry, providing a resource for the development of drugs such as phytomedicines and phytopharmaceuticals, and also as prototypes for the synthesis of new drugs (Barreiro & Bolzani Citation2009; Cragg & Newman Citation2013). The genus Garcinia is well known as a rich natural source of xanthones, biflavones and benzophenones, which show a wide range of biological and pharmacological properties, e.g. antioxidant, anti-inflammatory, antimicrobial and cytotoxic activities (Mbwambo et al. Citation2006; Yang et al. Citation2007; Naldoni et al. Citation2009; Al-Shagdari et al. Citation2013; Nontakham et al. Citation2013). Garcinia achachairu Rusby (Clusiaceae) grows in Brazil, where it is popularly known as ‘achachairu’ and is used in folk medicine to treat rheumatism, inflammation, pain and gastric disorders (Alves et al. Citation2000; Barbosa et al. Citation2008). In our previous investigations, we demonstrate that crude methanol extract and a pure compound, Guttiferone A, presented important antinociceptive, gastroprotector, antileishmanial and antimicrobial activities, using classical experimental models (Dal Molin et al. Citation2012; Niero et al. Citation2012; Cechinel-Filho et al. Citation2013; Melim et al. Citation2013). In addition, low genotoxicity was observed, which represents an important factor from a therapeutic point of view (Marques et al. Citation2012). In this study, we evaluate the chemical profile and antiproliferative effects of the methanolic extracts, fractions and two uncommon isolated xanthones, against several carcinoma cell lines in vitro.
Materials and methods
General experimental procedures
IR spectra were recorded on a BOMEM-100 with a Fourier-Transform Infrared (FT-IR) spectrometer, using KBr windows. EIMS were recorded on a Shimadzu Gas Chromatograph (Model QP-2010S series, Shimadzu Scientific Instruments, Kyoto, Japan) coupled with a mass spectrometric detector equipped with the NIST08 software database (iChemLabs, LLC, Somerset, NJ). 1H NMR spectra were recorded on a Bruker AV-300 and 300 MHz spectrometers (Bruker, Billerica, MA), while the 13C NMR spectra were recorded on a 75 MHz spectrometer (Bruker, Billerica, MA) in CDCl3 and CD3OD. Chemical shifts (δ) are expressed in ppm and coupling constants are given in Hz.
Chromatographic conditions
Recoated aluminium plates (silica gel 60F254; E. Merck, Kenilworth, NJ) were used for TLC. They were visualised under UV at 254 and 366 nm and by spraying with anisaldehyde sulphuric and FeCl3 reagents. Column silica gel (E. Merck, Kenilworth, NJ; 70–230 mesh) and flash silica gel (E. Merck, Kenilworth, NJ; 230–400 mesh) were used for column chromatography. Melting points were determined on a Microquímica APF-300 apparatus (Shimadzu Scientific Instruments, Kyoto, Japan) and were uncorrected. The purity of the isolated compound was examined by thin layer chromatography (TLC) using Merck silica gel pre-coated aluminium plates (thickness = 200 μm) and several solvent systems of different polarities. Doxorubicin was purchased from Fluka (Newport News, VA) with a purity ≥ 99%. All the reagents and solvents used were of analytical grade.
Plant material
The material plant of G. achachairu was collected in the town of Camboriú, SC, Brazil (Latitude 27°04′24.7′′S and longitude 48°42′45.1′′W) in March 2010 and identified by Dr. Oscar B. Iza (University of Vale do Itajaí). The material was collected on private land (Lord Walnir Machiavelli) which does not require specific permissions and does not involve endangered or protected species. A voucher specimen was deposited at the Barbosa Rodrigues Herbarium (Itajaí-SC) under number HBR 52637.
Extraction and isolation
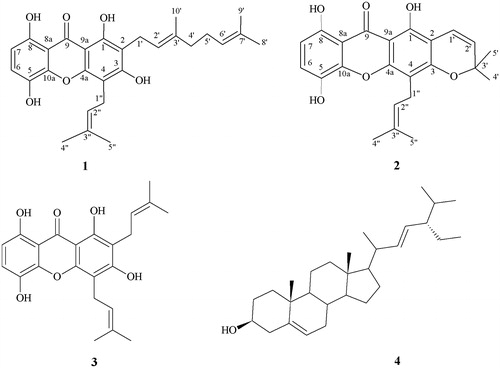
Air-dried powdered branches (1.6 kg) of G. achachairu were exhaustively extracted with methanol (3 L × 2) at room temperature for 7 d. The macerated was filtered and concentrated under reduced pressure in a rotatory evaporator, yield a dark-brown residue (168 g) referred to as the crude methanol extract (CME). All the extract was suspended in a methanol:water (90:10) mixture (900 mL) and subjected to liquid–liquid partition using solvents of increasing polarity: dichloromethane, ethyl acetate and butanol (300 mL; 5 × each). Part of the soluble dichloromethane fraction (10.04 g) was subjected to column chromatography (0.063–0.20 mm, 105.4 g, 3.5 × 50 cm, Merck, Kenilworth, NJ) over silica-gel and eluted with dichloromethane:methanol (100:0 →0:100) in increasing order of polarity to afford 168 fractions, which were combined based on TLC profiles. The fraction 28–35 (384.0 mg) was re-chromatographed using a solvent system of hexane:acetone, yielding new 94 sub-fractions. Sub-fractions 51–65 eluted with a mixture of hexane:acetone (80:20), presented as a yellow crystal (84.2 mg), and were identified as 3-demethyl-2-geranyl-4-prenylbellidypholine xanthone (1) by NMR, DEPT, HMBC and IR data in comparison with those reported previously (Ricaldez et al. Citation2000; Torrico et al. Citation2008).
The other fractions 36–56 (1.3 g) were combined and re-chromatographed using hexane:acetone (100:0 →0:100) as a solvent system, yielding 125 sub-fractions, which were grouped by a similar chromatographic profile. Sub-fractions 45–47 were eluted with hexane:acetone (70:30), presented as a pure yellow crystal (84.5 mg), and were identified as an uncommon 1,5,8-trihydroxy-4′,5′-dimethyl-2H-pyrane (2,3:3,2)-4-(3-methylbut-2-enyl) xanthone (2) based on IR, NMR, DEPT and HMBC data (Hano et al. Citation1993; Komguem et al. Citation2005; Yang et al. Citation2007). The similar fractions 114–120 yielded (155.9 mg), which were re-chromatographed using a solvent system hexane:acetone (100:0 → 0:100) yielding 33 sub-fractions, were grouped by a similar chromatographic profile. Sub-fractions 9–15 eluted with hexane:acetone (80:20) presented a yellow amorphous solid (37.7 mg) and identified as gartanin (3) by NMR spectra data in comparison with the literature (Govindachari et al. Citation1971; Parveen & Khan Citation1988; Hyung et al. Citation2010). The similar fractions 57–90 (3.4 g) from dichloromethane column were re-chromatographed as described above yielding 72 sub-fractions. Sub-fractions 6–19 (200.6 mg) eluted with 80:20 hexane:acetone presented as a white crystal were identified as stigmasterol (4) by direct comparison with authentic sample (Bezerra et al. Citation1994; Anjoo & Ajay Citation2011).
Spectroscopic data
3-Demethyl-2-geranyl-4-prenylbellidypholine xanthone (1): obtained as a yellowish solid; mp 146–148 °C; IR (KBr) λmax 3354, 2970, 1664, 1629 and 1220 cm − 1; 1H and 13C NMR data, see . EIMS m/z 464.5 [M]+; (calcd for C28H32O6).
Table 1. 1H and 13C NMR spectroscopic data of 1 at 300 MHz (for 1H NMR) and 75 MHz (for 13C NMR) in CDCl3, δ in ppm, J (in parenthesis) in Hz.
1,5,8-Trihydroxy-4′,5′-dimethyl-2H-pyrano(2,3:3,2)-4-(3-methylbut-2-enyl) xanthone (2): obtained as yellowish needles; mp 144–145 °C; IR (KBr) λmax 3350, 2976, 1602, 1211 and 819 cm − 1; 1H and 13C NMR data, see . EIMS m/z 394.4 [M]+; (calcd for C23H22O6).
In vitro anticancer activity assay
Human tumour cell lines of U-251 (glioma), MCF-7 (breast), NCI/ADR-RES (ovary expressing multi-drug resistance phenotype), 786-0 (kidney), NCI-H460 (lung, non-small cells), PC-3 (prostate) and HT-29 (colon) were kindly provided by the NCI, Bethesda, MD. Non-tumour cell line HaCat (human keratinocytes) was donated by Prof. Dr. Ricardo Della Coletta, FOP/UNICAMP. Stock cultures were grown in medium containing 5 mL RPMI 1640 (GIBCO BRL) supplemented with 5% foetal bovine serum. Penicillin:streptomycin (100 U/mL:100 μg/mL, 1 mL/L) was added to the experimental cultures. Cells in 96-well plates (100 μL cells well − 1) were exposed to sample concentrations in DMSO/RPMI (0.25, 2.5, 25 and 250 μg mL − 1) at 37 °C, 5% of CO2 in air for 48 h. The final DMSO concentration did not affect cell viability. Afterwards, cells were fixed with 50% trichloroacetic acid and cell growth was determined by spectrophotometry (540 nm) of cellular protein content using sulphorhodamine B assay (Monks et al. Citation1991). Doxorubicin chloridrate (0.1 mg/mg; Europharma, Green Bay, WI) was used as the positive control. The concentration–response curve for each cell line and the TGI values (total growth inhibition: concentration that elicits total growth inhibition) was determined through nonlinear regression analysis () using software ORIGIN 8.0 (OriginLab Corporation, Northampton, MA) (Shoemaker Citation2006).
ADMET parameter predictions
For the prediction of parameters involved in the molecular bioavailability, four methods were used: the bioavailability score (ABS), Egan violations count (EVC), Veber count violations (VVC) and the Lipinski rule of five (LRF) violations (Egan et al. Citation2000; Veber et al. Citation2002; Martin Citation2005). The methods used were executed by direct computer calculation by ChemDoodle (version 5.0.1-iChemLabs, LLC, Somerset, NJ) software. All structures were energetically minimised through HiperChem 7.1 software (iChemLabs, LLC, Somerset, NJ), using the sequence: energetic minimisation, molecular dynamics and energetic minimisation. For energetic minimising, the molecular mechanical method (MM) was used, by the Polak–Ribieri algorithm (RMS gradient 0.1 kcal/Å mol, up to 150 cycles under vacuum). The structures were then exported to formato mol using the ChemDoodle software (iChemLabs, LLC, Somerset, NJ), for the calculation of prediction methods.
Results and discussion
Repeated column chromatography of the CH2Cl2-soluble fraction of the branches of G. achachairu led to the isolation of two uncommon prenylated xanthones 1 and 2 and two known compounds (). The structures of the well-known compounds gartanin (3) and stigmasterol (4) were identified by comparing their physical and spectroscopic data (1H NMR, 13C NMR, DEPT and 2D NMR) with the published values (Bezerra et al. Citation1994; Komguem et al. Citation2005; Yang et al. Citation2007; Anjoo & Ajay Citation2011). Compound 1 was obtained as a yellow crystal and showed a [M]+ peak at m/z 464.5 in the electron impact ionisation mass spectroscopy (EIMS), corresponding to a molecular formula of C28H32O6. The IR spectrum showed the presence of hydroxyl groups (3354 cm − 1), a conjugated carbonyl group (1624 cm − 1) and aromatic rings (1500–1600 cm − 1). The 1H NMR spectrum (), revealed two downfield singlets at [δ H 11.22 and 12.16], suggesting the presence of two hydrogen bonded at phenolic hydroxyl groups. It also displayed the characteristic signals of two ortho-coupled aromatic protons [δ 6.67 (1H, d, J = 8.7 Hz) and 7.23 (1H, d, J = 8.7 Hz)], three olefinic protons [δ 5.28 (1H, m), 5.26 (1H, m), 5.06 (1H, s)] and five tertiary methyl group δ [1.85 (3H, s), 1.74 (3H, s), 1.68 (3H, s), 1.60 (3H, s) and 1.85 (3H, s)] suggestive attached geranyl and prenyl groups (Hano et al. Citation1993; Merza et al. Citation2004). The 13C NMR spectrum () of compound 1 revealed the presence of 28 signals, including five methyl, five methine, three methylene and 30 quaternary carbons, one of which was attributed to the carbonyl function (δ 184.6). In the HMBC spectrum (), the chelated phenolic protons observed at δ 12.36 and 11.26 showed correlations with aromatic carbons at δ 158.6 (C-1), 102.2 (C-2), 160.9 (C-9a) and δ 107.1 (C-8a), 109.3 (C-7) and 153.8 (C-8), respectively. The proton at δ 7.23 (d, J = 8.7 Hz, H-6) correlated with carbons at δ 135.7 (C-5), 142.8 (C-10a) and 153.8 (C-8). The proton signal δ 3.48 due to the geranyl moiety showed cross peaks with carbon signals at δ 158.6 (C-1), 109.2 (C-2), 161.7 (C-3), 120.9 (C-2') and 140.3 (C-3') demonstrating that the geranyl group was located at C-2 of ring A (). All its data in comparison with those reported previously and combined with other partial structures, leading to 3-demethyl-2-geranyl-4-prenylbellidypholine xanthone (1), which to our knowledge, are uncommon in the literature (Ricaldez et al. Citation2000; Komguem et al. Citation2005; Torrico et al. Citation2008).
Figure 2. Significant long-range correlations observed in 13C–1H HMBC for compound 1 (A) and 2 (B) isolated from branches of G. achachairu.
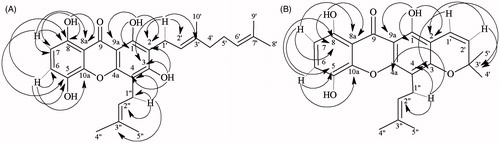
Compound 2 also was obtained as a yellow crystal and showed a [M]+ peak at m/z 394.4 in the electron impact ionisation mass spectroscopy (EIMS) suggesting a molecular formula C23H22O6. Compound 2 was a close analogue of 1, with characteristics of absorption on the IR spectrum in the presence of hydroxyl groups (3350 cm − 1), conjugated carbonyl group (1629 cm − 1), stretching characteristics of aromatic rings (1490–1600 cm − 1) and an ether group (1211 cm − 1) suggesting one dimethylchromene ring. Similarly 1H NMR spectrum () also showed the signals of two ortho-aromatic protons [δ 6.65 (1H, d, J = 8.7 Hz) and 7.21 (1H, d, J = 8.7 Hz)], two phenolic hydroxyl groups [δ 12.21 and 11.26 (1H, s)], one prenyl group [δ 3.45 (2H, d, J = 7.0 Hz), δ 5.20 (1H, t, J = 7.4 Hz), δ 1.85 (3H, s) and 1.71 (3H, s)] and a suggestive dimethylchromene ring [δ 1.48 (6H, s), and δ 5.62, 6.72 (each 1H, d, J = 10.0 Hz)]. The 13C NMR spectrum () showed signals between δ 122.8 and 109.9, typical aromatic C–H located at C-6 and C-7, signals between δ 30 and 18, attributed to the aliphatic carbons C-4′, C-5′, C-1′, C-4′ and C-5′ and a signal at δ 184.8 which was assigned to C-9 of a carbonyl group. The disappearance of four methylene group signal in the DEPT spectrum when compared with compound 1 also corroborate a possible closing of a prenyl group moiety, producing a dimethylchromene. This hypothesis was confirmed by HMBC spectra (), in which a correlation of the hydroxyl group was observed at position δ 11.20 (C-8) with 107.5 (C-8a) and 153.8 (C-8). Similarly, the hydroxyl group at δ 12.21 (C-1) showed correlations with δ 155.4 (C-1), 104.8 (C-2) and 102.2 (C-9a). In addition, δ 7.21 (H-6) correlated with δ 135.8 (C-5), 153.8 (C-8) and 142.7 (C-10a). The evidence that the dimethylchromene ring is connected at C-2 and C-3 is that the δ 6.72 (H-1′) is correlated with δ 78.6 (C-3′) and 159.0 (C-3). Likewise, the prenyl group is located at C-4 due to the correlation of the δ 3.45 (H-1″) with δ 107.9 (C-4) and 122, 4 (C-2″) and 153.8 (C4a) (). These data are in accordance with other partial structure of similar compounds in comparison with literature data (Hano et al. Citation1993; Komguem et al. Citation2005; Yang et al. Citation2007) and were identified as a uncommon 1,5,8-trihydroxy-4′,5′-dimethyl-2H-pyrane (2,3:3,2)-4-(3-methylbut-2-enyl) xanthone (2).
Table 2. 1H and 13C NMR spectroscopic data of 2 at 300 MHz (for 1H NMR) and 75 MHz (for 13C NMR) in CDCl3, δ in ppm, J (in parenthesis) in Hz.
Table 3. Antiproliferative activity of methanolic extract, fractions and pure compounds from branches of G. achachairu, against human cancer cell linesTable Footnotea (TGI (μg/mL)Table Footnoteb).
Extract, fractions and compounds 1 and 2 were tested for their antiproliferative activity against different human cell lines (), since previous studies with another species of this genus exhibited promising melanogenesis in B16-F10 murine melanoma cells (Campos et al. Citation2013). As shown in , the dichloromethane (DCM) was the most active tested fraction, demonstrating an interesting profile for most human cancer cell lines, with most active for glioma (U-251), ovary expressing multi-drugs resistance phenotype (NCI/ADR-RES), kidney (786-0) and prostate (PC-3) with total grown inhibition (TGI) values of 24.9, 28.7, 22.9 and 25.7 μg/mL, respectively. The methanolic extract, buthanolic (BuOH) and ethyl acetate (EA) fractions did not demonstrate significant activity when analysed in the same model. Considering that the compounds were isolated from DCM fraction, they were evaluated against the same cell lines. As can be observed, compound 1 presented in vitro selective antiproliferative effects for U-251, NCI/ADR-RES and PC-3 with TGI values of 5.2, 4.0 and 4.9 μg/mL, respectively, which were close to the positive control, doxorubicin (). The isolated compounds presented better in vitro antiproliferative activity than the DCM fractions, which suggests that these compounds (especially xanthone 1) might be more involved with the in vitro anticancer activity. Although some studies have demonstrated the anticancer activity of Garcinia genus, no reports were found for G. achachairu (Akao et al. Citation2008; Ee et al. Citation2008; Yu et al. Citation2009; Yang et al. Citation2010; Kuete et al. Citation2014).
Considering the very small structural difference between the two xanthones, molecular modelling was performed using a computational program in an attempt to find the possible physicochemical parameters responsible for the observed activity (). It was observed that, for the descriptor ‘Egan violations count’ (EVC), the two compounds under study showed no violation. This means that these compounds have good conditions for passive intestinal absorption, which can be decisive for some drugs, such as omeprazole (Delgado & Remers Citation1998; Egan et al. Citation2000). The same occurs for the descriptor ‘Veber violations count’ (VVC), reinforcing the conclusion that the subject compounds possess good oral bioavailability profile (Veber et al. Citation2002). In the evaluation by the ‘Lipinski rule of five’ (LRF), it is noted that both compounds possessed no violation relating to logP. Also none of the compounds had violations for the descriptor total polar surface area (TPSA) which showed, in both cases, less than 140 Å2 (111.130 and 100.130, respectively). It is worth remembering here that the assignment of limits of TPSA, as a parameter for violation of the ‘Lipinski rule of five’, was not part of the original work that showed the parameterisation of that rule and is still the subject of additional studies (Lipinski Citation2004; Keller et al. Citation2006). Therefore, according to Lipinski, it can be concluded that there is no one limitation for cellular permeation observed in the studied compounds, but when two or more violations of the rule are observed, the compounds will certainly show low profile of cell permeation (Campos et al. Citation2010).
Table 4. Descriptors involved in ADMET evaluation.
The bioavailability score (ABS) states that this score indicates the probability of a compound to show bioavailability F > 10% in vivo studies (on mice) and in vitro studies (on Caco-2). It will be 0.11 for anions for which TPSA > 150 Å2; 0.56 if TPSA is between 75 and 150 Å2 and 0.85 if TPSA < 75 Å2. For the remaining compounds (neutral, cationic or zwitterionic), the ABS is 0.55, in which case, the compound passes in LRF (as observed with both compounds tested in this work). When the compounds fail this rule, the score is 0.17 (Martin Citation2005). Thus, in general, it is observed that the ADMET data indicate a good bioavailability and consequently that the two molecules exhibit characteristics that make them future drug candidates.
In conclusion, the results demonstrated, for the first time, a pronounced antiproliferative effect against different cell lines, particularly the isolated xanthones from barks of G. achachairu which justified, at least in part, their use as prototypes for the development of a new promising medicinal agent with anticancer profile. Further experiments are underway to determine the possible mechanisms involved in the antiproliferative action observed in the extract, fraction and isolated compounds.
Acknowledgements
The authors are also grateful to Dr. Eliseo Soprano and Mr. Walnir Machiavelli for providing the plant material and Dr. Oscar Benigno Iza for the botanical support.
Declaration of interest
The authors report that they have no conflicts of interest. The authors are grateful to the Network Ribecancer 212RT 0464 (CYTED/CNPq), ProPPEC/UNIVALI and FAPESC-Brazil for their financial support.
References
- Akao Y, Nakagawa Y, Iinuma M, Nozawa Y. 2008. Anti-cancer effects of xanthones from pericarps of mangosteen. Int J Mol Sci. 9:355–370.
- Al-Shagdari A, Alarcón AB, Cuesta-Rubio O, Piccinelli AL, Rastrelli L. 2013. Biflavonoids, main constituents from Garcinia bakeriana leaves. Nat Prod Commun. 8:1237–1240.
- Alves TMA, Silva AF, Brandão M, Grandi TSM, Smânia EF. 2000. Biological screening of Brazilian medicinal plants. Mem Inst Oswaldo Cruz. 95:367–373.
- Anjoo K, Ajay KS. 2011. Isolation of stigmasterol and β-sitosterol from petroleum ether extract of aerial parts of Ageratum conyzoides (Asteraceae). Int J Pharm Sci. 3:94–106.
- Barbosa W, Chagas EA, Martins L, Pio R, Tucci MLS, Artioli FA. 2008. Germinação de sementes e desenvolvimento inicial de plântulas de achachairu. Rer Bras Frut. 30:263–266.
- Barreiro EJ, Bolzani VS. 2009. Biodiversidade: fonte potencial para a descoberta de fármacos. Quim Nova. 32:679–688.
- Bezerra MZB, Campelo PA, Machado MIL, Mattos FJA, Braz-Filho R. 1994. Constituintes químicos isolados de três espécies do gênero Sclerolobium. Quim Nova. 17:205–209.
- Campos BF, Cechinel-Filho V, Corrêa R. 2010. Contribuiçãodaquímicamedicinalparaoplanejamentodenovosfármacos. In: Bresolin TMB, Cechinel-Filho V, editors. Fármacos e medicamentos. Uma abordagem multidisciplinar. São Paulo: Editora Santos. p. 417–425.
- Campos PM, Horinouchi CD, Prudente AS, Cechinel-Filho V, Cabrini DA, Otuki MF. 2013. Effect of a Garcinia gardneriana (Planchon and Triana) Zappi hydroalcoholic extract on melanogenesis in B16F10 melanoma cells. J Ethnopharmacol. 148:199–204.
- Cechinel-Filho V, Meyre-Silva C, Niero R, Mariano LNB, Nascimento GF, Vicente Farias I, Gazoni VF, Dos Santos SBC, Giménez A, Gutierrez-Yapu D, et al. 2013. Evaluation of anti-leishmanial activity of selected Brazilian plants and identification of the active principles. Evid-Based Comp Alter Med [Online]Available from: https://doi.org/http://www.hindawi.com/journals/ecam/2013/265025/ (Accessed 18 August 2015).
- Cragg MG, Newman JD. 2013. Natural products: a continuing source of novel drug leads. Biochim Biophys Acta. 3:3670–3695.
- Dal Molin MM, Silva S, Alves DR, Quintão NLM, Monache FD, Cechinel-Filho V, Niero R. 2012. Phytochemical analysis and antinociceptive properties of the seeds of Garcinia achachairu. Arch Pharm Res. 35:623–631.
- Delgado JN, Remers WA. 1998. Textbook of organic medicinal and pharmaceutical chemistry. Philadelphia: Lippincott Raven.
- Ee GC, Daud S, Izzaddin SA, Rahmani M. 2008. Garcinia mangostana: a source of potential anti-cancer lead compounds against CEM-SS cell line. J Asian Nat Prod Res. 10:475–499.
- Egan WJ, Merz KM, Baldwin JJ. 2000. Prediction of drug absorption using multivariate statistics. J Med Chem. 43:3867–3877.
- Govindachari TR, Kalyanaraman PS, Muthukumaraswamy N, Pai BR. 1971. Xanthones of Garcinia mangostana Linn. Tetrahedron 27:3919–3922.
- Hano Y, Okamoto T, Suzuki K, Negishi M, Nomura T. 1993. Components of the root bark of Morusinsignis Bur. Structures of three new isoprenylated xanthones morusignins I, J, and K and isoprenylated flavone morusignin L. Heterocycles 36:1359–1366.
- Hyung WR, Curtis-Long MJ, Jung S, Jin YM, Cho JK, Ryu Y, Lee WS, Park KH. 2010. Xanthones with neuraminidase inhibitory activity from these cases of Garcinia mangostana. Bioorg Med Chem. 18:6258–6264.
- Keller TH, Pichota A, Yin ZA. 2006. A practical view of ‘druggability’. Curr Opin Chem Biol. 10:357–361.
- Komguem J, Meli AL, Manfouo RN, Lontsi D, Ngounou FN, Kuete V, Kamdem HW, Tane P, Ngadjui BT, Sondengam BL, et al. 2005. Xanthones from Garcinia smeathmannii (Oliver) and their antimicrobial activity. Phytochemistry 66:1713–1717.
- Kuete V, Sandjo LP, Ouete JL, Fouotsa H, Wiench B, Efferth T. 2014. Cytotoxicity and modes of action of three naturally occurring xanthones (8-hydroxycudraxanthone G, morusignin I and cudraxanthone I) against sensitive and multidrug-resistant cancer cell lines. Phytomedicine 3:315–322.
- Lipinski CA. 2004. Lead and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol. 1:337–464.
- Marques ES, Silva S, Niero R, Andrade SF, Rosa PCPR, Perazzo EF, Maistro EL. 2012. Genotoxicity assessment of Garcinia achachairu Rusby (Clusiaceae) extract in mammalian cells in vivo. J Ethnopharmacol. 142:362–366.
- Martin YCA. 2005. A bioavailability score. J Med Chem. 48:3164–3170.
- Mbwambo ZH, Kapingu MC, Moshi MJ, Machumi F, Apers S. 2006. Antiparasitic activity of some xanthones and biflavonoids from the root bark of Garcinia livingstonei. J Nat Prod. 69:369–372.
- Melim C, Alves AD, Martins DTO, Bela Cruz A, Quintal ZRM, Guimarães K, Cechinel-Filho V, Niero R. 2013. Antimicrobial activity of extracts and fractions from aerial parts of selected plants (Garcinia achachairu, Macrosiphonia velame, Rubus niveus and Pilea microphylla) against some pathogenic microorganisms. Nat Prod Commun. 8:1567–1569.
- Merza J, Aumond MC, Rondeau D, Dumontet V, Ray Anne-Marie L, Seraphin D, Richomme P. 2004. Prenylated xanthones and tocotrienols from Garcinia virgata. Phytochemistry 65:2915–2920.
- Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A, et al. 1991. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst. 83:757–766.
- Naldoni FJ, Claudino AL, Cruz JW, Chavasco JK, Faria SPM, Veloso MP, Dos Santos MH. 2009. Antimicrobial activity of benzophenones and extracts from the fruits of Garcinia brasiliensis. J Med Food 12:403–407.
- Niero R, Dal Molin MM, Silva S, Damian NS, Maia LO, Delle Monache F, Cechinel-Filho V, Andrade SF. 2012. Gastroprotective effects of extracts and guttiferone A isolated from Garcinia achachairu Rusby (Clusiaceae) against experimentally induced gastric lesions in mice. Naunyn Schmiedebergs Arch Pharmacol. 385:1103–1109.
- Nontakham J, Charoenram N, Upamai W, Taweechotipatr M, Suksamrarn S. 2013. Anti-Helicobacter pylori xanthones of Garcinia fusca. Arch Pharm Res. 37:972–977.
- Parveen M, Khan NU. 1988. Two xanthones from Garcinia mangostana. Phytochemistry 27:3694–3696.
- Ricaldez FT, Vega G, Almanza R. 2000. Phytochemical study of Rheedia gardneriana. Rev Bol Quim. 17:9–14.
- Shoemaker RH. 2006. The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer. 6:813–823.
- Torrico F, Velasco P, Giménez A, Almanza RG. 2008. Xantonaspreniladasde Rheedia gardnerianay R. acuminata. In: Feliciano AS, Pérez AL, Del Olmo E, editors. Manual de determinación estructural de compuestos naturales. Programa Iberoamericano de Ciencia y Tecnologia. Bogota: CYTED. 1,5.
- Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD. 2002. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem. 45:2615–2623.
- Yang H, Figueroa M, Tom S, Baggett S, Jiang Basile MF, Weinstein IB, Kennelly EJ, et al. 2010. Benzophenones and biflavonoids from Garcinia livingstonei fruits. J Agric Food Chem. 58:4749–4755.
- Yang N-Y, Han Q-B, Cao X-W, Quio C-F, Song J-Z, Chen S-L, Yang D-J, Yiu H, Xu H-X. 2007. Two new xanthones isolated from the stem bark of Garcinia lancilimba. Chem Pharm Bull. 55:950–902.
- Yu L, Zhao M, Yang B, Bai W. 2009. Immunomodulatory and anticancer activities of phenolics from Garcinia mangostana fruit pericarp. Food Chem. 116:969–973.