Abstract
Context Withania somnifera (L.) Dunal is traditionally used for treating various ailments, but lacks scientific evaluation.
Objective This study evaluates Withania somnifera (WS) for its effect on platelet activity and inflammatory enzymes.
Materials and methods Aqueous and ethanolic (1:1) leaf extracts were subjected to in vitro indirect haemolytic activity using Naja naja venom, human platelet aggregation was quantified for lipid peroxidation using arachidonic acid (AA) as agonist and 5-lipoxygenase (5-LOX) levels were determined using standard spectrometric assays. Further, molecular docking was performed by the ligand fit method using molegro software package (Molegro ApS, Aarhus, Denmark).
Results The study found that aqueous and ethanol extracts have very negligible effect (15%) with an IC50 value of 13.8 mg/mL on PLA2 from Naja naja venom. Further, extracts of WS also had very little effect (18%) with an IC50 value of 16.6 mg/mL on malondialdehyde (MDA) formation. However, a 65% inhibition of 5-LOX with an IC50 value of 0.92 mg/mL was observed in 1:1 ethanol extracts. The same was evident from SAR model with the active ingredient withaferin A binding predominantly on Phe 77, Tyr 98, Arg 99, Asp 164, Leu 168, Ser 382, Arg 395, Tyr 396 and Tyr 614 with an atomic contact energy value of −128.96 compared to standard phenidone (−103.61). Thus, the current study validates the application of WS for inflammatory diseases.
Conclusion This study reveals the inhibitory potential of W. somnifera on inflammatory enzymes and platelet aggregation. Thus, WS can serve as a newer, safer and affordable medicine for inflammatory diseases.
Introduction
Withania somnifera (L.) Dunal (Solanaceae) (WS), commonly known as ashwagandha, is an important traditional medicinal plant. Research studies have shown that it has anti-arthritic, anti-rheumatic, anti-inflammatory, antitumour, antistress, antioxidant, immunomodulatory and rejuvenating properties (Mishra et al. Citation2000). Chemical studies have shown that it is enriched with alkaloids isopelletietine, anaferine, steroidal lactones (withanolides, withaferins–withaferin A-WA) and saponins (Rastogi & Mehrotra Citation1994).
Phospholipases are a group of enzymes that catalyse the cleavage of phospholipids. Phospholipases-A2 (PLA2) specifically recognises and hydrolyses phospholipids at the sn-2 position, such as of arachidonic acid (AA). PLA2 hydrolyses membrane phospholipids to release AA. During inflammatory conditions, this enzyme plays a vital role in metabolising the release of eicosanoids (Raghavendra et al. Citation2006).
WS has been found to be composed of glycoprotein (WSG), which can inhibit the phospholipase A2 activity of cobra venom (Naja naja) completely, but failed to neutralise the toxicity of the molecule. However, it reduced the toxicity as well as prolonged the death time of the experimental mice approximately 10 times when compared with venom alone. The WSG also inhibited several other PLA2 isoforms from the venom to varying extent (Gomes et al. Citation2010). Further, the other important constituent is withaferin A, which mainly contributes to inflammation via necrotic factor-kappaB pathway (Heyninck et al. Citation2014) and has also been involved in the amelioration of oxidative stress in inflammatory arthritis (Khan et al. Citation2015).
Platelets or thrombocytes are small disc-shaped, anuclear cells in blood. There are many agonists for the activation of platelets which leads to their aggregation. These agonists act by different molecular pathways. The AA acts by cyclooxygenase (COX), releasing thromboxanes which in turn causes platelet aggregation. Polyphenolic compounds from herbs have been reported to reduce the risk of cardiovascular disease and also modulate platelet function (Raghavendra & Naidu Citation2011).
Lipoxygenases (LOXs) are non-heme, iron-containing enzymes that are widely distributed throughout plants, animals, fungi and some bacteria. The 5-LOX, a Ca2+ - and ATP-requiring enzyme, catalyses the first two steps in the biosynthesis of the peptide-LTs and the chemotactic factor LTB4. Expression of 5-LOX protein is mainly restricted to myeloid cells, including granulocytes, monocytes/macrophages, mast cells and B-lymphocytes (Raghavendra et al. Citation2006).
In recent years, in silico modelling has become a necessary tool for drug design. Virtual screening and molecular docking studies are of high priority in drug discovery and development. Most of the molecular docking algorithms assume the protein as rigid object which leads to inappropriate correlation of the docking scores. There is no single docking algorithm or scoring function that can correctly predict the binding affinities of ligand in molecular interaction. For these reasons, in the present study, a highly validated docking program such as the ligand fit method in molegro package (Molegro ApS, Aarhus, Denmark) was used to investigate and identify the interaction of ligand molecule in the active region of the protein and to predict the binding affinity between the ligand and the receptor protein molecule using atomic contact energy (ACE) values (Lakshmi Ranganatha et al. Citation2013).
In this study, we have attempted to analyse the effect of WS extracts on secretary phospholipase A2 (sPLA2) from Naja naja venom for their anti-inflammatory effect followed by platelet aggregation. Further, the predominant bioactive molecule being withaferin A in WS, we also explored the structure–activity relationship (SAR) with anti-inflammatory enzymes, namely, COX, sPLA2 and 5-LOX with withaferin A compared with the standard drugs (aspirin, quercetin and phenidone) currently available in the market for inflammatory complications.
Materials and methods
All chemicals were of analytical reagent grade and procured by Merck Chemical Co., Bangalore, India. Adenosine triphosphate (ATP), arachidonic acid (AA), dithiothreitol (DTT), trichloroacetic acid (TCA) and phenidone were purchased from Sigma Chemical Co., St. Louis, MO.
Preparation of WS extracts (aqueous and ethanol)
Fresh and healthy leaves of WS were collected from in and around Mysore, Karnataka, India, during March 2014 and were used for taxonomical identification, and the same is deposited as voucher specimen and verified in Department of Studies in Botany, University of Mysore, Karnataka, India. The harvested WS leaves were dried at room temperature and the sample was powdered to 60 meshes. Aqueous extract was prepared using 50 g of powdered WS leaves with 100 mL of distilled water (w/v). The mixture was centrifuged at 1600 rpm for 10 min and the supernatant was used as WS aqueous extract to study their inhibitory effects (Yu & Dahlgren Citation2000). In the same manner, 1:1 ethanol and water (v/v) was used for second extraction as mentioned above.
Indirect haemolytic assay
Isolation of erythrocytes and indirect haemolytic assay
Blood was collected from healthy volunteers in plastic tubes containing tri-sodium citrate (9:1 v/v) as an anti-coagulant. To these tubes, phosphate buffer saline (PBS) was added and centrifuged at 1500 rpm for 15 min at room temperature (RT). RBCs were washed three times with PBS by centrifugation at 850 × g for 10 min. Washed RBC were re-suspended in PBS to a final concentration of 2.5% (v/v) and used for haemolytic activity assays. Human erythrocytes (1 mL) washed with PBS then mixed with 1 mL of egg yolk and PBS in the ratio of 1:1:8. Later 1 mL of this mixture was incubated with Naja naja venom (10 μg) for 30 min at RT. Reaction was stopped by adding ice cold PBS (9 mL) and centrifuged at 4 °C for 10 min at 1500 rpm. Inhibition assay was carried by incubating venom with the WS extract for 10 min before adding to the reaction mixture. The amount of haemolysis was estimated by measuring haemoglobin at 540 nm (Dhananjaya et al. Citation2011). Protein estimation was determined using bovine serum albumin (BSA) as a standard (Lowry et al. Citation1951; Bowman & Kaletta Citation1957).
Effects of WS extract on in vitro platelet aggregation
Venous blood was drawn from healthy human volunteers who were not on any medication (aspirin or other NSAIDs) for the past 15 d. Blood was collected with 3.8% sodium citrate (9:1, v/v). Platelet-rich plasma (PRP) was separated by centrifuging the blood at 150 × g for 20 min at RT (25 °C ± 2). Remaining blood was centrifuged at 750 × g for 20 min to obtain platelet poor plasma (PPP). Platelet count was adjusted to 1.6 × 108 platelets per μL of PRP. Experiments were performed within 2 h of PRP preparation. Platelet aggregation was monitored in dual channel chronolog aggregometer (Model 400; Chronolog Corporation, Manchester, UK) using 0.45 mL aliquots of PRP further incubated with WS extracts for 5 min, and aggregation was induced by the addition of agonist 1.0 mM of AA (optimum concentrations were used). Vehicle concentration was maintained at 5 μL in 0.5 mL of reaction mixture (with respect to 50% ethanol extract). The resulting platelet aggregation was measured as the increase in light transmission for 5 min (Raghavendra & Naidu Citation2011).
Effect of WS extract on lipid peroxidation in platelets
Lipid peroxidation in aggregated platelets was measured by the thiobarbituric acid reactive substance (TBARS) method. To the aggregated platelets, 1% BHT was added to arrest further lipid peroxidation. Proteins were precipitated by adding 10% TCA and centrifuged at 7000 × g for 20 min. The malondialdehyde (MDA) formed during the oxidation of platelets was reacted with thiobarbutric acid (TBA) and TBARS were measured at 535 nm spectrophotometrically. The lipid peroxidation product MDA was expressed as μmoles of MDA formed (Raghavendra & Naidu Citation2011).
Separation and isolation of 5-LOX from PMNLs of human blood
Blood was collected from healthy individuals using EDTA as an anticoagulant. Polymorphonuclear leukocytes (PMNLs) were separated from blood by the Ficoll gradient method. Blood was layered on top of ficoll (Histopaque 1077) at a ratio of 1:1 and centrifuged at 1600 rpm for 30 min. The buffy coat containing white blood cells (WBCs) was separated and washed with 3% NaCl to remove any contaminating red blood cells (RBCs) by centrifugation at 7000 rpm (4 °C) for 10 min. Finally, the white pellet of leukocytes was separated and washed with PBS. Isolated PMNLs were sonicated for 20–30 s at 20 kHz to release the cytosolic 5-LOX enzyme into solution. This solution was centrifuged at 10 000 rpm, for 30 min at 4 °C. The supernatant was taken as a source of enzyme and protein was estimated by using BSA standard (Bowman & Kaletta Citation1957; Raghavendra et al. Citation2006).
Molecular docking for structure–activity relationship (SAR)
The crystal structures of COX, 5-LOX and sPLA2 (accession: 1EQG, 3V92 and 1AYP) were built using CPH Models server 3.0 (CBS Corporation, New York, NY). Energy computations were performed on the molecule using GROMOS96 implementation of Swiss-PDB Viewer (GlaxoSmithKline R&D S.A. Raleigh, NC). Electrostatic point charges on the molecules were calculated. The ligands were docked into the active site using the molecular docking software PATCH Dock (SAIC-Frederick Inc., Frederick, MD) with default parameters. PATCH Dock is an algorithm for calculating the docking modes of small molecules into protein-binding sites based on their shape complementarity. In this, we have used ChemScore, a scoring function that is derived from regression against receptor–ligand binding free energies. The structures of the ligands for the current study were constructed using Dundee PRODRG server (Thomsen & Christensen Citation2006) which optimises the conformation of the side chains and minimises the energy. The minimum energy conformers of ligands were interactively docked into close proximity with the enzyme active site pocket. The possibility of binding, precise location of binding sites and the mode of ligand binding was carried out using an automated docking software, molegro virtual docker 2008, version 3.2.1 (Molegro ApS, Aarhus, Denmark, http://molegro.com), that is based on guided differential evolution and a force field-based screening function (Schüttelkopf & Aalten Citation2004). Possible binding conformation and orientations were analysed by clustering methods, embedded in molegro molecular viewer 2008, version 1.2.0 (Molegro ApS, Aarhus, Denmark). Docking studies were carried out using the enzymatic model. The enzyme was visualised using sequence option. The binding site was computed within spacing such that the binding site was well sampled with a grid resolution of 0.3 Å. The ligand was docked into this grid using the MolDock optimiser algorithm and its interactions were monitored using detailed energy estimates. A maximum population of 100 and maximum interactions of 10 000 were used for each run and the five best poses were retained. The software was utilised to identify hydrogen bonds and hydrophobic interactions between residues at the active site and the ligand.
Statistical analysis
All experiments were carried out in triplicates and repeated in three independent sets of experiments. Data are shown as means ± SD. The SPSS 16 version for windows (SPSS Software, Inc., Chicago, IL) computer program was used for statistical analysis. The significance of the study was assessed by one-way ANOVA, followed by post hoc comparison test. Correlations between quantitative properties were evaluated by calculating the Duncan and Dennett’s coefficient. Statistical significance value was set at p < 0.05.
Results and discussion
It is well documented that withaferin A (WA), steroidal lactone, is abundantly present in WS. Earlier studies have proved that this molecule is a major contributor for anti-angiogenesis property of WS. It is also proved that WA also affects the NF-κB and calcium signalling (Jilani et al. Citation2013). The studies have shown that WA has anti-oxidant and anti-lipid peroxidation effects (Manoharan et al. Citation2009). In our studies, WS extracts has shown slight inhibition on MDA formation and platelet aggregation. This may be because of the involvement of COX and/or by altering Ca2+ influx as previously explained by Jilani et al. (Citation2013). Along with WA another molecule withanone (WN) are two structurally similar withanolides isolated from WS. The results of the current study indicate that both water extract and 50% ethanol extract of WS are equipotent in the inhibition of sPLA2 activity. The same was observed in the case of platelet aggregation, MDA formation and 5-LOX activity. The study proves that WS extracts are more potent in inhibiting 5-LOX activity with nearly 65% inhibition (). Docking studies revealed that withaferin A has higher potency of affinity than the standard drugs on the inflammatory enzymes COX-1, sPLA2 and 5-LOX () and which is evident through their hydrogen bonding with atomic contact energy (ACE) values as indicated () along with their respective docking domain sites. The same was evident from SAR model with the active ingredient withaferin A binding predominantly on Phe 77, Tyr 98, Arg 99, Asp 164, Leu 168, Ser 382, Arg 395, Tyr 396 and Tyr 614 with an atomic contact energy value of −128.96 as compared with standard phenidone (−103.61). Similar studies on bioactives have been mainly studies for retro synthesis mechanism (Begum et al. Citation2013; Lakshmi Ranganatha et al. Citation2013). Hence, the current study may thus emphasise the role of withaferin A as they offer better contribution in understanding the inflammatory enzyme activities besides their stability under physiological conditions.
Figure 1. (A) Effect of WS on PLA2 activity, (B) effect of WS on platelet aggregation, (C) effect of WS on MDA formation and (D) effect of WS on 5-LOX activity. Note: (1) Water extract and (2) 50% ethanol extract. Values are mean ± SD of three independent determinations.
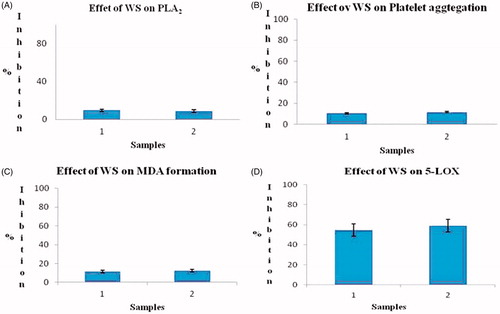
Figure 2. Molecular docking of COX-1, sPLA2 and 5-LOX with withaferin A and the standard anti-inflammatory drugs (aspirin, quercetin and phenidone), respectively.
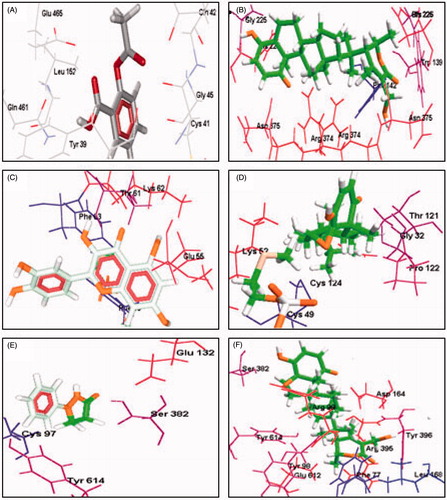
Table 1. Hydrogen bonding between withaferin A with COX-1, sPLA2 and 5-LOX and standard anti-inflammatory drugs (aspirin, quercetin and phenidone), respectively, indicating the comparative atomic contact energy (ACE) values.
Conclusion
Current researchers have shown great interest in documenting the ethanomedical data and scientific research on medicinal plants. Once these local preparations are evaluated precisely, a better efficacious herbal drug treatment would be developed. Henceforth, eco-friendly herbs would alleviate human suffering and inflammation related diseases. Thus, withaferin A may find better application in management of inflammation and in anti-inflammatory therapeutic engineering.
Acknowledgements
F. Z. and R. H. gratefully acknowledge Prof. Dr. K. V. Prabhakara, Principal, SBRR First Grade College, Mysore, and the management, Mahajana Education Society and Research Foundation, for their constant support and inspiration. All authors are grateful to Mr. Kamran Waseem, Department of Studies in Chemistry, University of Mysore, for the language corrections and suggestions. D. B. L. acknowledge the financial assistance of DST, Govt of India for the International Grant – Indo-Srilankan (DST/INT/SLP/P-007/2012, dated 5th May, 2014), and European Union for International Grant – Indo-European – Marie Curie IRSES - (PIRSES-GA-2013-612131, dated 15th Nov, 2014). DBL would like to place on record the deepest gratitude towards Dr. Chenraj Roychand, President Jain University trust and Dr. Krishna Venkatesh, CEO, Centre for Emerging Technologies (CET) for their constant motivation and support.
Declaration of interest
The authors report no conflicts of interest.
References
- Begum AB, Begum M, Ranganatha VL, Prashanth T, Zameer F, Hegdekatte R, Khanum SA. 2013. Synthesis, antioxidant and xanthine oxidase inhibitory activities of 5-[4-[2-(5-ethyl-2-pyridinyl) ethoxy] phenyl] methyl]-2,4-thiazolidinedione derivatives. Arch Pharm (Weinheim). 346:1–9.
- Bowman H, Kaletta U. 1957. Chromatography of rattlesnake venom; a separation of three phosphodiesterases. Biochim Biophys Acta. 24:619–631.
- Dhananjaya BL, Zameer F, Girish KS, D’Souza CJ. 2011. Anti-venom potential of aqueous extract of stem bark of Mangifera indica L. against Daboia russellii (Russell's viper) venom. Indian J Biochem Biophys. 48:175–183.
- Gomes A, Das R, Sarkhel S, et al. 2010. Herbs and herbal constituents active against snake bite. Indian J Exp Biol. 48:865–878.
- Heyninck K, Lahtela-Kakkonen M, Vander Veken P, Haegeman G, Vanden Berghe W. 2014. Withaferin A inhibits NF-kappaB activation by targeting cysteine 179 in IKKβ. Biochem Pharmacol. 91:501–509.
- Jilani K, Lupescu A, Zbidah M, Shaik N, Lang F. 2013. Withaferin A stimulated Ca2+ entry, ceramide formation and suicidal death of erythrocytes. Toxicol in Vitro 27:52–58.
- Khan MA, Subramaneyaan M, Arora VK, Banerjee BD, Ahmed RS. 2015. Effect of Withania somnifera (Ashwagandha) root extract on amelioration of oxidative stress and autoantibodies production in collagen-induced arthritic rats. J Complement Integr Med. 12:117–125.
- Lakshmi Ranganatha V, Zameer F, Meghashri S, Rekha ND, Girish V, Gurupadaswamy HD, Khanum SA. 2013. Design, synthesis and anticancer properties of novel benzophenone-conjugated coumarin analogs. Arch Pharm (Weinheim). 346:901–911.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem. 193:265–275.
- Manoharan S, Panjamurthy K, Menon VP, Balakrishnan S, Alias LM. 2009. Protective effect of Withaferin-A on tumour formation in 7,12-dimethylbenz[a]anthracene induced oral carcinogenesis in hamsters. Indian J Exp Biol. 47:16–23.
- Mishra LC, Singh BB, Dagenais S. 2000. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med Rev. 5:334–346.
- Raghavendra RH, Diwakar BT, Lokesh BR, Naidu KA. 2006. Eugenol-the active principle from cloves inhibits 5-lipoxygenase activity and leukotriene-C4 in human PMNL cells. Prostaglandins Leukot Essent Fatty Acids. 74:23–27.
- Raghavendra RH, Naidu KA. 2011. Eugenol and n-3 rich garden cress seed oil as modulators of platelet aggregation and eicosanoids in Wistar albino rats. Open Neutraceut J. 4:144–150.
- Rastogi RP, Mehrotra BN. 1994. Compedium of Indian medicinal plants. Lucknow, New Delhi, India: Central Drug Research Institute, NISC. p. 9, 15.
- Schüttelkopf AW, Aalten DMFV. 2004. PRODRG-a tool for high throughput crystallography of protein ligand complexes. Acta Crystallogr. 68:1355–1363.
- Thomsen R, Christensen MH. 2006. MolDock: a new technique for high accuracy molecular docking. J Med Chem. 49:3315–3321.
- Yu Z, Dahlgren RA. 2000. Evaluation of methods for measuring polyphenols in copper foliage. J Chem Ecol. 26:2119–2140.
