Abstract
Context Litsea cubeba (Lour.) Pers. (Lauraceae) has long been used as a folk remedy in Traditional Chinese Medicine (TCM) for the treatment of rheumatic diseases. Previous studies from our laboratory indicated that L. cubeba extract showed anti-arthritic activity in rats.
Objective To study L. cubeba chemically and biologically and to find the potential constituents responsible for its anti-arthritic effect.
Materials and methods The compounds were isolated from the root of L. cubeba by column chromatography which eluted with PE:EtOAc gradient system, and the structures were elucidated by detailed spectroscopic data analysis; the anti-inflammatory activity of the isolated compounds was evaluated by lipopolysaccharide (LPS)-induced RAW 264.7 cells and the TNF-α and NO level were measured by ELISA (commercial kit); The iNOS and COX-2 mRNA expression were measured by RT-PCR and the phosphorylation of IκBα, IKKβ, P38 and Akt were determined by western blots.
Results A novel 9-fluorenone, 1-ethoxy-3,7-dihydroxy-4,6-dimethoxy-9-fluorenone (1), together with 4 known compounds, namely pinoresinol (2), syringaresinol (3), 9,9′-O-di-(E)-feruloyl-meso-5,5′-dimethoxysecoisolariciresinol (4) and lyoniresinol (5) were isolated from the root of L. cubeba for the first time. The IC50 for NO inhibition on compounds 1 and 4 were 56.1 ± 1.2 and 32.8 ± 2.3 μM, respectively. The IC50 for TNF-α inhibition were 28.2 ± 0.9 and 15.0 ± 1.0 μM, respectively. Both 1 and 4 suppress mRNA expression of iNOS, COX-2 and protein phosphorylation of IκBα, IKKβ in LPS-induced RAW 264.7 cells.
Discussion and conclusion Compounds 1 and 4 isolated from L. cubeba exhibited potent anti-inflammatory activity through the NF-κB signal pathway.
Introduction
Rheumatoid arthritis (RA) is a common, immune-mediated disease characterized by chronic progressive inflammation and destruction of joints and associated structures, as well as systemic symptoms (Tarner & Müller-Ladner Citation2008; Praveen & Suchita Citation2013). It is generally considered as a disease caused by genetic and external factors involved in predisposing genes, lifestyle factors such as smoking, infectious agents, occupational exposures and exposure to pets (Yang et al. Citation2010). Currently, the pathogenesis of RA has not been completely understood, however, it is clear that inflammatory cytokines such as TNF-α, IL-1β, IL-6 and IL-17A as well as inflammatory mediators such as cyclooxygenases (COX) and NO play an important role (Annunziato et al. Citation2009). The glucocorticoids, non-steroidal anti-inflammatory drugs (NSAIDs), immunosuppressant and biologic are the main drugs employed for the treatment of RA in clinic. However, the use of these drugs always associated with long-term side-effects, toxicity, high cost and incurs hypersensitivity to medications and infections (Feldmann et al. Citation2001). In fact, herbal medicine is being widely used virtually around the world for the treatment of rheumatic and arthritic diseases, in recent decades, considerable advances have been made in both clinical and basic research on the treatment of RA (Soeken et al. Citation2003; Lin et al. Citation2013).
Litsea cubeba (Lour.) Pers. (Lauraceae) has long been used as a folk remedy in Traditional Chinese Medicine (TCM) for the treatment of rheumatic diseases (Feng et al. Citation2009). Many pharmacological activities of L. cubeba extract and its compounds have been reported such as anticancer, antibacterial, antioxidant and anti-inflammatory immunological activities. Our previous study also proved that L. cubeba exhibited remarkable anti-arthritic activity on Freund’s adjuvant induced arthritis in rats (Lin et al. Citation2013). So far, the anti-arthritic active constituent has not been discovered yet. As part of our ongoing effort to discover the bioactive compounds from this medical plant, the chemical constituents in the root of L. cubeba were investigated in the present study. Macrophages play a central role in organizing the release of inflammatory mediators, including nitric oxide (NO) and tumor necrosis factor-alpha (TNF-α). Due to their highly reproducible response to lipopolysaccharide (LPS), the RAW 264.7 mouse macrophage cell line is widely used for inflammation studies (Ji et al. Citation2012). In the present study, the anti-inflammatory activities were evaluated in vitro employing LPS-induced RAW 264.7 cells. Furthermore, the probable mechanism was examined by real-time PCR and western blot analysis.
Materials and methods
General experimental procedures
NMR spectra were measured with Bruker Avance 600 spectrometer (Billerica, MA) with solvent peak as references. IR Spectra were recorded in KBr pellets on a Bruker Vector 22 spectrophotometer (Billerica, MA). ESI-MS was taken on a Waters Q-TOF MS (Milford, MA). UV Spectra were obtained in MeOH on a Varian Cary Eclipse 300 spectrophotometer (Palo Alto, CA). Column chromatography was performed on silica gel (100–200 or 200–300; Qingdao Marine Chemical Factory) and Sephadex LH-20 (Amersham Biosciences, Uppsala, Sweden). TLC was carried out on silica-gel plates (Yan-Tai Institute of Chemical Technology, 0.2 mm thickness, 5 cm × 10 cm), then visualised under UV light and by spraying with 10% H2SO4, followed by heating.
Plant material
The roots of L. cubeba (dry slices) were purchased from an Herbal Medicinal Materials Company of Anton Pharmaceutical Co., Ltd (Bozhou, Anhui Province, China), and identified by Prof. B.K. Huang (The Second Military Medical University, Republic of China). A voucher specimen has been deposited in School of Pharmacy, Second Military Medical University, Shanghai, China (No. 20110820).
Extraction and purification
Sliced dried roots of L. cubeba (50 kg) were extracted three times (3 h each time) with 75% ethanol. The extract was then combined and evaporated to dryness under reduced pressure, which yielded 650 g residues. The residues were then suspended in water (5 L) and partitioned with petroleum ether (PE), CH2Cl2, EtOAc and n-BuOH successively, to obtain respective fractions (PE fraction 50 g, CH2Cl2 fraction 200 g, EtOAc fraction 56 g, n-BuOH fraction 200 g). The CH2Cl2 fraction (50 g) was subjected to silica gel column chromatography (1 kg) and eluted with PE:EtOAc gradient system (25:1, 20:1, 15:1, 10:1, 5:1, 2.5:1, 1:1, successively) to give seven fractions (Frs.C1-C7). Fraction C5 was fractionated on silica gel column chromatography eluted with PE:EtOAc (7:1) and purified by Sephadex LH-20 with MeOH to afford compounds 1 (70 mg), 2 (50 mg) and 3 (200 mg). Fraction C4 was fractionated on silica gel column chromatography eluted with PE:EtOAc (20:1) and further purified by preparative HPLC to give 4 (35 mg) and 5 (22 mg).
Cell culture
The RAW 264.7 cell was grown in Dulbecco’s modified Eagle’s medium (Hyclone) supplemented with 10% fetal bovine serum (Gibco) and 100 units/mL penicillin/streptomycin sulfate. The cells were incubated in a humidified 5% CO2 atmosphere at 37 °C.
Determination of NO and TNF-α production
The cells were plated at a density of 5 × 105 cells/mL in 96-well plates for 24 h and then stimulated with LPS (1 μg/mL) in the presence or absence of different concentrations of compounds 1–5 for 24 h. The supernatants were collected for the NO and TNF-α assays. NO was determined by measuring the amount of nitrite with Griess reagent (a mixture of equal amounts of reagents A and B, A: 1% sulfanilamide in 5% H3PO4, B: 0.1% naphthylethylene diamine dihydrochloride, Sigma-Aldrich). NO was measured by the detection of its stable oxidative metabolite, nitrite, as described previously (Zhao et al. Citation2013). The TNF-α concentrations were measured using radioimmunoassay according to the manufacturer’s protocol (R&D Systems, Minneapolis, MN).
RNA extraction and real-time polymerase chain reaction (real-time PCR) analysis
Real-time quantitative polymerase chain reaction (RT-PCR) analysis of iNOS, COX-2 and GAPDH mRNA were performed as described previously (Xu et al. Citation2010). For PCR amplification, the following mouse-specific sense and antisense primers were used: iNOS, 5′-TGGAGTCACAGAAGGAGTGGCTAAC-3′ (forward) and 5′-TCTGACCACAGTGAGGAATGTCCAC-3′ (reverse); COX-2, 5′-GGAGAGACTAAGATAGTGATC-3′ (forward) and 5′-ATGGTCAGTAGACCTTTACAGCTC-3′ (reverse); GAPDH, 5′-GGGGAGCCAAAAGGGTCATC-3′ (forward) and 5′-GACGCCTGCTTCACCACCTTCTTG-3′ (reverse).
Western blotting analysis
Western blot analysis was performed as described previously (Wang et al. Citation2012). The concentrations of proteins from whole-cell lysates were determined by Bradford assay. Briefly, equal amounts of protein were separated by SDS-PAGE and transferred onto nitrocellulose membranes. The blots were then blocked overnight with 5% (m/v) nonfat dry milk, and probed with phospho-specific antibodies to IKBα, Ikkβ, p38 and akt antibodies in 5% (m/v) bovine serum albumin dissolved in TBST [20 mM Tris-HCl buffer, pH 7.6, containing 137 mM NaCl and 0.05% (v/v) Tween-20]. With the use of horseradish peroxidase-conjugated secondary anti-rabbit or anti-mouse antibody, bound antibodies were detected by enhanced chemiluminescence.
Results and discussion
Structures of isolated compounds
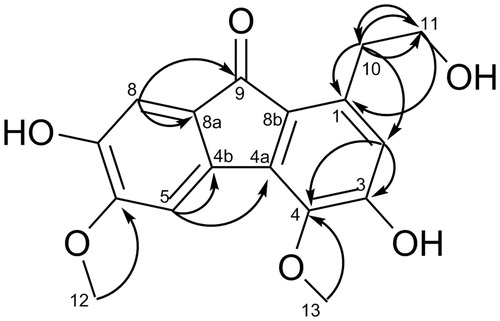
Compound 1, a faint yellow powder, was determined to have a molecular formula of C17H16O6 by ESI-MS (m/z 317.18, [M + H]+; m/z 315.04, [M − H]−) and NMR data analysis. The IR absorption suggested the presence of hydroxyl (3467 cm−1), carbonyl (1673.2 cm−1) and phenyl groups (1602.2, 1504.6 cm−1). The 1H NMR spectrum () of 1 exhibited signals characteristic for two methoxys at δH [3.91 (s, 3H), 3.97 (s, 3H)], one 1, 3, 4, 5, 6-penta-substituted aromatic ring at δH 6.44 (1H, s), and a 1, 2, 4, 5-tetra-substituted benzene ring at δH [7.34 (1H, s, H-5) and 6.94 (1H, s, H-8)]. In addition, the chemical shifts at δH 3.73 (2H, t, J = 7.2 Hz, H-10), δH 3.07 (2H, t, J = 7.2 Hz, H-11) suggested that compound 1 exhibited an ethoxy (–CH2–CH2–OH), which were confirmed by the HMBC spectrum () correlations from H-10 to C-11 and from H-11 to C-10.
Table 1. 1H (600 MHz) and 13C (150 MHz) NMR spectroscopic data of compound 1 in MeOD.
In the 13C NMR and DEPT spectrum, the 12 sp2-hybridised carbons include three tertiary carbons at δC 116.7 (C-2), 106.6 (C-5), 110.0 (C-8), and four oxygenated quaternary carbons at δC 157.2 (C-3), 141.7 (C-4), 152.2 (C-6), 146.6 (C-7), as well as five further quaternary carbons at δC 137.5 (C-1), 128.5 (C-4a), 135.1 (C-4b), 123.3 (C-8a), 136.8 (C-8b), and their chemical shifts were indicative of a 9-fluorenone skeleton when the carbonyl group (δC 193.1, C-9) was taken into consideration (Yang et al. Citation2004). This hypothesis was supported by the HMBC spectrum, in which the correlations from the aromatic proton H-8 (δH 6.94) to C-8b and C-9, from H-5 (δH 7.34) to C-4a and C-4b were observed. The two methoxys were linked to C-4 and C-6, respectively, while the ethoxy was connected to C-1, as evidence by the HMBC () correlations from H-12 (δH 3.91) to C-6 (δC 152.2), from H-13 (δH 3.97) to C-4 (δC 141.7), and from H-10/11 to C-1 (137.5). All proton and carbon signals were assigned based on HSQC, HMBC and 1H-1H COZY spectra. Thus, based on the above evidences, compound 1 was established as 1-ethoxy-3,7-dihydroxy-4,6-dimethoxy-9-fluorenone ().
1-Ethoxy-3,7-dihydroxy-4,6-dimethoxy-9-fluorenone (1), C17H16O6, faint yellow powder; IR (KBr) νmax 3467.0, 2937.8, 1673.2, 1602.2, 1578.7, 1504.6, 1265.9, 1135.5, 793.8 cm−1; ESI-MS m/z 317.18, [M + H]+; m/z 315.04, [M − H]−; 1H NMR (MeOD, 600 MHz), 13C NMR (MeOD, 150 MHz), HMBC data ().
Identification of known compounds
The structures of the following constituents, pinoresinol (2), syringaresinol (3), 9,9′-O-di-(E)-feruloyl-meso-5,5′-dimethoxysecoisolariciresinol (4), lyoniresinol (5) were identified by comparison of their spectral data with the literature.
Pinoresinol (2), C20H22O6, white powder, ESI-MS m/z: 372.4 [M]+. 1H NMR (600 MHz, CDCl3, δ/ppm): 6.8 0–6.89 (6H, m, H-2,2′, 5,5′, 6,6′), 4.73 (2H, d, J = 5.6 Hz, H-7, 7′), 3.90 (6H, s, 2 × OMe), 3.8 6–4.25 (4H, m, H-9, 9′), 3.09 (2H, m, H-8, 8′). 13C NMR (150 MHz, CDCl3, δ/ppm): 148.1 (C-3, 3′), 146.6 (C-4, 4′), 134.3 (C-1, 1′), 120.3 (C-6, 6′), 115.6 (C-5, 5′), 110.0 (C-2, 2′), 87.2 (C-7, 7′), 73.0 (C-9, 9′), 57.3 (3,3′-OMe), 55.5 (C-8, 8′) (Meagher et al. Citation1999).
Syringaresinol (3), C22H26O8, white powder, 1H NMR (600 MHz, MeOD, δ/ppm,): 6.57 (4H, s, H-2, 6, 2′, 6′), 5.49 (2H, s, 4, 4′-OH), 4.72 (2H, d, J = 4.0 Hz, H-7, 7′), 4.28 (2H, dd, J = 6.8, 9.0 Hz, H-9a, 9′a), 3.47 (2H, s, H-9b, 9′b), 3.90 (12H, s, 3, 3′, 5, 5′-OMe), 3.08 (2H, m, H-8, 8′); 13C NMR (150 MHz, MeOD, δ/ppm): 148.5 (C-4, 4′), 135.6 (C-3, 3′, 5, 5′), 133.5 (C-1, 1′), 104.0 (C-2, 6, 2′,6′), 87.4 (C-7, 7′), 73.1 (C-9, 9′), 57.7 (3, 3′, 5, 5′-OMe), 55.7 (C-8, 8′) (Sharp et al. Citation2001).
9,9′-O-Di-(E)-feruloyl-meso-5,5′-dimethoxysecoisolariciresinol (4), C42H46O14, white powder, ESI-MS m/z: 775.12 [M]+. 1H NMR (600 MHz, DMSOd6, δ/ppm): 7.53 (1H × 2, d, J = 15.9 Hz, H-7″, 7‴), 7.00 (1H × 2, dd, J = 1.8, 8.2 Hz, H-6″,6‴), 6.95 (1H × 2, d, J = 1.8 Hz, H-2″, 2‴), 6.84 (1H × 2, d, J = 8.2 Hz, H-5″,5‴), 6.23 (1H × 2, d, J = 15.9 Hz, H-8″,8‴), 6.21 (2H × 2, s, H-2, 6, 2′,6′), 4.40 (1H × 2, dd, J = 5.5, 11.3 Hz H-9b, 9b′), 4.14 (1H × 2, dd, J = 5.8, 11.3 Hz, H-9a, 9a′), 3.86 (3H × 4, s, H-3, 5, 3′, 5′), 3.72 (3H × 2, s, H-3″,3‴), 2.67 (1H × 2, dd, J = 7.1, 14.0 Hz, H-7b,7b′), 2.63 (1H × 2, dd, J = 7.9, 14.0 Hz, H-7a,7a′), 2.10 (1H × 2, m, H-8,8′); 13C NMR (150 MHz, DMSO, δ/ppm): 166.6 (C-9″, 9‴), 149.3 (C-3″, 3‴), 147.9 (C-4″, 4‴), 147.7 (C-3, 3′, 5, 5′), 145.0 (C-7″, 7‴), 133.6(C-4, 4′), 129.9 (C-1, 1′), 125.4 (C-1″, 1‴), 123.1 (C-6″, 6‴), 115.4 (C-8″, 8‴), 114.3 (C-5″, 5‴), 111.1 (C-2″, 2‴), 106.1 (C-2, 6, 2′,6′), 63.8 (C-9, 9′), 55.7 (3, 5, 3′, 5′-OMe), 55.6 (3″,3‴-OMe), 40.3 (C-8, 8′), 34.3 (C-7,7′) (Kuroda et al. Citation2011).
Lyoniresinol (5), C22H28O8, yellow powder, 1H NMR (600 MHz, MeOD, δ/ppm): 6.45 (1H, s, H-2), 6.34 (2H, s, H-2′, 6′), 5.36 (1H, s, 3-OH), 5.34 (1H, s, 4′-OH), 4.02 (1H, d, J = 7.8 Hz, H-7), 3.5 6–3.79 (4H, m, H-9, H-9′), 3.88 (3H, s, 3-OMe), 3.79 (6H, s, H-3′, 5′-OMe), 3.30 (3H, s, 5-OMe), 2.70 (1H, dd, J = 4.0, 16.0 Hz, H-7a), 2.58 (1H, t, J = 9.2 Hz, H-7b), 1.63 (1H, m, H-8), 1.96 (1H, dd, J = 4.4, 10.8 Hz, H-8); 13C NMR (150 MHz, MeOD, δ/ppm): 129.9 (C-1), 107.8 (C-2), 147.5 (C-3), 138.3 (C-4), 146.8 (C-5), 126.3 (C-6), 34.8 (C-7), 41.7 (C-8), 68.1 (C-9), 139.6 (C-1′), 106.7 (C-2′, C-6′), 148.1 (C-3′, C-5′), 134.5 (C-4′), 44.4 (C-7′), 50.8 (C-8′), 65.3 (C-9′), 57.79 (3′, 5′-OMe), 60.8 (5-OMe), 57.4 (3-OMe) (Vecchietti et al. Citation1979).
Anti-inflammatory activity
All the isolated compounds were evaluated for their inhibitory effects both on NO and TNF-α production on RAW 264.7 cell induced by LPS. The IC50 value of each compound on NO and TNF-α production was listed in . The results showed that the new compound, 1-ethoxy-3,7-dihydroxy-4,6-dimethoxy-9-fluorenone (1) and compound 4 exhibited potent anti-inflammatory activity with IC50 values of 56 and 32 μM on NO and of 28 and 15 μM on TNF-α, respectively. Compounds 2, 3 and 5 also showed modest anti-inflammatory activity with IC50 values in the range of 93–221 μM. These results indicated that the synergistic anti-inflammatory effect of multiple components may be responsible for the anti-arthritic activity of L. cubeba.
Table 2. The IC50 of TNF-α and NO production.
Effect of C1 and C4 on iNOS and COX-2 mRNA expression in RAW 264.7 cells
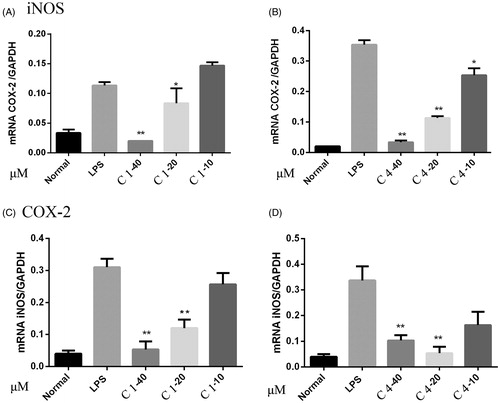
During the inflammatory response, activated macrophages secrete large amounts of pro-inflammatory mediators, such as NO and prostaglandin E2 (PGE2), via the inducible isoforms of NO synthase (iNOS) and cyclooxygenase-2 (COX-2), respectively, as well as pro-inflammatory cytokines, such as TNF-α, IL-6 (Jung et al. Citation2009). Most NO are synthesised through the oxidative deamination of l-arginine by inducible NOS (iNOS), and high levels of NO cause inflammatory diseases, such as bowel disease, RA (Blantz & Munger Citation2002). Current results showed that compounds 1–5 exhibited inhibitory effect both on NO and TNF-α production on RAW 264.7 cell induced by LPS, and the mRNA expression of iNOS and COX-2 was also investigated by RT-PCR. As showed in , compounds 1 and 4 strongly suppressed the mRNA expression levels of both iNOS and COX-2. These data suggested that pretranslational events were involved in L. cubeba inhibition of LPS-induced expression of iNOS and COX-2 (GAPDH as a control gene), and compounds 1 and 4 showed the most potent inhibitory effect among the isolated compounds.
Figure 2. RT-PCR analysis of the expression of iNOS and COX-2 mRNA. RAW 264.7 cells were pretreated with C1 and C4 (40, 20, 10 μM) for 2 h, and then incubated with LPS for 18 h. The cells were lysed, and total RNA was subjected to RT-PCR with the primers iNOS or COX-2 with GAPDH as internal control. These experiments were repeated three times with similar results. *p < 0.05, **p < 0.01 indicate statistically significant differences from the LPS-treated group.
Effect of C1 and C4 on IκBα, IKKβ, P38 and Akt protein phosphorylation in RAW 264.7 cells
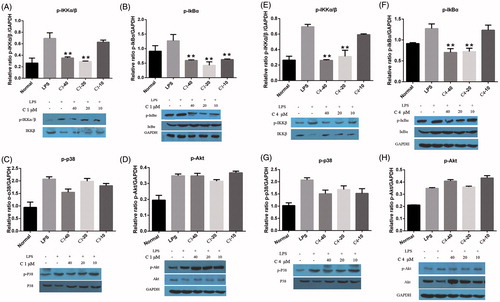
Inflammatory processes play a fundamental role in the damage of articular tissues, many in vitro and in vivo studies have examined the contribution of components of the NF-κB signaling pathways to the pathogenesis of various rheumatic diseases, in particular, of osteoarthritis (OA) and RA. The NF-κB also seems to be responsible for inducing iNOS and COX-2 in inflammatory cells (Roman Blas & Jimenez Citation2006). Activation of NF-κB by LPS is induced by a cascade of events leading to the activation of inhibitor κB (IκB) kinases (IKKs), which in turn phosphorylates IκB and leads to the degradation of NF-κB and its translocation to the nucleus (Griscavage et al. Citation1996). The phosphorylation and degradation of IκB are crucial steps for NF-κB activation in activated macrophages. In addition, the phosphorylation of NF-κB also regulated by (ERK)1/2, P38 MAPK, and PI3K/Akt pathways (Chandrasekar et al. Citation2004). The effect of compounds 1 and 4 on protein expression and phosphorylation of IκBα, IKKβ, P38 and Akt were evaluated by western blot assay. As shown in , the phosphorylated forms of IκBα and IKKβ, were barely detectable in the unstimulated RAW 264.7 cells. However, the IκBα and IKKβ phosphorylation level was increased significantly after the LPS (2 μg/mL) treatment for 30 min. A pretreatment with compound 1 significantly inhibited the LPS-mediated IκBα and IKKβ phosphorylation at 40, 20, 10 μM and 40, 20 μM, respectively (). However, compound 1 had no effect on the phosphorylation level of P38 and Akt (). As showed in , compound 4 significantly inhibited both phosphorylation level of IκBα and IKKβ at 40, 20 μM, and no significant effect on the phosphorylation level of P38 and Akt.
Figure 3. Effect of C1 and C4 on LPS-induced phosphorylation and degradation of IκBα, IKKβ, P38 and Akt. RAW 264.7 cells were pretreated with C1 and C4 (40, 20, 10 μM) for 2 h, and then incubated with LPS for 30 min. The cellular lysates were prepared and analysed for content of IκBα, p-IκBα, IKKβ, p-IKKβ, P38, p-P38, Akt, p-Akt and GAPDH by western blot. These experiments were repeated three times with similar results. *p < 0.05, **p < 0.01 indicate statistically significant differences from the LPS-treated group.
Conclusion
In summary, the anti-inflammatory activity of L. cubeba was characterised by the suppression mRNA expression of iNOS, COX-2, and various cytokines, such as TNF-α and NO, through the NF-κB signal pathway. These results showed that compounds 1 and 4, two main active compounds of L. cubeba, inhibit the LPS-induced expression of iNOS and COX-2 at the mRNA levels as well as the expression of TNF-α, NO transcripts in macrophage RAW 264.7 cells. These suppressive effects are mediated by inhibiting the phosphorylation of IκBα and IKKβ which are crucial steps for NF-κB activation. These results indicate that the synergistic anti-inflammatory effect of multiple components may be contributed to the anti-arthritic activity of L. cubeba.
Declaration of interest
The authors report no declarations of interest. This work was supported by the National Natural Science Foundation of China (Grant Nos. 81573696, U1203104, 81403162, 81202865), Youth Scholars Breeding Project for Medical Science of PLA (Grant No. 13QNP098) and Shanghai Municipal Committee of Science and Technology (Grant Nos. 13401900102, 14401902900).
References
- Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. 2009. Type 17 T helper cells-origins, features and possible roles in rheumatic disease. Nat Rev Rheumatol. 5:325–331.
- Blantz RC, Munger K. 2002. Role of nitric oxide in inflammatory conditions. Nephron 90:373–378.
- Chandrasekar B, Marelli-Berg FM, Tone M, Bysani S, Prabhu SD, Murray DR. 2004. Beta-adrenergic stimulation induces interleukin-18 expression via beta2-AR, PI3K, Akt, IKK, and NF-kappaB. Biochem Biophys Res Commun. 319:304–311.
- Feldmann M, Brennan F, Bondeson J, Paleolog E, Foxwell B, Maini R. 2001. Analysis of cytokine expression in rheumatoid synovium has provided new insights into the pathogenesis of rheumatoid arthritis and new therapeutic opportunities. Transplant P. 33:2085–2086.
- Feng T, Xu Y, Cai XH, Du ZZ, Luo XD. 2009. Antimicrobially active isoquinoline alkaloids from Litsea cubeba. Planta Med. 75:76–79.
- Griscavage JM, Wilk S, Ignarro LJ. 1996. Inhibitors of the proteasome pathway interfere with induction of nitric oxide synthase in macrophages by blocking activation of transcription factor NF-kappa B. Proc Natl Acad Sci USA. 93:3308–3312.
- Ji G, Zhang Y, Yang Q, Cheng S, Hao J, Zhao X, Jiang Z. 2012. Genistein suppresses LPS-induced inflammatory response through inhibiting NF-κB following AMP kinase activation in RAW 264.7 macrophages. PLoS One 7:e53101.
- Jung HW, Seo UK, Kim JH, Leem KH, Park YK. 2009. Flower extract of Panax notoginseng attenuates lipopolysaccharide-induced inflammatory response via blocking of NF-kappaB signaling pathway in murine macrophages. J Ethnopharmacol. 122:313–319.
- Kuroda M, Sakurai K, Mimaki Y. 2011. Chemical constituents of the stems and twigs of Lindera umbellata. J Nat Med. 65:198–201.
- Lin B, Zhang H, Zhao XX, Rahman K, Wang Y, Ma XQ, Zheng CJ, Zhang QY, Han T, Qin LP. 2013. Inhibitory effects of the root extract of Litsea cubeba (Lour.) Pers. on adjuvant arthritis in rats. J Ethnopharmacol. 147:327–334.
- Meagher LP, Beecher GR, Flanagan VP, Li BW. 1999. Isolation and characterization of the lignans, isolariciresinol and pinoresinol, in flaxseed meal. J Agric Food Chem. 47:3173–3180.
- Praveen DK, Suchita M. 2013. Herbal sources of anti-arthritic potential: a comprehensive review. Int J Pharma. 4:88–92.
- Roman Blas J, Jimenez S. 2006. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthr Cartilage. 14:839–848.
- Sharp H, Thomas D, Currie F, Bright C, Latif Z, Sarker SD, Nash RJ. 2001. Pinoresinol and syringaresinol: two lignans from Avicennia germinans (Avicenniaceae). Biochem Syst Ecol. 29:325–327.
- Soeken K, Miller S, Ernst E. 2003. Herbal medicines for the treatment of rheumatoid arthritis: a systematic review. Rheumatology 42:652–659.
- Tarner IH, Müller-Ladner U. 2008. Drug delivery systems for the treatment of rheumatoid arthritis. Expert Opin Drug Deliv. 5:1027–1037.
- Vecchietti V, Ferrari G, Orsini F, Pelizzoni F. 1979. Alkaloid and lignan constituents of Cinnamosma madagascariensis. Phytochemistry 18:1847–1849.
- Wang ZQ, Jiang W, Zhang Z, Qian M, Du B. 2012. Nitidine chloride inhibits LPS-induced inflammatory cytokines production via MAPK and NF-kappaB pathway in RAW 264.7 cells. J Ethnopharmacol. 144:145–150.
- Xu CQ, Liu BJ, Wu JF, Xu YC, Duan XH, Cao YX, Dong JC. 2010. Icariin attenuates LPS-induced acute inflammatory responses: involvement of PI3K/Akt and NF-kap signaling pathway. Eur J Pharmacol. 642:146–153.
- Yang H, Chou GX, Wang ZT, Hu ZB, Xu LS. 2004. Two new fluorenones from Dendrobium chrysotoxum. J Asian Nat Prod Res. 6:35–38.
- Yang M, Xiao C, Wu Q, Niu M, Yao Q, Li K, Chen Y, Shi C, Chen D, Feng G, et al. 2010. Anti-inflammatory effect of Sanshuibaihu decoction may be associated with nuclear factor-kappa B and p38 MAPK alpha in collagen-induced arthritis in rat. J Ethnopharmacol. 127:264–273.
- Zhao F, Chen L, Zhang M, Bi C, Li L, Zhang Q, Shi C, Li M, Zhou S, Kong L. 2013. Inhibition of lipopolysaccharide-induced iNOS and COX-2 expression by indole alkaloid, 3-(hydroxymethyl)-6,7-dihydroindolo[2,3-a]quinolizin-(12H)-one, via NF-κB inactivation in RAW 264.7 macrophages. Planta Med. 79:782–787.