Abstract
Context 3β-Acetoxyurs-11-en-13β,28-olide (I), a triterpenoid, is found in most plant species. Pharmacologically triterpenes are very effective compounds with potent anticancer, anti-HIV and antimicrobial activities.
Objectives Microbial transformation of 3β-acetoxyurs-11-en-13β,28-olide (I) was performed in order to obtain derivatives with improved pharmacological potential.
Materials and methods Compound (I, 100 mg) was incubated with Aspergillus niger culture for 12 d. The metabolite formed was purified through column chromatography. Structure elucidation was performed through extensive spectroscopy (IR, MS and NMR). In vitro α- and β-glucosidase inhibitory, and antiglycation potentials of both substrate and metabolite were evaluated.
Results Structure of metabolite II was characterized as 3β-acetoxyurs-11,12-epoxy-13β,28-olide (II). Metabolite II was found to be an oxidized product of compound I. In vitro α- and β-glucosidases revealed that metabolite II was a potent and selective inhibitor of α-glucosidase (IC50 value = 3.56 ± 0.38 μM), showing that the inhibitory effect of metabolite II was far better than compound I (IC50 value = 14.7 ± 1.3 μM) as well as acarbose (IC50 value = 545 ± 7.9 μM). Antiglycation potential of compound II was also high with 82.51 ± 1.2% inhibition. Thus, through oxidation, the biological potential of the substrate molecule can be enhanced.
Conclusion Biotransformation can be used as a potential tool for the production of biologically potent molecules.
Introduction
Triterpene is a diverse class of organic compounds, mostly found in the plant kingdom. It has been reported that triterpenes and their derivatives show interesting biological activities such as anti-HIV (Mayaux et al. Citation1994; Kashiwada et al. Citation2000; Zhu et al. Citation2001), HIV protease enzyme inhibition (Ma et al. Citation1999), antibacterial (Wolska et al. Citation2010) and anticancer effects against various cancer cell lines (Lee et al. Citation1988; Lin et al. Citation1990; Liu Citation1995). Strategies have been used to derivatize triterpenes in order to improve their biological potentials. Synthetic transformation has a number of shortcomings as it is restricted only to the activated position in the molecule. Besides this, there are little chances of stereoselectivity in reaction following the method of chemical transformation. On the other hand, enzymes have extraordinary strength of stereo and regioselectivity without protecting groups. Through biocatalysis a number of less toxic bioactive derivative are produced in fairly good quantity which would be difficult to obtain either from biological system or chemical synthesis (Rasor & Voss Citation2001). A number of publications are available regarding the biotransformation of triterpenes. In one experiment, using Cunninghamella species, betulinic acid was transformed into its glycosylated product (Chatterjee et al. Citation1999), similarly in another report, different hydroxylated products of betulinic acid were produced using Bacillus megaterium (Chatterjee et al. Citation2000).
Microbial transformation is a successful tool to predict metabolic pathways and the fate of drugs in the human body. This is because of the fact that fungi are eukaryotic organisms having an enzyme system similar to that of humans (Rosazza et al. Citation1986). As far as the development of new drugs is concerned, the technology of biotransformation is very useful because the derivative product may either have an improved property compared with the starting compound or may be similar to those metabolites produced during metabolism when administered to mammals. Keeping in view the literature available on the biotransformation of triterpenes an attempt was made to transform 3β-acetoxyurs-11-en-13β,28-olide, an ursane type of triterpene with Aspergillus niger. The main objective of the present research work was to obtain pharmacologically useful derivatives of 3β-acetoxyurs-11-en-13β,28-olide.
Materials and methods
General experimental details
Optical rotation was determined on a Perkin-Elmer model 341 polarimeter (PerkinElmer Health Sciences, Inc., Shelton, CT). UV/visible spectra were measured on a Hitachi U-2001 UV/Vis spectrometer (Hitachi America, Ltd., Troy, MI). IR spectra were recorded on a Bruker FT-IR model IFS-88 spectrometer (Bruker Corporation, New Orleans, LA). 1H and 13C NMR spectra were performed on an AVANCE AV-600 NMR spectrometer, using TMS or solvent peaks as a reference standard. MS spectra were obtained on a JEOL MS Route spectrometer. Incubation of micro-organisms and biotransformation was performed on an Incubator Shaker JS-FS-2500 (Johnsam Co., Inchon, Korea). All solvents used in this study were of analytical grade; silica gel for column chromatography was product of E. Merck, Darmstadt, Germany.
The micro-organism used in this study was obtained from the Pathology Department, Agriculture University, Peshawar, and was maintained on Sabaroud dextrose agar (SDA) at 4 °C in a refrigerator.
Isolation of 3β-acetoxyurs-11-en-13β,28-olide (I)
The bark of R. arboreum was collected in the month of February, 2011, at Seran valley of Hazara division and was identified by Dr. Rashid Department of Botany, University of Peshawar; a voucher specimen 7212/Bot. was deposited at the natural herbarium of Peshawar University for future reference. The shade-dried bark (5 kg) was crushed into small pieces and finally pulverized into fine powder. The plant materials were soaked in methanol with occasional shaking, at room temperature. After 15 d, the methanol soluble materials were filtered. The filtrate was concentrated under vacuum at low temperature using a rotary evaporator. The crude methanol extract of bark was redissolved in distilled water and successively extracted with hexane, chloroform, ethyl acetate and n-butanol to afford the corresponding extracts (Harborne Citation1998). The ethyl acetate fraction was subjected to column chromatography using column grade silica gel (80 g). Eluting the column with hexane/CHCl3 and finally with CHCl3/CH3OH afforded 21 sub-fractions (EA-EU). Purification of sub-fraction ER through flash column chromatography using hexane/EtOAc as a solvent system afforded a white crystalline compound, which was characterized as 3β-acetoxyurs-11-en-13β,28-olide (I).
Fungi and culture medium for experiment
The broth medium used for A. niger for conducting the biotransformation experiments composed of 5.0 g glucose, 2.5 g KH2PO4, 2.5 g peptone, 2.5 g yeast extracts, 2.5 g NaCl and 2.5 g glycerol in 500 ml of distilled water.
General procedure for biotransformation
Stage II fermentation protocol was used for conducting all types of biotransformation reactions. Spores from a freshly prepared 2-day-old culture was inoculated aseptically into 250 mL flask having sterile broth medium (100 mL), which was then incubated on shaking table for 48 h at 30 °C at 200 rpm. Stage I culture from seed flask was aseptically transferred into 10 flasks each having 100 mL of pre-autoclaved broth media. All these flasks were incubated on shaking table for a period of 48 h at 30 °C at 200 rpm. Compound I (100 mg) was dissolved in 5 mL ethyl acetate so that a transparent solution was formed. The solution was equally distributed among 10 flasks. All flasks were incubated in a rotary shaker at 30 °C and 200 rpm for a period of 12 d. Two kinds of controls were run in all experiments, i.e. substrate control (having only substrate without biomass) for checking stability of substrate and culture control (having biomass without substrate) for checking metabolites endogenously produced by the fungi.
Isolation and characterization of biotransformed products
Fungal mycelia were removed from the broth media through filtration and were thoroughly washed with ethyl acetate (1 L). Extraction of organic portion from filtrate was performed with ethyl acetate (5 L). Whole organic part was dried with anhydrous Na2SO4 and was finally concentrated under reduced pressure with the help of rotary evaporator to afford brown gummy material (500 mg). In a similar way, the controls were also harvested. Biotransformation of substrate was confirmed with the help of thin layer chromatography. The crude material was purified through column chromatography using n-hexane:ethyl acetate (90:10) solvent system to afford a white amorphous powder (10 mg) identified as 3β-acetoxyurs-11,12-epoxy-13β,28-olide (II) using 1D and 2D spectroscopic techniques.
3β-Acetoxyurs-11,12-epoxy-13β,28-olide (II)
White amorphous solid; mp; 287 °C, UV λmax (MeOH); 275 nm, Rf = 0.5 [acetone: hexane; 8:2]. The 1H NMR (CDCl3, 600 MHz) and 13C NMR (CDCl3, 150 MHz) are given in ; HREI-MS m/z 513.72 [M + H]+ (calculated for C32H48O5, 512.3502).
Table 1. 1H and 13C NMR chemical shift assignments of 3β-acetoxyurs-11,12-epoxy-13β, 28-olide (II) (600 and 150 MHz, respectively, CDCl3).
Biological potential of metabolite
α-Glucosidase inhibition study
Assay for α-glucosidase inhibition was performed by slight modification of a previously published method (Ma et al. Citation2011). Briefly, solutions of α-glucosidase (from Saccharomyces cerevisiae) and its substrate p-nitrophenyl α-d-glucopyranoside (pNPG) were prepared in phosphate buffer (70 mM, pH 6.8). Methanol was used as a preferred solvent for the preparation of inhibitor solutions. The inhibition assays were conducted by adding inhibitor solution (10 μL) to 70 μL buffer and 10 μL of enzyme solution (2.5 unit/mL) in 70 mM phosphate buffer (pH 6.8) followed by preincubation at 37 °C for 5 min. After preincubation, 10 μL of 10 mM substrate (pNPG) prepared in phosphate buffer was added to the mixture to initiate enzymatic reaction. The reaction mixture was incubated at 37 °C for 30 min, and the reaction was stopped by the addition of 80 μL of 0.2 M Na2CO3. Acarbose was used as a positive control. The α-glucosidase activity was determined by measuring the p-nitrophenol released from pNPG at 405 nm using an Elx 800 Micro plate reader (PerkinElmer Health Sciences, Inc., Shelton, CT). The % inhibition was calculated using the following equation:
The IC50 value of the potent inhibitor was determined by testing 10–12 serial dilutions of inhibitor and was calculated by using the program PRISM 5.0 (Graph Pad Inc., San Diego, CA).
β-Glucosidase inhibition study
To determine the inhibitory activity against β-glucosidase, the assay was performed with slight modification of the previously published method (Pérez et al. Citation2008). β-Glucosidase (from sweet almonds) and p-nitrophenyl β-d-glucopyranoside (pNPG) as a substrate were prepared in 0.07 M phosphate buffer (pH 6.8). The inhibition assays were conducted by adding inhibitor solution (10 μL) to 70 μL buffer and 10 μL of enzyme solution (2.0 unit/mL) in 0.07 M phosphate buffer (pH 6.8) followed by preincubation at 37 °C for 5 min. The reaction was started by adding 10 μL of substrate (p-nitrophenyl glucopyranoside (pNPG) (10 mM)) in phosphate buffer to the preincubated reaction mixture. The reaction mixture was then incubated at 37 °C for 30 min and stopped by adding 80 μL of 0.2 M Na2CO3. Negative control contained 10 μL of distilled water instead of inhibitor. Acarbose was used as a positive control.
Antiglycation study
Antiglycation activity was determined using a previously described method with appropriate modifications (Xi et al. Citation2008). Briefly, to 250 μL of bovine serum albumin (1 mg/mL), an equal amount of 500 mM glucose was added. Test compound dissolved in DMSO (50 μL) was added to this mixture and the contents were subjected to incubation at 60 °C for 24 h. TCA of 100% (10 μL) was added to each sample to stop the reaction. Samples were centrifuged at 10 000 rpm 4 °C for 15 min. Supernatant was removed and pellets were collected. Phosphate buffer saline (PBS) at pH 10 (50 μL) was added to dissolve the pellets. Finally, the fluorescence intensity was measured at 360 nm excitation and 460 nm emissions using a Biotek Flx 800 spectrofluorometer (BioTek Instruments, Inc., Winooski, VT).
Results and discussion
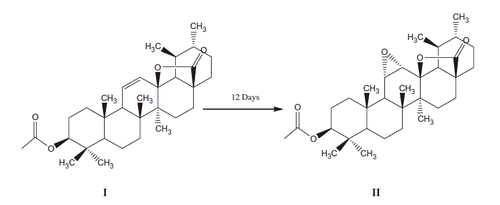
Aspergillus niger a filamentous fungi was studied for its ability to transform 3β-acetoxyurs-11-en-13β,28-olide (I). It was confirmed from the screening scale transformation experiment that A. niger had great ability to transform compound (I) at specific position on the main frame work of the substrate to an epoxy derivative, i.e., 3β-acetoxyurs-11,12-epoxy-13β,28-olide (II) (Scheme 1).
Structure of transformed product II was elucidated as 3β-acetoxyurs-11,12-epoxy-13β,28-olide on the basis of detailed physical and spectroscopic data. The electron impact mass spectrometry (EIMS) showed the molecular ion peak at m/z 512 was 16 mass units higher than the starting material indicating the formation of an oxidized derivative of the substrate. Similarly the high resolution electron impact mass spectrometry (HREI MS) of the metabolite (II) showed the M+ at m/z 512.3485 correspond to the molecular formula C32H48O5 (calculated. 512.3502).
1H NMR spectrum of metabolite II showed singlets at 0.83, 1.14, 0.84, 1.14, 1.03 and 2.03 due to C-23, C-24, C-25, C-26, C-27 and C-2′ tertiary methyl groups, respectively, while the secondary methyl protons at C-29 and C-30 appeared as doublet at resonances 0.96 (d, J = 6 Hz) and 1.19 (d, J = 6 Hz), respectively. A downfield methine signal appeared as double doublet at resonance 4.5 (dd, J = 6 Hz) was assigned to C-3 having the acetoxy group. The acetoxy group at C-3 was given β-configuration on the basis of the magnitude of coupling constant and chemical shift values. In addition, two downfield signals resonating at 2.93 (d, J = 6 Hz) and 3.09 s were assigned to C-11 and C-12 epoxy methine proton, respectively. The detailed 1H NMR data of metabolite (II) are presented in .
13C NMR spectra (broadband decoupled and DEPT) of metabolite II revealed the presence of 8 methyl, 8 methylene, 8 methine and 8 quaternary carbons. The resonances at 27.69, 16.19, 17.22, 17.3, 20.22, 19.51 and 17.29 were ascribed to the methyl carbons at C-23, C-24, C-25, C-26, C-27, C-29 and C-30, respectively, while the resonance at 21.3 was assigned to the acetoxy methyl carbon. The resonances at 89.03 and 45.10 were assigned to C-13 and C-17 quaternary carbons having the lactonic group, respectively. A downfield signal resonating at 80.42 was ascribed to C-3 methine carbon having the acetoxy group. Similarly two other downfield signals resonating at 56.15 and 54.75 were assigned to the C-11 and C-12 methine carbons, respectively, having the epoxy group. In addition, another downfield signal resonating at 171.06 was assigned to the ester carbonyl carbon. The detailed 13C NMR assignment of metabolite (II) is shown in . Based on the above data, the compound (II) was characterized as 3β-acetoxyurs-11,12-epoxy-13β,28-olide.
The biotransformed compound II along with the starting compound I was tested against the yeast α-glucosidase enzyme. Compound II (3β-acetoxyurs-11,12-epoxy-13β,28-olide), which is a triterpene, was found to be the most potent inhibitor of the cited enzyme with an IC50 value of 3.56 ± 0.38 μM. The inhibitory effect of compound II was far better than compound I and was interestingly higher than acarbose (standard inhibitor), which was 545 ± 7.9 μM. In order to find the selectivity of compound II, it was treated against β-glucosidase of sweat almond origin. The inhibitory potential of compound II was less than 13% when tested at end concentration of 0.1 mM, which showed that neither the substrate nor the metabolite can inhibit β-glucosidase enzyme (). Hence these compounds can be studied further as a potential antidiabetic agent with selective inhibition against α-glucosidase.
Table 2. Inhibition potential of compounds I and II against α-glucosidase, β-glucosidase and glycation.
Antiglycation potential of compound II was also determined which was surprisingly high with an inhibition of 80.5 ± 6.3% (). When compared with compound I, the activity of compound II was also high, thus showing that oxidation of the substrate by the microbes enhances its biological potential.
Conclusion
Filamentous fungus A. niger was screened for its ability to transform compound I, in order to improve its biological potential. However, it was found to be the most active organism for transformation of the title compound. Only one bioactive transformation product was obtained in fairly good quantity. α-Glucosidase inhibitory potential of metabolite II was higher compared with the standard as well as starting compound I, while β-glucosidase inhibitory potential was less than 13% showing that metabolite II was a selective inhibitor of α-glucosidase. The antiglycation potential of compound II was also higher than the standard (rutin) showing that microbial technology is a useful tool for the production of metabolites with improved biological potential. The results show that metabolite II is a potent antidiabetic compound that can lead to the discovery of novel potential drug for diabetes with dual action.
Declaration of interest
The authors report that they have no conflicts of interest. The authors are greatly indebted to the Higher Education Commission of Pakistan for financial support under its indigenous PhD fellowship scheme.
References
- Chatterjee P, Kouzi SA, Pezzuto JM, Hamann MT. 2000. Biotransformation of the antimelanoma agent betulinic acid by Bacillus megaterium ATCC 13368. Appl Environ Microbiol. 66:3850–3855.
- Chatterjee P, Pezzuto JM, Kouzi SA. 1999. Glucosidation of betulinic acid by Cunninghamella species. J Nat Prod. 62:761–763.
- Harborne JB. 1998. Phytochemical methods. London: Chapman and Hall.
- Kashiwada Y, Nagao T, Hashimoto A, Ikeshiro Y, Okabe H, Cosentino LM, Lee KH. 2000. Anti-AIDS agent 38. Anti-HIV activity of 3-O-acetyl ursolic acid derivatives. Nat Prod. 63:1619–1622.
- Lee KH, Lin YM, Wu TS, Zhang DC, Yamagishi T, Hayashi T, Hall IH, Chang JJ, Wu RY, Yang TH. 1988. The cytotoxic principles of Prunella vulgaris, Psychotria serpens, and Hyptis capitata: ursolic acid and related derivatives. Planta Med. 54:308–311.
- Lin CN, Lu CM, Cheng MK, Gan KH, Won SJ. 1990. Alkylated flavanones from the bark of Cryptocarya chartacea as dengue virus NS5 polymerase inhibitors. J Nat Prod. 53:513–516.
- Liu J. 1995. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol. 49:57–68.
- Mayaux JF, Bousseau A, Pauwels R, Huet T, Henin Y, Dereu N, Evers M, Soler F, Poujade C, De Clercq E, et al. 1994. Triterpene derivatives that block entry of human immunodeficiency virus type I into cells. Proc Natl Acad Sci USA. 91:3564–3568.
- Ma C-M, Nakamura N, Hattori M, Shimotohno K. 1999. Inhibitory effects of constituents from Cynomorium songaricum and related triterpene derivatives on HIV-1 protease.
- Ma HY, Gao HY, Sun L, Huang J, Xuand XM, Wu LJ. 2011. Constituents with α-glucosidase and advanced glycation end-product formation inhibitory activities from Salvia miltiorrhiza Bge. J Nat Med. 65:37–42.
- Pérez M, Muñoz FJ, Muñoz E, Fernández M, Sinisterra JV, Hernáiz MJ. 2008. Synthesis of novel glycoconjugates and evaluation as inhibitors against β-glucosidase from almond. J Mol Catal B: Enzym. 52–53:153–157.
- Rasor JP, Voss E. 2001. Enzyme-catalyzed processes in pharmaceutical industry. Appl Catal A: Gen. 221:145–158.
- Rosazza JPN, Duel MW, Brossi A. (Ed.). 1986. Alkaloids: chemistry and pharmacology. Vol. 27. New York: Academic Press. p. 39192.
- Verma N, Behera BC, Sharma BO. 2012. Glucosidase inhibitory and radical scavenging properties of lichen metabolites salazinic acid, sekikaic acid and usnic acid. Hacett J Biol Chem. 40:7–21.
- Wolska KI, Grudnaik AM, Fiecek B, Dowjat AK, Kurek A. 2010. Antibacterial activity of oleanolic and ursolic acids and their derivatives. Cent Eur J Biol. 5:543–553.
- Xi M, Hai C, Tang H, Chen M, Fang K, Liang X. 2008. Antioxidant and antiglycation properties of total saponins extracted from traditional Chinese medicine used to treat diabetes mellitus. Phytother Res. 22:228–237.
- Zhu Y-M, Shen J-K, Wang H-K, Cosentino L-M, Lee K-H. 2001. Synthesis and anti-HIV activity of oleanolic acid derivatives. Bioorg Med Chem Lett. 11:3115–3118.