Abstract
Context Quercetin (QE), a bioflavonoid present abundantly in fruits and vegetables, has been reported to possess antioxidant properties. Acrylamide (ACR) is formed in foods during cooking and is known to be neurotoxic.
Objective The present study was designed to evaluate the protective effect of QE against neurotoxicity induced by ACR.
Materials and methods Four groups of Wistar rats consisting of six rats each: (i) control group; (ii) acrylamide treated group (50 mg/kg body weight as single dose); (iii) quercetin group: rats were treated intraperitoneally (i.p.) with QE (10 mg/kg body weight alone every day for 5 d); (iv) quercetin + acrylamide group: quercetin (10 mg/kg bw) was given i.p. every day for 5 d followed by acrylamide i.p. injection (50 mg/kg bw) on fifth day (single dose). Rats were killed after 48 h.
Results Administration of ACR (50 mg/kg bw) in Wistar rats resulted in significant increase of dopamine, interferon-γ and 8-hydroxyguanosine with concomitant decrease of serotonin (p < 0.001) in the rat brain. Treatment of rats with QE intraperitonealy (10 mg/kg body weight) before ACR assault resulted in the diminution of ACR-mediated neurotoxicity as evident from decreased levels of dopamine, interferon-γ (p < 0.001) and 8-hydroxyguanosine with concomitant restoration of serotonin levels (p < 0.001).
Discussion and conclusion On the basis of the above results, the present study suggests that quercetin may be a potential therapeutic agent for restoration of oxidative damage to neurons.
Introduction
Acrylamide (ACR), a byproduct of the Maillard reaction, can be generated from food components during heat treatment where it is produced as a result of the formation of the reactive carbonyl group on the sugar and amino group of the amino acid (Mottram et al. Citation2002). Humans are susceptible to exposure because acrylamide is produced naturally as a result of cooking of starch rich food at high temperatures during baking or frying. It is also likely to be produced by grilling and roasting of food. In industry, most acrylamide is used to synthesize polyacrylamides, which finds uses as water-soluble thickeners. These include use in wastewater treatment, gel electrophoresis (SDS-PAGE), papermaking, ore processing, tertiary oil recovery and the manufacture of permanent press fabrics. Some ACR is used in the manufacture of dyes and the manufacture of other monomers. Acrylamide is considered to be a genotoxic carcinogen because it has the potential to cause cancer by interacting with the genetic material (DNA) in cells. Though under physiological conditions ACR does not cross the blood–CSF barrier, it has been shown that ACR exposure damages the blood–cerebrospinal fluid barrier and impairs secretory and transport functions (Yao et al. Citation2014). ACR also promotes neurodegenerative diseases such as Parkinson’s and Alzheimer’s disease (Lopachin et al. Citation2002).
Dietary polyphenols have been proposed to be promising functional food additives due to their potent antioxidant capacity and other health-beneficial bioactivities (Xu et al. Citation2014; Li et al. Citation2015). Concomitantly, we indicated in our previous studies that, quercetin scavenges reactive oxygen species and reduces oxidative DNA damage in liver and kidney by reversal of biochemical oxidative stress markers (Zargar et al. Citation2015). Administration of quercetin 2 h before injection of CdF2 in mice resulted in reversal of indoleamine 2,3-dioxygenase activity in both liver and kidney to near normal levels (Zargar et al. Citation2014). Various reports suggest that quercetin passes through the blood–brain barrier and influences the neuronal cells directly. A higher concentration of quercetin metabolites appear in the brain after several hours of quercetin administration (Day et al. Citation2001). These reports may provide an understanding of the neuroprotective role of quercetin, emphasizing the influence of this flavonoid in the diet for human health. The present study evaluates the beneficial effects of quercetin on ACR-induced oxidative damage of brain.
Materials and methods
Chemicals
All the chemicals used, including quercetin, were purchased from Sigma Chemical Co., St. Louis, MO.
Animals
Healthy young approximately 21 d old western albino rats (male/female) were obtained from the Animal Breeding Laboratory, King Saud University, Riyadh, Saudi Arabia, and kept in the Central Animal House Facility of the institute. Rats were housed in polypropylene cages at room temperature, 60 ± 15% relative humidity and with a 12 h light–dark cycle. Animals were provided standard laboratory chow and purified water ad libitum. The study was approved by the Institutional Animal Ethics Committee. Ethical guidelines for the care and use of laboratory animals in experiments were strictly followed. After 1 week of acclimatization, the rats were randomly divided into different groups consisting of six rats each as described below.
Experimental protocol
Healthy Wistar rats weighing from 45 to 60 g were divided randomly into four groups consisting of six rats each were studied: (i) control group; (ii) rats treated with single i.p injection of 50 mg/kg bw ACR on the fifth day; (iii) rats treated with five i.p. injections of QE (10 g/kg bw); (iv) rats treated with five i.p. injections of 10 mg/kg bw of QE followed by i.p. injection of ACR (50 mg/kg bw) on the fifth day.
Sample preparation
All the groups were sacrificed by asphyxiation with carbon dioxide after 24 h. The dissected brains were placed into the Eppendorf tubes and weighed. Afterwards each brain tissue was homogenised with an ultrasonic cell disrupter (Vibracell 72434, Bioblock, Illkrich-Cedex, France) in 150 μL 0.1 M perchloric acid containing 0.4 mM sodium metabisulphite. The homogenates were then centrifuged at 10 000 × g for 25 min at 4 °C and the supernatants were filtered through a 0.22 μm filter (Sigma, Chicago, IL) and frozen at −70 °C until analysis.
Determination of brain monoamines
Determination of brain serotonin and dopamine was carried out using high-performance liquid chromatography (HPLC) by the method of Zagrodzka et al. (Citation2000). Filtrate was injected into the HPLC system. The HPLC system consisted of a quaternary gradient delivery pump Model HP 1050 (Hewlett-Packard, Palo Alto, CA), a sample injector Model 7125 (Rheodyne, Berkeley, CA), and an analytical column ODS 2 C18, 4.6 × 250 mm, particle size 5 μm (Hewlett-Packard, Palo Alto, CA) protected by guard column (Lichnospher 100 RP-18, 4 × 4 mm, Gelman Sciences Inc., Ann Arbor, MI), particle size 5 μm (Hewlett-Packard, Palo Alto, CA). The electrochemical detector model HP 1049 A (Hewlett-Packard, Palo Alto, CA) with glassy carbon working electrode was used at a voltage setting of +0.65V for monoamines. The detector response was plotted and measured using a chromatointegrator ver. 1.2 (Esoft, East Brunswick, NJ). The concentration of monoamines and their related metabolites in each sample were calculated from the integrated chromatographic peak area and expressed as ng/100 mg wet tissue. The mobile phase comprised a 0.15 M sodium dihydrogen phosphate, 0.1 mM EDTA, 0.5 mM sodium octanesulphonic acid, 10–12% methanol (v/v) and 5 mM lithium chloride. The mobile phase was adjusted to pH 3.4 with phosphoric acid, filtered through 0.22 μm filter (Sigma, Chicago, IL) and degassed with helium. A column temperature of 32 °C and a flow rate of 1.4 ml/min were used.
Assay of IFNγ
IFNγ was measured using an ELISA kit (Cloud-clone Corporation, Burlington, NC) according to the manufacturer’s instructions. This assay employs a quantitative sandwich enzyme immunoassay technique that measures IFNγ in less than 5 h. A polyclonal antibody specific for human IFNγ has been pre-coated onto a 96-well microplate with removable strips. IFNγ in standards and samples is sandwiched by the immobilized antibody and biotinylated polyclonal antibody specific for IFNγ, which is recognized by a streptavidin–peroxidase conjugate. All unbound material is then washed away and a peroxidase enzyme substrate is added. The colour development is stopped and the intensity of the colour is measured at 550 nm and subtracted from the absorbance at 450 nm. Each sample was measured in duplicate and the level of IFNγ was represented in pg/mg.
Assay of 8-hydroxy-2-deoxyguanosine (8-OHdG)
8-OHdG, a ubiquitous marker of oxidative stress-mediated DNA damage, was quantified using an ELISA kit from Abnova Corporation (Walnut, CA) according to the manufacturer’s instructions. Briefly, 50 μL of detection a working solution was added to each well and incubated for 1 h at 37 °C. After three washes by wash buffer, plate was inverted to dry and 100 μL of detection reagent B working solution was added to each well and incubated for 45 min at 37 °C. After five washes, 90 μL of substrate solution was added to each well. Incubation was done in dark for 15–30 min at 37 °C. Finally, 50 μL of stop solution was added to each well. Change in colour was determined in a microplate reader set to 450 nm. Each sample was measured in duplicate and the level of 8-OHdG was represented in ng/mg DNA against standard curve generated by reducing the data using computer software capable of generating a four parameter logistic (4-PL) curve-fit.
Statistical analysis
A computer SPSS program (SPSS Inc., Chicago, IL) was used, and the results were expressed as mean ± SD (n = 6). Comparisons were made by the one-way ANOVA between the control and treated groups. Dunnett’s test was used to compare between the groups.
Results
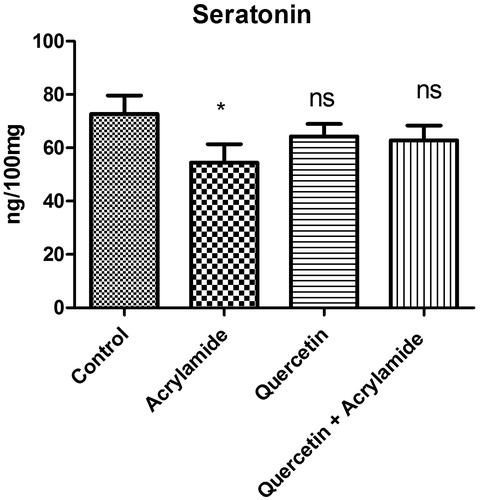
shows the effect of ACR, QE alone and QE with ACR on the serotonin levels in the cerebral cortex of Wistar rats. Administration of ACR alone caused a significant decrease in the levels of serotonin when compared with the control group (p <0.05). However, there were no significant differences in the level of serotonin in rats given quercetin before 5 d of ACR insult.
Figure 1. Effects of quercetin and acrylamide treatment on serotonin levels in the cerebral cortex of Wistar rats. Enzyme activity is expressed as ng/100 mg (n = 6). *p < 0.001 when compared with the control group (independent samples t-test between the control and the treated groups in brain). NSNon-significant as compared with the quercetin alone or quercetin and acrylamide (independent samples t-test between the control and the treated groups in brain).
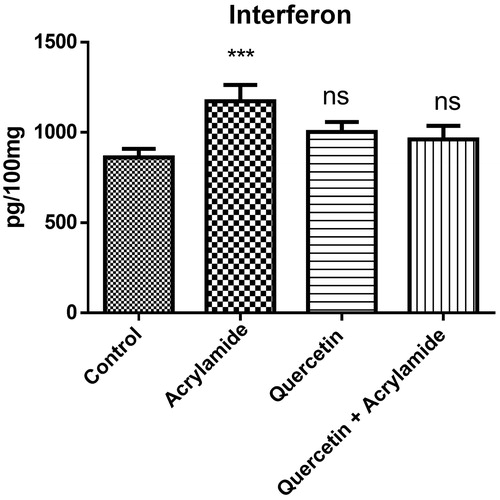
shows the effect of ACR, quercetin alone and in combination on the interferon-γ levels in the cerebral cortex of Wistar rats. Administration of ACR alone caused a significant increase in the levels of IFN-γ when compared with the control group (p <0.05). Prophylactic treatment with quercetin resulted in the near restoration of IFN-γ levels to normal values.
Figure 2. Effects of quercetin and acrylamide treatment on interferon-γ levels in the cerebral cortex of Wistar rats. Enzyme activity is expressed as pg/100 mg (n = 6). ***p < 0.0001 when compared with the control group (independent samples t-test between the control and the treated groups in brain). NSNon-significant as compared with quercetin alone or quercetin and acrylamide (independent samples t-test between the control and the treated groups in brain).
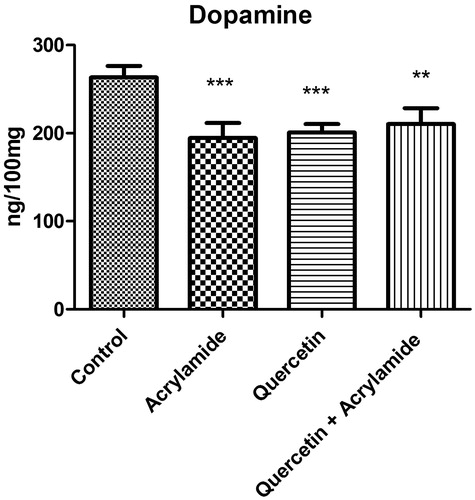
shows the effect of ACR, quercetin alone and quercetin with ACR on the dopamine levels in the cerebral cortex of Wistar rats. ACR caused significant decrease in the levels of dopamine when compared with the control group (p <0.05). Treatment of rats with quercetin alone and QE plus ACR caused a significant decrease in the levels of dopamine when compared with the control group.
Figure 3. Effects of quercetin and acrylamide treatment on dopamine levels in the cerebral cortex of Wistar rats. Enzyme activity is expressed as ng/100 mg (n = 6). *p < 0.001 when compared to control group, ***p < 0.0001 when compared to control group (independent samples t-test between the control and the treated groups in brain).
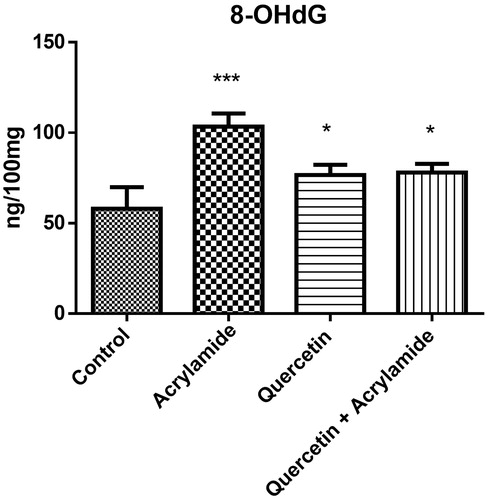
shows the effect of ACR, quercetin alone and quercetin along with ACR on the 8-hydroxyguanine levels in the cerebral cortex of Wistar rats. ACR caused significant increase (p <0.05) in the levels of 8-hydroxyguanine when compared with the control group. QE treatment alone and in combination also caused the significant increase in the levels of dopamine when compared with the control group and a non-significant increase in levels when compared with the ACR alone group. Although there was significant difference between the ACR and the QE + ACR group, the quercetin alone group and the QE + ACR group showed significant differences compared with the control group.
Figure 4. Effects of quercetin and acrylamide treatment on 8-hydroxyguanine levels in the cerebral cortex of Wistar rats. Enzyme activity is expressed as ng/100 mg (n = 6). *p < 0.001 when compared to control group, ***p < 0.0001 when compared to control group (independent samples t-test between the control and the treated groups in brain). There was found significant difference between ACR alone and quercetin+ACR group showing the partial protection in quercetin+ACR group.
Discussion
Acrylamide is a potent neurotoxin and capable of inducing CNS and PNS damage in humans and animals. ACR exposure induces ataxia, skeletal muscle weakness and weight loss in both experimental animal models and occupationally exposed humans (Lopachin Citation2005). Concomitantly, it was indicated that quercetin prevents alterations in oxidative stress parameters as well as neurotransmitter parameters (Abdalla et al. Citation2014). It has been shown that QE scavenges reactive oxygen species and reduces oxidative DNA damage in liver (Zargar et al. Citation2015). Various studies have indicated that QE passes through the blood–brain barrier and affects the neuronal cells directly. Also, a higher concentration of QE metabolites appears in the brain after several hours of QE administration (Day et al. Citation2001). In this study, QE caused a significant recovery in the levels of serotonin decreased by ACR. It was shown that serotonin and its precursor have powerful antioxidant properties and recovery of this neurotransmitter concentration in brain was related to the reduction of lipid peroxide generation and improved antioxidant status (Munoz Castaneda et al. Citation1990). QE also showed protective effect on IFN-γ levels which were increased by ACR. Siddiqi et al. (Citation2012) reported the increased levels of IFN-γ in cerebral cortex of rats induced with gold nanoparticle toxicity and concluded that levels of IFN-γ can be used as potential biomarker for oxidative stress. ACR-induced dopamine depletion was found in present study. Consistently dopamine depletion in neurotoxic effects was also found by Prasad and Muralidhara (Citation2012). In present study, QE significantly restored dopamine levels in treated groups. Further, QE partially reversed 8-OhdG levels in treated groups. 8-OhdG is one of the indicators reflecting oxidative damage caused by ROS (Halliwell and Gutteridge Citation1999). The level of 8-OhdG in tissues is a good indicator of cellular oxidative stress occurring due to degenerative diseases such as cancer (Yao et al. Citation2004). In consistent to this study, Zhang et al. (Citation2013) reported the increase of 8-OhdG in the urine of the people exposed to formaldehyde. An increase in 8-OhdG in the urine of children with acute leukaemia was seen in relation to the oxidative DNA damage (Yang et al. Citation2008). However, no change in the amount of 8-OhdG in urine and plasma was reported by Vaughan et al. (Citation1986).
Conclusion
The results from present study may contribute to a better understanding of quercetin in the neuroprotection, emphasizing its influence in the human diet for better health, possibly by preventing brain injury associated with ACR. QE exhibited protective effects against ACR-induced neurotoxicity in Wistar rats through increase of serotonin levels and reduction of IFN-γ levels in cerebral cortex. QE also exhibited partial neuroprotection with respect to dopamine and 8-OHdG. To our knowledge, this is the first study to report on protective effect of QE in ACR-induced neurotoxicity in Wistar rats. However, more antioxidants need to be studied to prove complete neuroprotection by QE.
Declaration of interest
The authors declare that they have no conflict of interest. This research project was supported by a Grant from the “Research Center of the Center for Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University.
References
- Abdalla FH, Schmatz R, Andréia MC, Baldissarelli J, de Oliveira JS, Rosa MM, Nunes MAG, Maribel AR, da Cruz IBM, Barbisan F, et al. 2014. Quercetin protects the impairment of memory and anxiogenic-like behavior in rats exposed to cadmium: possible involvement of the acetylcholinesterase and Na(+), K(+)-ATPase activities. Physiol Behav. 135:152–167.
- Day AJ, Mellon F, Barron D, Sarrazin G, Morgan MR, Williamson G. 2001. Human metabolism of dietary flavanoids: identification of plasma metabolites of quercetin. Free Radic Res. 35:941–952.
- Halliwell B, Gutteridge JMC. 1999. Free radicals, other reactive species and disease. In: Halliwell B, Gutteridge JMC, editors. Free radicals in biology and medicine. 3rd ed. New York, (NY): Oxford University Press. p. 617–783.
- Li Z, Jiang H, Xu C, Gu L. 2015. A review: using nanoparticles to enhance absorption and bioavailability of phenolic phytochemicals. Food Hydrocoll. 43:153–164.
- LoPachin RM. 2005. Acrylamide neurotoxicity: neurological, morphological and molecular endpoints in animal models, in advances in experimental medicine and biology. Adv Exp Med Biol. 561:21–37.
- LoPachin RM, Ross JF, Reid ML, Das S, Mansukhani S, Lehning EJ. 2002. Neurological evaluation of toxicaxonopathies in rats: acrylamide and 2,5-hexanedione. Neurotoxicology 23:95–110.
- Mottram DS, Wedzicha BL, Dodson AT. 2002. Food chemistry: acrylamide is formed in the Maillard reaction. Nature 419:448–449.
- Munoz Castaneda JR, Montilla P, Padillo FJ, Bujalance I, Munoz MC, Muntane J, Tunez I. 1990. Role of serotonin in cerebral oxidative stress in rats. Acta Neurobiol Exp (Wars). 66:1–6.
- Prasad S, Muralidhara N. 2012. Evidence of acrylamide induced oxidative stress and neurotoxicity in Drosophila melanogaster – its amelioration with spice active enrichment: relevance to neuropathy. Neurotoxicology 33:1254–1264.
- Siddiqi NJ, Abdelhalim MAK, El-Ansary AK, Alhomida AS, Ong WY. 2012. Identification of potential biomarkers of gold nanoparticle toxicity in rat brains. J Neuroinflamm. 9:123–127.
- Vaughan TL, Strader C, Davis S, Daling JR. 1986. Formaldehyde and cancers of the pharynx, sinus and nasal cavity. II. Residential exposures. Int J Cancer. 38:685–688.
- Xu C, Yagiz Y, Borejsza-Wysocki W, Lu J, Gu L, Ramírez-Rodrigues MM, Marshall MR. 2014. Enzyme release of phenolics from muscadine grape (Vitis rotundifolia Michx.) skins and seeds. Food Chem. 157:20–29.
- Yang Y, Jin XM, Yan CH, Tian Y, Tang JY, Shen XM. 2008. Urinary level of nickel and acute leukaemia in Chinese children. Toxicol Ind Health. 24:603–610.
- Yao QH, Mei SR, Weng QF, Zhang PD, Yang Q, Wu CY, Xu GW. 2004. Determination of urinary oxidative DNA damage marker 8-hydroxy-2′-deoxyguanosine and the association with cigarette smoking. Talanta 63:617–23.
- Yao X, Yan L, Yao L, Guan W, Zeng F, Cao F, Zhang Y. 2014. Acrylamide exposure impairs blood–cerebrospinal fluid barrier function. Neural Regen Res. 9:555–560.
- Zagrodzka J, Romaniuk A, Wieczorek M, Boguszewski P. 2000. Bicuculline administration into ventromedial hypothalamus: effects on fear and regional brain monoamines and GABA concentrations in rats. Acta Neurobiol Exp. 60:333–343.
- Zargar S, Siddiqi NJ, Al Daihan SK, Wani TA. 2014. Effect of cadmium fluoride and quercetin on in vivo activity of indoleamine 2,3-dioxygenase in mice liver and kidney. Fluoride 47:31–42.
- Zargar S, Siddiqi NJ, Khan TH, Elredah IE. 2015. Protective effects of quercetin on cadmium fluoride induced oxidative stress at different intervals of time in mouse liver. Acta Biochim Pol. 62:207–213.
- Zhang BY, ShiYQ, Chen X, Dai J, Jiang ZF, Li N. 2013. Protective effect of curcumin against formaldehyde-induced genotoxicity in A549 Cell Lines. J Appl Toxicol. 33:1468–1473.
