Abstract
Context Hypericum perforatum L. (Hypericaceae), used in moderate depression treatment, is active in experimental tests for antidepressant activity. For H. connatum Lam., a South American species lacking hyperforin, antidepressant effects have not been demonstrated.
Objective This study evaluates the antidepressant-like effect of H. connatum in rats and identifies the components involved in this activity.
Materials and methods First, the effects of acute and 14-d oral administrations of an extract derived from H. connatum aerial parts were studied using the Escape Deficit (ED) test. Next, methanol-extracted flavonoid-enriched fractions B and C and fraction-purified flavonoids (quercetin, rutin and isoquercitrin) were evaluated in the ED test after acute administration. To rule out possible confounding effects of the flavonoids, we examined nociceptive threshold using the tail-flick test and anxious behaviour using the elevated plus maze (EPM) test.
Results Hypericum connatum increased reactivity of unavoidable stress-exposed rats after acute (0.5 and 1 g/kg: ED = 18.6/30 and 19.8/30, respectively) and repeated administration (0.5 g/kg twice daily: ED = 17.8/30). Protective effects were observed for fractions B and C (250 mg/kg: ED = 18.1/30 and 18.8/30, respectively), quercetin (2.5, 5 and 10 mg/kg: ED = 15.3/30, 18.3/30 and 21.6/30, respectively), rutin (5 and 10 mg/kg: ED = 15.4/30 and 13.0/30, respectively) and isoquercitrin (2.5 mg/kg: ED = 19.2/30). The flavonoids did not modify nociceptive threshold or performance in the EPM test.
Discussion and conclusion Hypericum connatum showed protective activity in the ED test, a correlate of potential antidepressant-like effects that appeared to be related to the flavonoid components of this species.
Introduction
The genus Hypericum L. (Hypericaceae) encompasses 484 species and it is one of the 100 large angiosperm genera (Scotland Citation2000). Chemical investigation of the genus has led to the isolation of more than 100 compounds such as flavonoids, xanthones, naphthodianthrones and phloroglucinol derivatives that are differently represented in the diverse species. In the last decades, the genus has received widespread attention mainly in relation to the antidepressant activity of Hypericum perforatum L. (commonly known as St. John’s wort), that is widely used today in the treatment of mental depression (Linde et al. Citation1996, Citation2008; Kasper et al. Citation2010). Hypericum perforatum extracts are active in a large number of behavioural and biochemical models which are indicative of antidepressant activity (Okpanyi and Weischer Citation1987; Butterweck et al. Citation1997; Chatterjee et al. Citation1998a; De Vry et al. Citation1999; Gambarana et al. Citation1999). Intriguingly, H. perforatum extract has an antidepressant-like activity after acute and repeated administration (Gambarana et al. Citation1999), at variance with the results obtained with standard antidepressant drugs that are ineffective after a single administration (Gambarana et al. Citation2001a; Grappi et al. Citation2003; Rauggi et al. Citation2005). On one hand, the mechanisms of H. perforatum central actions are still debated. Several of its components have been shown to have antidepressant and anxiolytic-like effects in animals, or interact with neurotransmitter systems believed to be involved in depression, anxiety and other psychiatric disorders (Chatterjee et al. Citation1998b; Di Carlo et al. Citation2001; Gambarana et al. Citation2001b; Butterweck and Schmidt Citation2007; Caccia and Gobbi Citation2009; Kasper et al. Citation2010), but experimental and clinical studies point to the phloroglucinol derivative hyperforin as the main active component endowed with antidepressant activity (Laakmann et al. Citation1998; Chatterjee et al. Citation1998b; Di Carlo et al. Citation2001; Gambarana et al. Citation2001b). On the other hand, little is known about possible antidepressant effects of other Hypericum species, that may also contain different secondary metabolites compared with H. perforatum. Among the Hypericum spp., those native of South America have only recently been investigated and, similar to what is known for H. perforatum, a variety of biological effects have been reported for the extracts of several of these species, such as antiviral, antimicrobial, antinociceptive and central nervous system activities in in vitro and in vivo tests (Gnerre et al. Citation2001; Viana et al. Citation2006; Centurião et al. Citation2014). One of the South American species is Hypericum connatum Lam., widely used in traditional medicine as tonic, astringent, and to treat mouth wounds (Corrêa Citation1984). In Argentina, this plant is used as a cardiac tonic and commercialised under the name cabotoril (Fusco et al. Citation2007). A recent paper by Fratianni et al. (Citation2013) describes the biochemical composition of two extracts of H. connatum. Interestingly, these extracts do not contain appreciable amounts of hyperforin but have a high content in polyphenols.
Depression is a leading cause of disability worldwide and although different classes of antidepressant drugs are available and efficacious, about one-third of depressed patients do not satisfactorily respond to treatment or suffer from significant side effects (Trivedi et al. Citation2006). In this context, natural products of traditional use are actively studied as alternative sources of potential new antidepressant compounds with low toxicity and novel mechanisms of action. Thus, we considered of interest to investigate whether the extract of H. connatum, that lacks the main active component of H. perforatum, i.e., hyperforin, was also endowed with an antidepressant-like activity in rats.
To this end, we investigated the property of H. connatum extract to prevent in rats the development of consequences of unavoidable stress exposure using the acute escape deficit (ED) test (Gambarana et al. Citation2001a). The ED test, that we extensively characterized (Gambarana et al. Citation1995, Citation1999, Citation2001a; Grappi et al. Citation2003; Rauggi et al. Citation2005), is derived from the learned helplessness model (Overmier and Seligman Citation1967); it is easy to perform, has high reproducibility and yield (about 90–95% of the rats exposed to the acute unavoidable stress develop the escape deficit) and we have shown that it is endowed with good predictive validity of putative antidepressant-like activity (Gambarana et al. Citation2001a). The effects of H. connatum were compared with the effects of H. perforatum or fluoxetine after single or repeated treatment, respectively, as standard antidepressants belonging to different classes are inactive after a single administration in the ED test (Gambarana et al. Citation1995, Citation2001a; Grappi et al. Citation2003; Rauggi et al. Citation2005), similar to what observed in other experimental tests and in the clinical setting. Since the extract showed a protective effect, a second set of experiments was performed to identify the active components with subsequent fractionation and purification. In particular, we tested on the ED test two flavonoid-enriched fractions and then three fraction-purified flavonoids. Moreover, in order to rule out the existence of possible confounding factors in the anti-stress effects observed in the ED test, we examined nociceptive threshold using the tail-flick test and anxious behaviour using the elevated plus maze (EPM) test.
Materials and methods
Plant material
Aerial parts of H. connatum were collected in October 2012 in Argentina, province of San Luis, Dpt. Junín, Valle de Concarán, 32°20′36′′ S, 65°00′49′′ W, altitude 864 m, and botanically authenticated by Prof. Maria del Rosario Fusco. A voucher specimen of the plant was kept in the Herbarium of the National University of San Luis under the number MRF/324. No permission is required to collect H. connatum.
Animals
Experiments were carried out on male Sprague–Dawley rats (Charles River, Calco, Italy), weighing 200–225 g when the experimental procedures began, allowing 10 d of habituation to the animal colony. Animals were housed 4–5 per cage (bedding Lignocel® 3/4S, Harlan Laboratories, San Pietro al Natisone, Italy) in an environment maintained at constant temperature and humidity (21 ± 2 °C, 50 ± 10%), with free access to food (4RF21, Mucedola, Settimo Milanese, Italy) and water. A 12 h reverse light/dark cycle (7:00 a. m. lights off, 7:00 p. m. lights on) was used. Experiments were carried out from 9:00 am to 5:00 pm under a red light and controlled noise conditions. All animals were daily manipulated and exposed to sham drug administration by experimenters during the 10-d habituation to the animal facility, and when the experiments began they were well adapted to handling. The procedures used were in accordance with the European legislation on the use and care of laboratory animals (EU Directive 2010/63), and were approved by the University of Siena Ethics Committee (CELAOUS 28/02/2012). All efforts were made to minimize the number of animals used and their suffering.
Extracts preparation
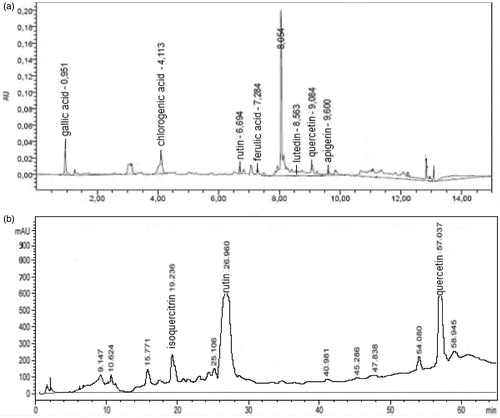
The aerial parts of the plant (5 kg; stems: 80%, leaves 15%, flowers 5%) were air-dried and then extracted with methanol for 2 d at room temperature, yielding 840 g of residue (DER = 5.9:1). This residue was divided into the fractions soluble in ethyl acetate and in ethanol, generating 235 g and 350 g of residue, respectively. The ethanol soluble fraction was analyzed by ultrahigh-performance liquid chromatography (UPLC) (). An ACQUITY Ultra Performance LCTM system (Waters, Milford, MA) linked to a PDA 2996 photodiode array detector (Waters, Milford, MA) was used and the analysis was performed at 30 °C using a reverse-phase column (BEH C18, 1.7 mm, 2.1 mm × 100 mm, Waters, Milford, MA). The mobile phase consisted of Solvent A (7.5 mM acetic acid) and Solvent B (acetonitrile) at a flow rate of 250 mL/min. Twelve gradient elution was employed starting from 5% B to 100% B within 12 min of run. The pressure ranged from 6000 to 8000 psi (41.37–55.16 MPa) during the chromatographic run. The effluent was analyzed with an LC detector (scanning range 210-400 nm; resolution 1.2 nm). The injection volume was 5 mL, and the peaks were monitored at 280 nm. The phenolic compounds were identified by qualitatively and quantitatively comparing the peak areas on the chromatograms of samples with those of diluted standard solutions, through Empower software (Empower Software Solutions, Orlando, FL).
Figure 1. Chromatograms of the ethanolic extract (a) and fraction B (b) of H. connatum. (a) UPLC chromatogram of the ethanolic extract of H. connatum. (b) RPHPLC chromatogram of fraction B of the ethanolic extract of H. connatum and identification of potential active compounds, quercetin, rutin and isoquercitrin. Retention times are expressed in min.
Ethanolic extract purification of H. connatum and isolation of active compounds
The ethanolic extract of H. connatum was fractionated in H2O-n-BuOH (1:1): the butanolic part (2.66 g) was solubilized in methanol and then separated by gel permeation chromatography on a Sephadex LH-20 column, eluting with MeOH. One hundred and sixty-eight fractions of about 10 mL each were obtained and pooled in nine main fractions (I–IX) on the basis of their TLC similarity in n-BuOH–AcOH–H2O (12:3:5) and CHCl3–MeOH–H2O (80:18:2). Fraction B (fractions V + VI) and C (VII), which resulted active in the escape test, were further purified with RPHPLC on a C18 μ-Bondapack column (30 cm × 7.8 mm), eluting with MeOH–H2O (70%:30%, 60 min, flow 1 mL/min. From fraction B (), pure isoquercitrin (32 mg), quercetin (43 mg) and rutin (76 mg) were isolated and structural determination was performed by analyses of 1H NMR, 13C NMR and 13C NMR DEPT data and their comparison with literature data (Agrawal Citation1989).
Behavioural tests
Acute stress protocol
The experimental procedure, previously described in detail (Gambarana et al. Citation2001a), consisted in the induction of ED. Rats were immobilized with a flexible wire net and administered about 80 tail shocks (1 mA × 5 s, one every 30 s). After 24 h, rats were exposed to an avoidable shock-escape test. In our experimental conditions, naive rats (which had not received the unavoidable stress) scored between 22 and 30 escapes out of 30 trials. More than 95% of unavoidable stress-exposed rats developed an ED state, defined by a score of 0–6 escapes out of 30 trials, when tested 24 h after the unavoidable shock session.
Locomotor activity
Locomotor activity was measured by an apparatus consisting of eight compartments (40 × 45 × 50 cm) with a transparent Perspex cage (23 × 33 × 19 cm) in each compartment (Imetronic, Pessac, France). Motor activity was detected by a system of photocell infrared beams, dividing the floor area into a rear and a front sector. The interruption of two photocell beams belonging to two different sectors was recorded as an ambulatory activity count. Infrared photocell beams placed at a 15 cm height detected rearing activity. On test day, rats were placed in the apparatus and activity was recorded for 30 min after a 10 min habituation period.
Antinociceptive test
Pain threshold was assessed by the radiant heat tail-flick method according to D'Amour and Smith (Citation1941). The radiant heat intensity in the tail-flick apparatus (Ugo Basile, Comerio, Italy) was adjusted to obtain average latency values of 4 s in control rats. A 10-s cutoff point was employed to prevent tissue damage to the tail. The mean values obtained in the control group after vehicle treatment were compared with the mean values obtained in the experimental groups 60 min following treatment.
Elevated plus maze (EPM) test
The EPM test was performed as described by Pellow et al. (Citation1985) in order to assess the possible anti-anxiety effects of the compounds under study. The percentage of time spent in open or closed arms was calculated by the following formula: (time in open or closed arms/time in open + closed arms) × 100.
Drugs
Hypericum perforatum extract (0.3% hypericin content) was supplied by Comifar S.p.A., Milanese MI, Italy; H. connatum extract was obtained as described above. Morphine was purchased from SALARS (Como, Italy), fluoxetine from ACEF (Fiorenzuola d’Arda, Italy), diazepam from Tocris Bioscience (Bristol, UK) and all other chemicals were purchased from Sigma-Aldrich S.r.l. (Milano, Italy). Fluoxetine (Gambarana et al. Citation2001a) and morphine (Scheggi et al. Citation2000) were dissolved in deionized/distilled water and administered in a final volume of 1 mL/kg. Diazepam (Harada et al. Citation2006) was suspended in distilled water with 0.3% Tween 80 and administered in a final volume of 1 mL/kg. Hypericum perforatum extract was mixed with methyl-cellulose (1:0.04 w/w) and dissolved in water; H. connatum extract and its fractions were not soluble in methyl-cellulose and water and were dissolved in DMSO/water (50:50 v/v); quercetin, rutin and isoquercitrin were dissolved in corn oil (Sekaran et al. Citation2012; Selvakumar et al. Citation2013). Solutions of extracts, fractions and purified compounds were freshly prepared before use and administered p.o. in a final volume of 2 mL/kg rat body weight. Rats in each control group received the corresponding volume of the appropriate vehicle.
Statistical analyses
Statistical analyses were performed on commercially available software (GraphPad Prism statistical package, GraphPad, San Diego, CA). Data are expressed as mean ± SEM and were subjected to one-way analysis of variance (ANOVA). Post hoc analyses were performed by the Dunnett’s multiple comparisons test, when p < 0.05.
Experimental protocols
Effect of a single administration of H. connatum on acute ED development
The purpose of the experiment was to examine the potential antidepressant-like effect of H. connatum extract after acute administration at two different doses, that is, the ability to prevent the development of the behavioural consequences of unavoidable stress exposure. Sixty minutes before the unavoidable stress session rats were divided into six groups: two groups were administered vehicle p.o. (methyl-cellulose and water, n = 4, or DMSO/water, n = 4, stress groups); four groups of five rats each received H. perforatum, or H. connatum at the doses of 0.5 or 1 g/kg. Doses were selected based on the results obtained with H. perforatum extract in order to obtain a robust protective effect in H. perforatum-treated rats (Gambarana et al. Citation1999). Twenty-four hours later, all animals were tested for escape. Rats treated with H. perforatum extract were used as the positive control group as classical antidepressant drugs are inactive in this test after single administration. In addition, eight more rats received vehicle (methyl-cellulose and water, n = 4, or DMSO/water, n = 4) and 24 h later they were tested for escape (Naive groups). Rats receiving the two different vehicles performed similarly and the results obtained in the subgroups of naive or stress animals were pooled into a single naive and a single stress group.
Effect of repeated treatment with H. connatum extract on acute ED development
The purpose of this experiment was to evaluate whether the protective effect of a single administration of H. connatum extract on ED development after unavoidable stress exposure was maintained after repeated treatment; as the positive control group, fluoxetine-treated rats were used. Rats received twice daily p.o. vehicle (n = 12) or H. connatum (0.5 g/kg, n = 8), or were administered i.p. once a day fluoxetine (5 mg/kg, n = 8) for 14 d. On day 14, spontaneous locomotor activity was assessed in order to evaluate a possible motor stimulant activity of the extract, which would constitute a bias in evaluating an escape deficit behaviour. On day 15, four vehicle-treated rats were exposed only to the escape test (naive); the remaining vehicle-treated rats (stress) and the H. connatum- and fluoxetine-treated groups were exposed to the unavoidable stress session and, 24 h later, to the escape test. Fluoxetine dose was selected based on published results (Gambarana et al. Citation2001a); for H. connatum, the lowest tested dose active after acute administration was used. Behavioural tests were performed 18 h after the evening administration, in order to avoid possible acute effects of the treatment.
Effect of single administration of fractions of H. connatum extract on acute ED development
The purpose of this experiment was to evaluate whether the protective activity of H. connatum extract was retained in the fractions chromatographically obtained as described above (fractions B and C). For this purpose, an acute administration was chosen as the experiments with the extract indicated that the acute effect was predictive of a sustained activity after repeated administration. Rats received vehicle p.o. and 24 h later underwent the escape test, without unavoidable stress exposure (naive, n = 6); the remaining rats received p.o. vehicle (stress, n = 7), H. connatum (0.5 g/kg, n = 5), fraction B (250 mg/kg, n = 9) or fraction C (250 mg/kg, n = 6) 60 min before the unavoidable stress session, and they underwent the escape test 24 h later. The doses of fractions were chosen based on a rough estimate of the enrichment in possible active compounds compared with the extract.
Effect of single administration of quercetin, rutin or isoquercitrin on the acute ED development
The acute administration was chosen for these experiments as the results obtained with the extract indicated that the acute effect was predictive of a sustained activity after repeated administration. In order to evaluate the effect of quercetin, six rats received vehicle p.o. and 24 h later underwent the escape test, without unavoidable stress exposure (naive); eight rats received vehicle p.o. (stress) and five groups of rats each received quercetin p.o. at the doses of 2.5 (n = 6), 5 (n = 6), 10 (n = 6), 20 (n = 4) or 40 mg/kg (n = 4) 60 min before the unavoidable stress session and were tested for escape on the following day. In order to evaluate the effect of rutin, five rats received vehicle p.o. and 24 h later underwent the escape test, without unavoidable stress exposure (naive); five rats received p.o. vehicle (stress) and three subgroups of rats each received rutin at the doses of 5 (n = 5), 10 (n = 8) and 20 mg/kg (n = 6) 60 min before the unavoidable stress session and were tested for escape on the following day. Finally, to evaluate the effect of isoquercitrin, five rats received vehicle p.o. and 24 h later underwent the escape test, without unavoidable stress exposure (Naive); five rats received vehicle p.o. (stress) and five rats received isoquercitrin (2.5 mg/kg) 60 min before the unavoidable stress session and were tested for escape on the following day. Doses of quercetin, rutin and isoquercitrin were selected based on the published results (Butterweck et al. Citation2000; Machado et al. Citation2008; Kawabata et al. Citation2010).
Effect of a single administration of quercetin, rutin and isoquercitrin on the EPM test
To assess whether quercetin, rutin and isoquercitrin modified anxious behaviour at the doses active in the escape test, groups of five rats each were tested in the EPM test 60 min after a single p.o. administration of vehicle, quercetin (10 mg/kg), rutin (10 mg/kg) or isoquercitrin (2.5 mg/kg). As a positive control group, five rats received diazepam (1 mg/kg, p.o.) 60 min before the test. The diazepam dose was selected based on the published results (Harada et al. Citation2006).
Effect of a single administration of quercetin, rutin or isoquercitrin on pain threshold
To exclude an analgesic activity of the compounds, which would constitute a major bias in evaluating a reduction of the behavioural consequences of exposure to noxious stimuli, groups of rats were administered p.o. vehicle (n = 5), quercetin (10 mg/kg, n = 5), rutin (10 mg/kg, n = 5) or isoquercitrin (2.5 mg/kg, n = 6), and 60 min later were tested with the radiant heat tail-flick method. As a positive control for a full analgesic effect, a group of morphine-treated rats was used (5 mg/kg s.c., 10 min before the test, n = 5). The morphine dose and the route of administration were chosen based on the previous results (Scheggi et al. Citation2000; Scheggi et al. unpublished results).
Results
Effect of a single administration of H. connatum on acute ED development
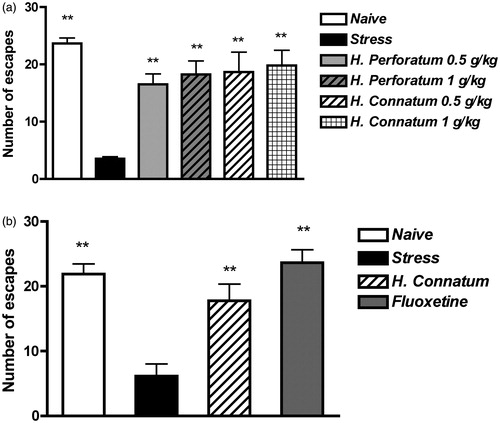
Hypericum connatum and H. perforatum were administered acutely p.o. at 0.5 and 1 g/kg, 1 h before unavoidable stress exposure. Analysis by one-way ANOVA of the number of escapes showed a significant difference between groups (F5,30 = 22.73, p < 0.001). Post hoc analysis demonstrated that the scores in H. perforatum- and H. connatum-treated groups, at both doses, were different from the score in the stress group (p < 0.01) (). Thus, H. connatum was active in the ED test and its effects were similar to those of H. perforatum in preventing the development of escape deficit, at the doses tested.
Figure 2. Effects of H. perforatum or H. connatum treatment on ED development. (a) Rats received vehicle (stress, n = 8), H. perforatum (0.5 g/kg or 1 g/kg, n = 5) or H. connatum (0.5 g/kg, n = 5, or 1 g/kg, n = 5) 60 min before the unavoidable stress session, and they underwent the escape test 24 h later. A group of vehicle-treated rats underwent the escape test, without unavoidable stress exposure (naive, n = 8). (b) Rats received vehicle (stress, n = 8), H. connatum (0.5 g/kg twice daily, n = 8), or fluoxetine (5 mg/kg/day, n = 8) for 14 d and, on the 15th day, they were exposed to unavoidable stress. Twenty-four hours later, rats were tested for escape. A group of vehicle-treated rats underwent the escape test, without unavoidable stress exposure (naive, n = 4). Data are expressed as mean ± SEM of the number of escapes/30 trials. **p < 0.01 versus the stress group.
Effect of repeated treatment with H. connatum extract on acute ED development
Rats treated with H. connatum (0.5 g/kg p.o. twice a day) or fluoxetine (5 mg/kg i.p. once a day) for 14 d were exposed to unavoidable stress exposure and escape test. One-way analysis of variance of escape numbers demonstrated a significant difference between the groups (F3,24 = 11.91, p < 0.001). Post hoc analysis demonstrated that repeated treatments with H. connatum or fluoxetine were able to prevent the condition of ED induced by unavoidable stress exposure (p < 0.01) (). To ascertain whether the activity of the extract on ED was related to a non-specific activating effect, spontaneous locomotor activity was monitored in the different groups of rats at day 14. Analysis by one-way ANOVA of the data did not show any statistical difference in horizontal locomotor activity or rearing number between groups (data not shown).
Effect of single administration of two fractions of H. connatum extract on acute ED development
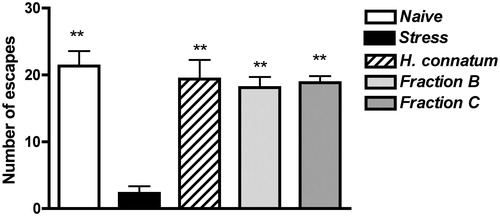
Fractions B and C chromatographically obtained from H. connatum were administered acutely p.o. at a dose 250 mg/kg 60 min before unavoidable stress exposure. Analysis of the number of escapes by one-way ANOVA indicated a significant difference between the groups (F4,28 = 19.95, p < 0.001); post hoc analysis demonstrated that both fractions B and C were able to prevent the development of ED induced by unavoidable stress exposure (p < 0.01; ).
Figure 3. Effects of acute treatment with H. connatum fractions on ED development. Rats received vehicle (stress, n = 7), H. connatum (0.5 g/kg, n = 5), fraction B (250 mg/kg, n = 9) or fraction C (250 mg/kg, n = 6) 60 min before the unavoidable stress session, and they underwent the escape test 24 h later. A group of vehicle-treated rats underwent the escape test, without unavoidable stress exposure (naive, n = 6). Data are expressed as mean ± SEM of the number of escapes/30 trials. **p < 0.01 versus the stress group.
Effect of single administration of quercetin, rutin or isoquercitrin on the acute ED development
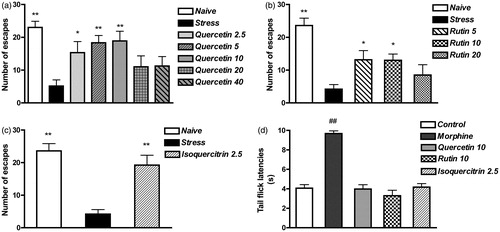
Since the extract and fractions of H. connatum were efficacious in preventing the development of the deficits produced by acute unavoidable stress exposure even after a single administration, the possible activity on the prevention of ED of the main compounds isolated from fraction B (quercetin, rutin and isoquercitrin) was examined in order to establish their contribution to the antidepressant like-effect. In the quercetin experiment, analysis of the number of escapes by one-way ANOVA indicated significant differences between groups (F6,33 = 7.38, p < 0.001). Post hoc analysis demonstrated a clear-cut escape deficit in the stress group (p < 0.01 versus the naive group), while the performance of the 2.5, 5 and 10 mg/kg quercetin-treated groups was significantly different from that of the stress group (p < 0.05 for 2.5 mg/kg; p < 0.01 for 5 and 10 mg/kg) (). In the rutin experiment, analysis of the number of escapes by one-way ANOVA demonstrated significant differences between groups (F4,24 = 9.11, p < 0.001). Post hoc analysis demonstrated a clear-cut ED in the stress group, while the performance of the 5 and 10 mg/kg rutin-treated groups was different from that of the stress group (p < 0.05, both doses) (). Finally, in the isoquercitrin experiment, analysis of the number of escapes by one-way ANOVA demonstrated significant differences between groups (F2,12 = 18.88, p < 0.001); post hoc analysis demonstrated a clear-cut ED in the stress group, while the per-formance of the isoquercitrin-treated group was different from that of the stress group (p < 0.01) ().
Figure 4. Effects of acute treatment with quercetin, rutin and isoquercitrin on ED development and pain threshold. (a) Rats received vehicle (stress, n = 8), quercetin (2.5, 5, 10, 20 and 40 mg/kg, n = 4–8) 60 min before the unavoidable stress session and underwent the escape test 24 h later. A group of vehicle-treated rats underwent the escape test, without unavoidable stress exposure (naive, n = 6). (b) Rats received vehicle (stress, n = 5), rutin (5, 10 and 20 mg/kg, n = 5–8) 60 min before the unavoidable stress session and underwent the escape test 24 h later. A group of vehicle-treated rats underwent the escape test, without unavoidable stress exposure (naive, n = 5). (c) Rats received vehicle (stress, n = 5), isoquercitrin (2.5 mg/kg, n = 5) 60 min before the unavoidable stress session and underwent the escape test 24 h later. A group of vehicle-treated rats underwent the escape test, without unavoidable stress exposure (naive, n = 5). Data are expressed as mean ± SEM of the number of escapes/30 trials. (d) Rats received quercetin (10 mg/kg, n = 5), rutin (10 mg/kg, n = 5), isoquercitrin (2.5 mg/kg, n = 5) or vehicle (1 mL/kg, n = 5) and were tested in the tail flick apparatus 60 min later. As a positive control group, rats were treated with morphine (5 mg/kg s.c., n = 5) 10 min before the test. Data are expressed as mean ± SEM of tail flick latencies in s. **p < 0.01, *p < 0.05 versus the corresponding stress group; ## p < 0.01 versus the control group.
Effect of a single administration of quercetin, rutin and isoquercitrin on the EPM test
To assess whether quercetin, rutin and isoquercitrin modified anxious behaviour at the doses active in the escape test, rats were tested in the EPM test 60 min after a single oral administration of vehicle, diazepam (1 mg/kg), quercetin (10 mg/kg), rutin (10 mg/kg) and isoquercitrin (2.5 mg/kg). The time spent in the open arms (as compared with closed arms) was utilized as an index of an anti-anxiety-like effect because there were no differences between groups in the number of closed, open or total arm entries (data not shown). Analysis of the percentage of time spent in the open and closed arms by one-way ANOVA showed differences between groups (time in open arm: F4,24 = 5.45 p < 0.01; time in closed arm: F4,24 = 3.29 p < 0.05). In particular, post hoc analysis demonstrated a significant increase in time spent in the open arm (p < 0.01) and a significant decrease in time spent in the closed arm (p < 0.05) in the diazepam-treated rats, while the performance of quercetin-, rutin- and isoquercitrin-treated groups was not different from that of the vehicle group (). Moreover, the absence of significant changes in the total numbers of entries into arms observed in the EPM test, suggesting that none of the substances administered modified locomotor activity in the test.
Table 1. Effects of quercetin, rutin or isoquercitrin acute treatment on the performance in the EPM test.
Effect of a single administration of quercetin, rutin or isoquercitrin on pain threshold
In order to verify whether the flavonoids modified the nociceptive threshold, rats were tested in the tail-flick test after vehicle, quercetin (10 mg/kg), rutin (10 mg/kg), isoquercitrin (2.5 mg/kg) and morphine (5 mg/kg) administration. Analysis of tail-flick latencies by one-way ANOVA showed significant differences between groups (F4,21 = 40.80, p < 0.001). Post-hoc analysis demonstrated that the tail-flick latency in morphine-treated rats was higher than in the control rats (p < 0.01), while quercetin, rutin or isoquercitrin administration did not modify the tail-flick latency. Thus, these compounds did not show analgesic properties that could have confounded the effects observed in the escape test ().
Discussion
This study shows that H. connatum extract was active, at the doses tested, in preventing the development of the ED induced by acute exposure to unavoidable stress following a single acute treatment and after a 14-d treatment, similar to what was observed previously with H. perforatum (Gambarana et al. Citation1999). Moreover, H. connatum extract did not modify locomotor activity (data not shown), that is, the observed protective activity was not mediated by non-specific activating effects of the extract. Acute ED is a sensitive test for screening compounds with a possible protective activity on the behavioural consequences of unavoidable stress exposure and antidepressants belonging to different classes are active on this test only after repeated treatment (Gambarana et al. Citation2001a; Grappi et al. Citation2003; Rauggi et al. Citation2005). The protective activity of H. perforatum extract in this test is primarily related to hyperforin (Gambarana et al. Citation2001b). On the other hand, while H. perforatum extract has a hyperforin content of about 4.5%, the extract of H. connatum used in this study had an undetectable hyperforin content. Thus, the activity of H. connatum in the ED test did likely stem from the effects of different compounds. Since polyphenols represent an important component of H. connatum extract (Fratianni et al. Citation2013), in order to evaluate whether these compounds and, more specifically, flavonoids were involved in the observed activity, the effects of some purified flavonoid-enriched fractions were studied on acute ED. A single administration was chosen to study the possible effects of fractions and flavonoids as the experiments with the extract indicated that the acute effect was predictive of a sustained activity after repeated administration. The fractions tested, enriched in flavonoids, showed a marked protective effect after acute administration on stress-induced ED development. We then studied the possible anti-stress activity of quercetin, isoquercitrin or rutin that we isolated from H. connatum. A significant protective activity in the ED test was observed with isoquercitrin, quercetin and rutin, in agreement with the results obtained in different tests for antidepressant-like activity after acute (Butterweck et al. Citation2000; Machado et al. Citation2008; Kawabata et al. Citation2010) or repeated administrations (Rinwa and Kumar Citation2013; Holzmann et al. Citation2015). These results indicate that the protective effects on stress exposure observed after the administration of the extract or its flavonoid-enriched fractions were related to the presence of these flavonoids, suggesting that the active principles responsible of H. connatum central activity are likely different from those involved in the antidepressant effects of H. perforatum.
The administration of increasing doses of rutin showed decreasing protective effects in the ED test. Similar results were obtained with the two higher doses of quercetin tested (10 and 20 mg/kg) and although we have to acknowledge that a limited number of rats received these doses, the lack of protective activity in the ED test was clear. Similar effects of rutin and quercetin administration were observed in the tail suspension and forced swimming test in mice (Machado et al. Citation2008; Holzmann et al. Citation2015). However, neither compound reduced locomotor activity in the EPM test (this study) or in the open field test (Machado et al. Citation2008). These results seem to suggest that rutin plays a secondary role in the antidepressant-like effect of H. connatum. Isoquercitrin, quercetin and rutin did not modify the pain threshold of treated rats, thus excluding a reduced perception of noxious stimuli in the ED test. Isoquercitrin, quercetin and rutin did not modify the performance in the EPM test at the doses that had protective effect on stress-induced ED. These results are at variance with other studies showing that flavonoids are endowed with in vivo anxiolytic effects in rodents (Herrera-Ruiz et al. Citation2008; Fernandez et al. Citation2009; Grundmann et al. Citation2009). This discrepancy may arise from species differences, as many studies were performed in mice (Herrera-Ruiz et al. Citation2008; Fernandez et al. Citation2009; Grundmann et al. Citation2009).
In conclusion, this study shows the activity of H. connatum extract in a test responsive to antidepressant compounds. Hypericum connatum extract was endowed with a protective effect on stress induced ED after acute administration and tolerance did not develop to this effect. Fractions enriched in flavonoids and the major compounds isolated were efficacious in preventing the behavioural consequences of unavoidable stress exposure, suggesting that they play a relevant role in the effect of H. connatum extract. On the other end, the isolated flavonoids did not modify the nociceptive threshold or the behaviour in the EPM test in our experimental setting, at the doses active in the ED test. Thus, the protective effect of H. connatum extract on consequences of stress exposure is a likely correlate of a potential antidepressant-like activity that appears to be related to the flavonoid components of this plant.
A limitation of this study is the use of a single test as an index of the potential antidepressant-like activity of H. connatum extract and its components. Therefore, the antidepressant properties of isoquercitrin and quercetin observed with the acute ED test will be further characterized using validated models of depressive symptoms (Gambarana et al. Citation2001a; Marchese et al. Citation2013; Scheggi et al. Citation2015).
Declaration of interest
The authors report that they have no conflicts of interest. This work was supported by a FARB 2012 Grant to Prof. V. De Feo.
References
- Agrawal PK. 1989. Carbon-13 NMR of flavonoids. Amsterdam, The Netherlands: Elsevier.
- Butterweck V, Jurgenliemk G, Nahrstedt A, Winterhoff H. 2000. Flavonoids from Hypericum perforatum show antidepressant activity in the forced swimming test. Planta Med. 66:3–6.
- Butterweck V, Schmidt M. 2007. St. John's wort: role of active compounds for its mechanism of action and efficacy. Wien Med Wochenschr. 157:356–361.
- Butterweck V, Wall A, Liefländer-Wulf U, Winterhoff H, Nahrstedt A. 1997. Effects of the total extract and fractions of Hypericum perforatum in animal assays for antidepressant activity. Pharmacopsychiatry 30:117–124.
- Caccia S, Gobbi M. 2009. St. John’s wort components and the brain: uptake, concentrations reached and mechanisms underlying pharmacological effects. Curr Drug Metab. 10:1055–1065.
- Centurião FB, Braga A, Machado FR, Tagliari B, Müller LG, Kolling J, Poser GV, Wyse AT, Rates SM. 2014. Study of antidepressant-like activity of an enriched phloroglucinol fraction obtained from Hypericum caprifoliatum. Pharm Biol. 52:105–110.
- Chatterjee SS, Bhattacharya SK, Wonnemann M, Singer A, Müller WE. 1998b. Hyperforin as a possible antidepressant component of hypericum extracts. Life Sci. 63:499–510.
- Chatterjee SS, Nöldner M, Koch E, Erdelmeier C. 1998a. Antidepressant activity of Hypericum perforatum and hyperforin: the neglected possibility. Pharmacopsychiatry 31:7–15.
- Corrêa MP. 1984. Dicionário das Plantas Úteis do Brasil e das Exóticas Cultivadas. Ri ode Janeiro, Brasil: Instituto Brasileiro de Desenvolvimento Florestal.
- D'Amour FE, Smith DL. 1941. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 72:74–79.
- De Vry J, Maurel S, Schreiber R, de Beun R, Jentzsch KR.. 1999. Comparison of Hypericum extracts with imipramine and fluoxetine in animal models of depression and alcoholism. Eur Neuropsychopharmacol. 9:461–468.
- Di Carlo G, Borrelli F, Ernst E, Izzo AA. 2001. St. John’s wort: Prozac from the plant kingdom. Trends Pharmacol Sci. 22:292–297.
- Fernandez SP, Nguyen M, Yow TT, Chu C, Johnston GAR, Hanrahan JR, Chebib M. 2009. The flavonoid glycosides, myricitrin, gossypin and naringin exert anxiolytic action in mice. Neurochem Res. 34:1867–1875.
- Fratianni F, Nazzaro F, Marandino A, Fusco MR, Coppola R, De Feo V, De Martino L. 2013. Biochemical composition, antimicrobial activities, and anti-quorum-sensing activities of ethanol and ethyl acetate extracts from Hypericum connatum Lam. (Guttiferae). J Med Food. 16:454–459.
- Fusco MR, Sosa A, Petenatti ME, Juárez A, Del Vitto L, Petenatti EM. 2007. Medicamentos herbarios en el centro-oeste argentino. VII. Caracterización farmacognóstica y actividad cardiotónica de Hypericum connatum (Clusiaceae). Lat Am J Pharm. 26:209–214.
- Gambarana C, Ghiglieri O, de Montis MG. 1995. Desensitization of the D1 dopamine receptors in rats reproduces a model of escape deficit reverted by imipramine, fluoxetine and clomipramine. Prog Neuropsychopharmacol Biol Psychiatry. 19:741–755.
- Gambarana C, Ghiglieri O, Tolu P, De Montis MG, Giachetti D, Bombardelli E, Tagliamonte A. 1999. Efficacy of an Hypericum perforatum (St. John's wort) extract in preventing and reverting a condition of escape deficit in rats. Neuropsychopharmacology 21:247–257.
- Gambarana C, Scheggi S, Tagliamonte A, Tolu P, De Montis MG. 2001a. Animal models for the study of antidepressant activity. Brain Res Prot. 7:11–20.
- Gambarana C, Tolu P, Masi F, Rinaldi M, Giachetti D, Morazzoni P, De Montis MG. 2001b. A study of the antidepressant activity of Hypericum perforatum on animal models. Pharmacopsychiatry 34:S42–S44.
- Gnerre C, von Poser GL, Ferraz A, Viana A, Testa B, Rates SM. 2001. Monoamine oxidase inhibitory activity of some Hypericum species native to South Brazil. J Pharm Pharmacol. 53:1273–1279.
- Grappi S, Nanni G, Leggio B, Rauggi R, Scheggi S, Masi F, Gambarana C. 2003. The efficacy of reboxetine in preventing and reverting a condition of escape deficit in rats. Biol Psychiatry. 53:890–898.
- Grundmann O, Nakajima JI, Kamata K, Seo S, Butterweck V. 2009. Kaempferol from the leaves of Apocynum venetum possesses anxiolytic activities in the elevated plus maze test in mice. Phytomedicine 16:295–302.
- Harada K, Aota M, Inoue T, Matsuda R, Mihara T, Yamaji T, Ishibashi K, Matsuoka N. 2006. Anxiolytic activity of a novel potent serotonin 5-HT2C receptor antagonist FR260010: a comparison with diazepam and buspirone. Eur J Pharmacol. 553:171–184.
- Herrera-Ruiz M, Romàn-Ramos R, Zamilpa A, Tortoriello J, Jimenèz-Ferrer JE. 2008. Flavonoids from Tilia americana with anxiolytic activity in plus maze test. J Ethnopharmacol. 118:312–317.
- Holzmann I, da Silva LM, Correa da Silva JA, Steimbach VM, de Souza MM. 2015. Antidepressant-like effect of quercetin in bulbectomized mice and involvement of the antioxidant defenses, and the glutamatergic and oxidonitrergic pathways. Pharmacol Biochem Behav. 136:55–63.
- Kasper S, Caraci F, Drago F, Aguglia E. 2010. Efficacy and tolerability of Hypericum extract for the treatment of mild to moderate depression. Eur Neuropsychopharmacol. 20:747–765.
- Kawabata K, Kawai Y, Terao J. 2010. Suppressive effect of quercetin on acute stress-induced hypothalamic–pituitary–adrenal axis response in Wistar rats. J Nutr Biochem. 21:374–380.
- Laakmann G, Schule C, Baghai T, Kieser M. 1998. St. John's wort in mild or moderate depression: the relevance of hyperforin for the clinical efficacy. Pharmacopsychiatry 31(Suppl 1):54–59.
- Linde K, Bernr MM, Kriston L. 2008. St. John’s wort for major depression. Cochrane Database Syst Rev. 4:1–106, CD000448.
- Linde K, Ramirez G, Mulrow CD, Pauls A, Weidenhammer W, Melchart D. 1996. St. John’s wort for depression – an overview and meta-analysis of randomized clinical trials. BMJ 313:253–258.
- Machado DG, Bettio LE, Cunha MP, Santos AR, Pizzolati MG, Brighente IM, Rodrigues AL. 2008. Antidepressant-like effect of rutin isolated from the ethanolic extract from Schinus molle L. in mice: evidence for the involvement of the serotonergic and noradrenergic systems. Eur J Pharmacol. 587:163–168.
- Marchese G, Scheggi S, Secci ME, De Montis MG, Gambarana C. 2013. Anti-anhedonic activity of long-term lithium treatment in rats exposed to repeated unavoidable stress. Int J Neuropsychopharmacol. 16:1611–1621.
- Okpanyi SN, Weischer ML. 1987. Animal experiments on the psychotropic action of a Hypericum extract. Arzneimittelforschung 37:10–13.
- Overmier JB, Seligman ME. 1967. Effects of inescapable shock upon subsequent escape and avoidance responding. J Comp Physiol Psychol. 63:28–33.
- Pellow S, Chopin P, File SE, Briley M. 1985. Validation of open, closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 14:149–167.
- Rauggi R, Cassanelli A, Raone A, Tagliamonte A, Gambarana C. 2005. Study of mirtazapine antidepressant effects in rats. Int J Neuropsychopharmacol. 15:1–11.
- Rinwa P, Kumar A. 2013. Quercetin suppress microglial neuroinflammatory response and induce antidepressent-like effect in olfactory bulbectomized rats. Neuroscience 255:86–98.
- Scheggi S, Masi F, Tagliamonte A, Gambarana C, Tolu P, De Montis MG. 2000. Rats sensitized to morphine are resistant to the behavioral effects of an unavoidable stress. Brain Res. 853:290–298.
- Scheggi S, Pelliccia T, Ferrari A, De Montis MG, Gambarana C. 2015. Imipramine, fluoxetine and clozapine differently affected reactivity to positive and negative stimuli in a model of motivational anhedonia in rats. Neuroscience 291:189–202.
- Scotland RW. 2000. Taxic homology and three-taxon statement analysis. Syst Biol. 49:480–500.
- Selvakumar K, Bavithra S, Ganesh L, Krishnamoorthy G, Venkataraman P, Arunakaran J. 2013. Polychlorinated biphenyls induced oxidative stress mediated neurodegeneration in hippocampus and behavioral changes of adult rats: anxiolytic-like effects of quercetin. Toxicol Lett. 222:45–54.
- Sekaran S, Kandaswamy S, Gunasekaran K, Perumal E, Afsar Basha FY, Madhan Mohan BJ, Jagadeesan A. 2012. Protective role of quercetin on polychlorinated biphenyls (Aroclor-1254) induced oxidative stress and apoptosis in liver of adult male rats. J Biochem Mol Toxicol. 26:522–532.
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ. 2006. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 163:28–40.
- Viana AF, do Rego JC, Munari LDourmap N, Heckler AP, Costa TD, von Poser GL, Costentin J, Rates SM. 2006. Hypericum caprifoliatum (Guttiferae) Cham. & Schltdl.: a species native to South Brazil with antidepressant-like activity. Fundam Clin Pharmacol. 20:507–514.
