Abstract
Context Formononetin is a typical phytoestrogen, which is a bioactive component found in red clover plants. Previous studies have shown that formononetin inhibits the proliferation of several types of cancer cells, including prostate cancer and osteosarcoma. However, how formononetin affects the proliferation of CNE2 is not clear.
Objective The objective of this study is to investigate the effects of formononetin on nasopharyngeal carcinoma cells in vitro, along with the underlying mechanism.
Materials and methods CNE2 cells were incubated with various concentrations of formononetin (0, 0.1, 0.2, 0.3 and 1 μM) for 48 h. Cell proliferation was measured by [3-(4,5-dimethylthiazol-2-yl)]-2,5-diphenyltetrazolium bromide (MTT) assay, while the rate of apoptosis was measured by flow cytometry. Bcl-2 and bax mRNA expression levels were determined by real time polymerase chain reaction (RT-PCR), while p-ERK1/2 and bcl-2 protein expression levels were quantified by Western blotting.
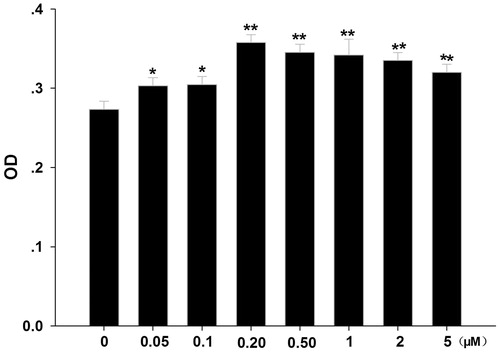
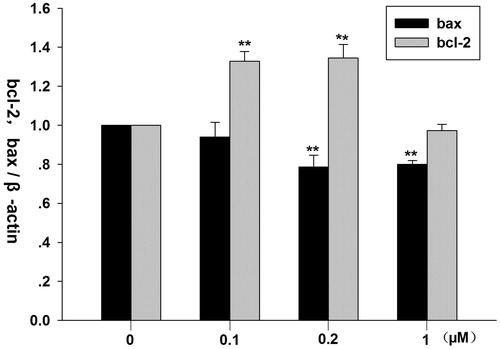
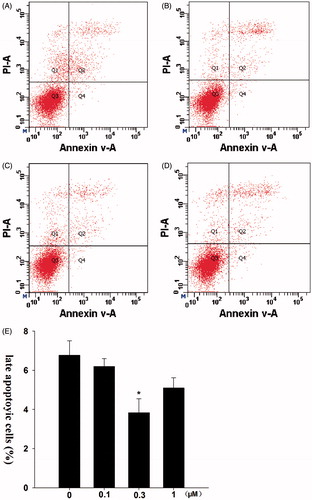
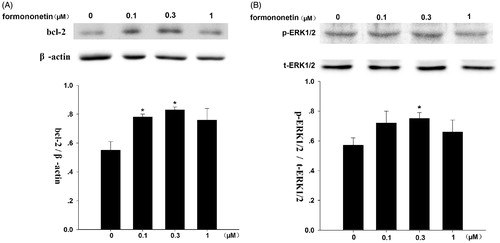
Results Formononetin promoted the proliferation of CNE2 cells at low concentrations (0, 0.05, 0.1, 0.2, 0.5, 1, 2 and 5 μM), OD values increased from 0.27 ± 0.01 to 0.30 ± 0.01, 0.30 ± 0.01,0.36 ± 0.01, 0.35 ± 0.01, 0.34 ± 0.01, 0.34 ± 0.01 and 0.32 ± 0.01, respectively. The percentage of late apoptosis declined from 6.77% ± 0.73% (0 μM group) to 6.2% ± 0.4% (0.1 μM group), 3.83% ± 0.71% (0.3 μM group) and 5.1% ± 0.52% (1M group). The mRNA levels of bax and bcl-2 were down- and upregulated, respectively, by formononetin. Bcl-2 and p-ERK1/2 protein levels were also upregulated.
Conclusions Formononetin stimulates CNE2 cell proliferation and has an inhibitory effect on CNE2 cells apoptosis, which is mediated by the activation of the ERK1/2 signaling pathways.
Introduction
Nasopharyngeai carcinoma (NPC) originates from the epithelial lining of the nasopharynx, and differs from other head and neck cancers in its aetiology, epidemiology and potential therapeutic options (Razak et al. Citation2010). NPC has a distinct ethnic and geographical distribution, with 80% of patients being diagnosed in China, based on the statistics of the World Health Organisation. Furthermore, NPC is more common in southern China, particularly Guangdong Province. At present, both the cause and pathogenesis of this cancer are not clear. It is widely believed that Epstein–Barr virus (EBV) infection contributes to its occurrence (Xu et al. Citation2013). Another study established a link between tobacco smoking and an increased risk of NPC (Xue et al. Citation2013). Environmental carcinogens and dietary components may also contribute to the development of NPC (Xie et al. Citation2014). Furthermore, some reports indicate that the positive expression of oestrogen receptor (ER) in NPC is strongly correlated with cell growth, invasion and distant metastasis (Mo et al. Citation2006; Huang et al. Citation2009).
Phytoestrogen (also called dietary estrogens) is a general term used to define classes of compounds that are non-steroidal, and are either of plant origin or derived from the in vivo metabolism of precursors present in several plants (Poluzzi et al. Citation2014). Due to their structural similarity to oestrogen, these compounds elicit biological effects and may mimic or modulate the actions of endogenous estrogens. Phytoestrogens include isoflavones, lignans, stilbenes and coumestans, which function as natural selective oestrogen receptor modulators (Liu et al. Citation2014).
Both formononetin and calycosin are typical phytoestrogens that belong to isoflavones. Formononetin, being ubiquitous in the plant kingdom, is the main active component of red clover, which has been widely used in clinics for diuretic therapy, anti-inflammation treatment and to improve immunity as a traditional Chinese medicinal herb. Previous studies have indicated that formononetin has antioxidative and estrogenic effects (Mu et al. Citation2009; Jia et al. Citation2014) and promotes the apoptosis of breast cancer, prostate cancer and osteosarcoma (Chen et al. Citation2013; Liu et al. Citation2014; Zhang et al. Citation2014) when given at high concentrations. High concentrations of calycosin have been reported to inhibit the growth of human breast cancer MCF-7 cells, while low concentrations promote the proliferation of MCF-7 cells via p-ERK1/2 activation in vitro and in vivo (Chen et al. Citation2011).
Thus, here, we aimed to determine how formononetin acts on NPC. We selected the oestrogen receptor positive human Nasopharyngeal Carcinoma Cell Line (CNE2) to investigate the effects of formononetin on NPC. We hypothesized that lower and higher concentrations of formononetin exert stimulatory and inhibitory effects on CNE2, respectively, by binding with ER. We investigated whether low concentrations of formononetin stimulate CNE2 growth.
Materials and methods
Drug preparation
Formononetin (purity > 98%, extracted by Phytomarker Ltd, Tianjin, China) was dissolved in dimethyl sulphoxide (DMSO). The formononetin stock (200 mM) was stored at 4 °C until use.
Cell culture
The human Nasopharyngeal Carcinoma Cell Line CNE2 was obtained from Shanghai Institute of Cell Biology (The Chinese Academy of Sciences, Shanghai, China). The cells were grown in RPMI-1640 medium (Gibco-BRL, Grand Island, NY), containing 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 100 kU/L penicillin and 100 mg/L streptomycin at 37 °C in a humidified atmosphere of 5% CO2. The cells were exposed to phenol red-free RPMI-1640 for at least 4 d before the experiments. The cells were starved with low-serum medium (contain 0.5% FBS) for 24 h for the cell proliferation assay.
MTT assay
The cells were seeded in 96-well dishes at a density of 6 × 103 cells/well and were cultured in low-serum medium. After 24 h, the cells were treated with different concentrations of formononetin and a control (without formononetin) for 48 h. Then, 20 μL MTT (Sigma Aldrich, St Louis, MO) was added to each well, and incubated for 4 h at 37 °C. Subsequently, the well contents were solubilized in DMSO. The optical density (OD) was measured at 490 nm.
Flow cytometry
To assay apoptotic cell numbers, CNE2 cells were treated with different concentrations of formononetin for 48 h. Subsequently, the cells were collected and washed with ice-cold PBS. The cells were then stained with annexin V–FITC and propidium iodide (PI), according to the manufacturer's instructions (BD Biosciences, San Jose, CA) for at least 15 min at room temperature. Early apoptotic cells stain positive for annexin V-FITC and negative for PI, whereas late apoptotic cells stain positive for both annexin V-FITC and PI.
Real-time PCR assay
CNE2 cells were treated with formononetin (0, 0.1, 0.2 and 1 μM) for 48 h before harvesting. Then, total RNA was isolated using TRIzol reagent (Gibco-BRL, Grand Island, NY) and cDNA was synthesized from total RNA using Revert Aid First Strand cDNA Synthesis Kit (Fermentas Inc., Glen Burnie, MD). Bax and bcl-2 mRNA expression was measured by quantitative real-time PCR using the ABI PRISM 7500 Sequence Detector System (Applied Biosystems, Carlsbad, CA). β-Actin was used as an internal control to normalize bax and bcl-2 mRNA levels. The following primer sequences were used: bax (forward 5′-GCGTCCACCCAAGAAGCTGAG-3′; reverse 5′-ACCACCCTGGTCTTGGATCC-3′), bcl-2 (forward 5′-CATGTGTGTGGAGAGCGTCAA-3′; reverse 5′-GCCGGTTCAGGTACTCAGTCA-3′) and β-actin (forward 5′-GGCATCCTCACCCTGAAGT-3′; reverse 5′-GGGATAGCACAGCCTGGAT-3′).
Western-blot assay
After treating the CNE2 cells with formononetin for the bax, bcl-2, p-ERK1/2 and t-ERK1/2 assays, the cells were harvested and total cell extracts were solubilized in ice-cold lysis buffer. The supernatant was collected by centrifugation at 12 000 rpm for 15 min at 4 °C and stored at −20 °C. The protein samples were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), after which they were transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA). The membranes were blocked with 5% non-fat milk for 2 h, and then incubated with primary antibodies of bax (1:200, Santa Cruz Biotechnology, Santa Cruz, CA), bcl-2 (1:200, Santa Cruz Biotechnology, Santa Cruz, CA), p-ERK1/2 (1:400, Abcam, Cambridge, UK), t-ERK1/2 (1:500, Abcam) and β-actin (1:1500, Jinqiao Zhongshan, Shanghai, China) at 4 °C with slow shaking. After 12 h, the membranes were prepared in the corresponding secondary antibody for 2 h, washed three times with TBST (tris-buffered solution, pH 7.6, 0.05% Tween 20), and the signals of binding activity were visualized with an electrochemiluminescence (ECL) reagent kit (Jinqiao Zhongshan, Shanghai, China). The band intensity was normalized with β-actin.
Statistical analyses
The results data are presented as mean ± standard error (SE). Student’s t-test was performed using SPSS 17.0 (SPSS Inc., Chicago, IL) to compare the different treatments. A p value < 0.05 was considered statistically significant.
Results
Effect of low formononetin concentrations on CNE2 cell proliferation
The MTT assay showed that in vitro formononetin stimulated CNE2 cell proliferation (). Compared with the control (0 μM), low concentrations of formononetin (0.05, 0.1, 0.2, 0.5, 1, 2 and 5 μM) increased CNE2 cell numbers. At formononetin concentrations below 0.2 μM, a dose-dependent increase was detected but not at higher concentrations.
Effect of low formononetin concentrations on CNE2 cell apoptosis
The number of late apoptotic cells of the control group was 6.7% (). In contrast, a lower percentage of late apoptotic cells were detected in the 0.1 μM group (6.2%), 0.3 μM group (3.8%) and 1 μM group (5.1%).
Figure 2. Effects of formononetin on the late apoptosis of CNE2 cells. 0.3 μM formononetin significantly inhibited the late apoptosis of CNE2 cells. (A) Control group, (B) 0.1 μM formononetin group, (C) 0.3 μM formononetin group, (D) 1 μM formononetin group and (E) rate of late apoptosis in CNE2 cells. *p < 0.05 versus control; n = 3.
Effect of low formononetin concentrations on bax and bcl-2 mRNA levels
The RT-PCR results showed that 0.1 and 0.2 μM formononetin significantly increased bcl-2 mRNA levels, while 0.2 and 1 μM formononetin significantly decreased bax mRNA levels ().
Effect of low formononetin concentrations on bax, bcl-2, p-ERK1/2 and t-ERK1/2 protein expression
Formononetin (0.1 and 0.3 μM) induced bcl-2 protein expression (), but had no significant effect on bax protein expression (results not shown). Formononetin of 0.3 μM noticeably induces p-ERK1/2 protein expression (), while t-ERK1/2 protein expression was not affected.
Figure 4. Effect of formononetin on the protein expression of bcl-2 and p-ERK1/2 in CNE2 cells. (A) Formononetin increased the expression levels of bcl-2 protein, β-actin was used as the loading control. *p < 0.05 versus control; n = 3. (B) Formononetin elevated the expression levels of p-ERK1/2 protein, t-ERK1/2 was used as the loading control. *p < 0.05 versus control; n = 4.
Discussion
Here, we confirmed that low concentrations of formononetin (up to 0.3 μM) promote CNE2 cell proliferation and inhibit CNE2 cell apoptosis. We also reported the possible molecular mechanism involved in this process through evaluating bcl-2 and p-ERK1/2 expression.
ERK belongs to a family of mitogen-activated protein kinases (MAPKs). The MAPK superfamily is composed of several signaling pathways that are involved in a variety of cellular processes, such as cell proliferation, differentiation and survival (Tulalamba & Janvilisri Citation2012; Chen et al. Citation2014). MAPKs include extracellular signal-regulated kinases (ERKs), c-jun NH2-terminal kinases and p38 MAPKs. Within the ERK group, eight isoforms are present (i.e., ERK1,2, 3, 4, 5, 6, 7 and 8) (Bogoyevitch & Court Citation2004). Out of these isoforms, ERK1/2 (also called the P44/42-MAPK) has been extensively studied. ERK activation is believed to be important for promoting cell-cycle progression and proliferation signals (Guo et al. Citation2014), because cell growth is inhibited when ERK1/2 pathways are negatively regulated (Peng et al. Citation2007; Fang et al. Citation2015). The ERK pathway may also be linked to apoptosis (Chen et al. Citation2011), with this view being supported by our results; whereby, p-ERK expression rose after CNE2 cell treatment with formononetin. Thus, low concentrations of formononetin may significantly promote CNE2 cell proliferation through p-ERK upregulation. This process subsequently modulates the activity of downstream targets, including some members of the bcl-2 protein family (Wang et al. Citation2015).
The bcl-2 family proteins are important for regulating cellular apoptosis (Huang et al. Citation2014). More members from the family are being found and isolated. These proteins are usually divided into three groups: (i) anti-apoptotic members, such as bcl-2 and bcl-xL; (ii) pro-apoptotic members, such as bax and bak; and (iii) BH3-only proteins. Bcl-2 prevents many forms of apoptotic cell death and some forms of necrotic cell death (Tsujimoto & Shimizu Citation2000). Two pathways activate the apoptotic cell death program: (i) the mitochondria-mediated pathway and (ii) the ligand-mediated pathway (Chen et al. Citation2011). The bcl-2 protein family changes the permeability of the outer mitochondrial membrane; thus, influencing the mitochondrial pathway (Pan et al. Citation2014).
The bcl-2 protein inhibits apoptotic cell death. The bcl-2 gene prolongs the length of a cell’s life-span and has therapeutic potential for heart disease (Gustafsson & Gottlieb Citation2007). In our study, we demonstrated that low concentrations of formononetin significantly upregulate both the mRNA and protein levels of bcl-2, while decreasing the percentage of late apoptosis. Thus, formononetin inhibits apoptosis of CNE2 cells by activating antiapoptotic bcl-2, which assists with the effect of calycosin on MCF-7 (Chen et al. Citation2011). The overexpression of bcl-2 may prevent the activation of bax (Gustafsson & Gottlieb Citation2007). Consequently, bax gene expression was decreased. However, the underlying mechanisms are associated a decline in the bax/bcl-2 ratio, which regulates whether cells enter the apoptotic program (Pan et al. Citation2014).
A previous study observed that phenol red noticeably stimulated oestrogen receptor-positive cell proliferation, due to its structural resemblance to certain non-steroidal estrogens (Berthois et al. Citation1986). Therefore, to avoid this issue in the current study, we cultured CNE2 cells in phenol red-free RPMI for at least 4 d before initiating the experiment.
In summary, we found that low concentrations of formononetin stimulate CNE2 cell proliferation. Our study is the first to demonstrate that red clover plants may be contribute to the occurrence and development of NPC, which remind clinic professionals should give special care for nasopharyngitis or NPC patients when red clover plants were used.
Funding information
This work was supported by the National Natural Science Foundation of China (No. 81260343), the National Natural Science Foundation of Guangxi (No. 2013GXNSFAA019122) and the higher Educational Science and Technology Program of Guangxi Province, China (No. KY2015YB224).
Disclosure statement
The authors report that they have no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. 1986. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci USA. 83:2496–2500.
- Bogoyevitch MA, Court NW. 2004. Counting on mitogen-activated proteinkinases-ERKs 3, 4, 5, 6, 7 and 8. Cell Signal. 16:1345–1354.
- Chen J, Hou R, Zhang X, Ye Y, Wang Y, Tian J. 2014. Calycosin suppresses breast cancer cell growth via ERβ-dependent regulation of IGF-1R, p38 MAPK and PI3K/Akt pathways. PLoS One. 9:e91245.
- Chen J, Liu L, Hou R, Shao Z, Wu Y, Chen X, Zhou L. 2011. Calycosin promotes proliferation of estrogen receptor-positive cells via estrogen receptors and ERK1/2 activation in vitro and in vivo. Cancer Lett. 308:144–151.
- Chen J, Xiong WB, Xiong Y, Wu YY, Chen XJ, Shao ZJ, Liu LT, Kuang WJ, Tan XS, Zhou LM. 2011. Calycosin stimulates proliferation of estrogen receptor-positive human breast cancer cells through downregulation of Bax gene expression and upregulation of Bcl-2 gene expression at low concentrations. J Parenter Enteral Nutr. 35:763–769.
- Chen J, Zhao X, Ye Y, Wang Y, Tian J. 2013. Estrogen receptor beta-mediated proliferative inhibition and apoptosis in human breast cancer by calycosin and formononetin. Cell Physiol Biochem. 32:1790–1797.
- Fang CY, Wu CC, Hsu HY, Chuang HY, Huang SY, Tsai CH, Chang Y, Tsao GS, Chen CL, Chen JY. 2015. EGCG inhibits proliferation, invasiveness and tumor growth by up-regulation of adhesion molecules, suppression of gelatinases activity, and induction of apoptosis in nasopharyngeal carcinoma cells. Int J Mol Sci. 16:2530–2558.
- Guo J, Zhou X, Chen Y, Bai M, Yang X, Zhao K, Hao W, Wei W, Zhang Y. 2014. mGluR3 promotes proliferation of human embryonic cortical neural progenitor cells by activating ERK1/2 and JNK2 signaling pathway in vitro. Cell Mol Biol (Noisy-le-Grand). 60:42–49.
- Gustafsson AB, Gottlieb RA. 2007. Bcl-2 family members and apoptosis, taken to heart. Am J Physiol Cell Physiol. 292:C45–C51.
- Huang C, Wu M, Tang Y, Li X, Ouyang J, Xiao L, Li D, Li G. 2009. NAG7 promotes human nasopharyngeal carcinoma invasion through inhibition of estrogen receptor alpha and up-regulation of JNK2/AP-1/MMP1 pathways. J Cell Physiol. 221:394–401.
- Huang WJ, Bi LY, Li ZZ, Zhang X, Ye Y. 2014. Formononetin induces the mitochondrial apoptosis pathway in prostate cancer cells via downregulation of the IGF-1/IGF-1R signaling pathway. Pharm Biol. 52:466–470.
- Jia WC, Liu G, Zhang CD, Zhang SP. 2014. Formononetin attenuates hydrogen peroxide (H2O2)-induced apoptosis and NF-κB activation in RGC-5 cells. Eur Rev Med Pharmacol Sci. 18:2191–2197.
- Liu Y, He J, Chen X, Li J, Shen M, Yu W, Yang Y, Xiao Z. 2014. The proapoptotic effect of formononetin in human osteosarcoma cells: involvement of inactivation of ERK and Akt pathways. Cell Physiol Biochem. 34:637–645.
- Mo L, Kuang G, Luo Y, Yang R. 2006. Relationship between the expression of estrogen and progestogen receptors in distant metastasis of nasopharyngeal carcinoma. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 20:494–495.
- Mu H, Bai YH, Wang ST, Zhu ZM, Zhang YW. 2009. Research on antioxidant effects and estrogenic effect of formononetin from Trifolium pratense (red clover). Phytomedicine. 16:314–319.
- Pan LL, Wang AY, Huang YQ, Luo Y, Ling M. 2014. Mangiferin induces apoptosis by regulating Bcl-2 and Bax expression in the CNE2 nasopharyngeal carcinoma cell line. Asian Pac J Cancer Prev. 15:7065–7068.
- Peng C, Liu HY, Zhou M, Zhang LM, Li XL, Shen SR, Li GY. 2007. BRD7 suppresses the growth of Nasopharyngeal carcinoma cells (HNE1) through negatively regulating beta-catenin and ERK pathways. Mol Cell Biochem. 303:141–149.
- Poluzzi E, Piccinni C, Raschi E, Rampa A, Recanatini M, De Ponti F. 2014. Phytoestrogens in postmenopause: the state of the art from a chemical, pharmacological and regulatory perspective. Curr Med Chem. 21:417–436.
- Razak AR, Siu LL, Liu FF, Ito E, O’Sullivan B, Chan K. 2010. Nasopharyngeal carcinoma: the next challenges. Eur J Cancer 46:1967–1978.
- Tsujimoto Y, Shimizu S. 2000. Bcl-2 family: life-or-death switch. FEBS Lett. 466:6–10.
- Tulalamba W, Janvilisri T. 2012. Nasopharyngeal carcinoma signaling pathway: an update on molecular biomarkers. Int J Cell Biol. 2012:594681.
- Wang M, Lu X, Dong X, Hao F, Liu Z, Ni G, Chen D. 2015. pERK1/2 silencing sensitizes pancreatic cancer BXPC-3 cell to gemcitabine-induced apoptosis via regulating Bax and Bcl-2 expression. World J Surg Oncol. 13:66.
- Xie M, Yi X, Wang R, Wang L, He G, Zhu M, Qi C, Liu Y, Ye Y, Tan S, et al. 2014. 14-Thienyl methylene matrine (YYJ18), the derivative from matrine, induces apoptosis of human nasopharyngeal carcinoma cells by targeting MAPK and PI3K/Akt pathways in vitro. Cell Physiol Biochem. 33:1475–1483.
- Xu T, Tang J, Gu M, Liu L, Wei W, Yang H. 2013. Recurrent nasopharyngeal carcinoma: a clinical dilemma and challenge. Curr Oncol. 20:e406–e419.
- Xue WQ, Qin HD, Ruan HL, Shugart YY, Jia WH. 2013. Quantitative association of tobacco smoking with the risk of nasopharyngeal carcinoma: a comprehensive meta-analysis of studies conducted between 1979 and 2011. Am J Epidemiol. 178:325–338.
- Zhang X, Bi L, Ye Y, Chen J. 2014. Formononetin induces apoptosis in PC-3 prostate cancer cells through enhancing the Bax/Bcl-2 ratios and regulating the p38/Akt pathway. Nutr Cancer. 66:656–661.