Abstract
Context Chebulae Fructus is used as an herbal remedy for diarrhoea in traditional Chinese medicine. However, there is no scientific evidence to support its antidiarrhoeal activity.
Objective This study evaluates the antidiarrhoeal properties of Chebulae Fructus aqueous extract (CFAE) and determines the active fraction.
Materials and methods The antidiarrhoeal effect of CFAE (200–800 mg/kg) was investigated by determining the wet dropping, intestinal transit in BALB/c mice and enteropooling in Wister rats. The protective effects of the CFAE on the intestinal and liver were tested by histopathological analyses. The antidiarrhoeal fraction was determined by castor oil-induced diarrhoea and its main constituents were identified by HPLC-ESI-MS.
Results The extract at doses of 200, 400 and 800 mg/kg reduced the diarrhoea by 9.1, 40.0 and 58.2% and inhibited intestinal transit by 18.3, 24.1 and 35.7%, respectively. Additionally, the CFAE (200, 400 and 800 mg/kg) decreased the volume of enteropooling by 47.1, 58.8 and 64.7%, respectively. Mice treated with castor oil presented morphological alterations in the small intestine and the liver. However, the lesions of mice treated with CFAE were alleviated. Moreover, the ethyl acetate fraction was the active fraction of CFAE, the fraction (41.7, 83.4 and 166.8 mg/kg) reduced the diarrhoea by 9.1, 38.2 and 54.5%, respectively. The major components of the ethyl acetate fraction were tannins, including gallic acid, 3, 4, 6-tri-O-galloyl-β-d-Glc, corilagin and ellagic acid according to the HPLC-ESI-MS analysis.
Conclusion The CFAE possessed antidiarrhoeal property and the ethyl acetate fraction was its main active fraction.
Introduction
Diarrhoea is the passage of abnormal liquid or unformed stool at an increased frequency. The disease may be caused by a wide array of agents such as infectious agents, some medications, plant and animal toxins, gastro-intestinal disorders and substances that increase gastrointestinal tract secretions (Shafi et al. Citation2014; Tadesse et al. Citation2014). Diarrhoea is a leading cause for morbidity and mortality in developing countries and is responsible for the death of millions of people each year, although there are improvements in public health and economic well being (Shafi et al. Citation2014).
The diarrhoea disease control program (DDC) has been launched by the World Health Organization (WHO); however, diarrhoea is still a big public health challenge in developing countries (Wansi et al. Citation2014). Around 2.5 million children die each year worldwide and 80% of which is reported in developing countries. Especially in crowded living conditions coupled with poor hygiene and malnutrition, diarrhoea disease is more common (Li et al. Citation2015).
Although currently pharmaceutical agents are available for the treatment and management of diarrhoea, drug resistance is also taken seriously (Mehmood et al. Citation2011). Thus, it is necessary to search for effective natural drugs that can be used as alternatives to commonly used antidiarrhoeal drugs. Additionally, the WHO has introduced a program for diarrhoea control which involves the use of traditional herbal medicines. There is growing interest in using natural compounds, such as extracts of spices and herbs, for antidiarrhoeal agents. In some cases, these compounds may be more specific and less toxic than those obtained by synthesis. Numerous plants from all continents have traditionally been used in the treatment of diarrhoeal disease (Xu et al. Citation2013; Agbor et al. Citation2014).
Chebulae Fructus (Hezi) is an important traditional Chinese medicine prepared from the fruits of Terminalia chebula Retz. (Combretaceae) and is developed for relieving diarrhoea, cough and sore throat (Committee Citation2010). In Synopsis of the Golden Chamber, Chebulae Fructus was used to cure dysentery and diarrhoea (Nanjing University of Traditional Chinese Medicine 2006). Chebulae Fructus, commonly known as black Myroblans (Saleem et al. Citation2002), has been traditionally used to produce medicines in the treatment of asthma, sore throats, vomiting, hiccoughing, diarrhoea, bleeding piles, gout and heart and bladder diseases (Rao & Nammi Citation2006). Currently, it is also reported that Chebulae Fructus possessed other various pleiotropic effects such as antioxidant, antidiabetic, renoprotective, hepatoprotective, immunomodulator and prokinetic effect (Chang & Lin Citation2012; Huang et al. Citation2012; Sarkar et al. Citation2012; Sasidharan et al. Citation2012). Additionally, a number of chemical constituents have been isolated from the plant that include chebulin, ellagic acid, 2,4-chebulyl-d-glucopyranose, arjungenin, chebulinic acid, gallic acid, terflavin A, terchebin, luteolin and tannic acid (Pfundstein et al. Citation2010; Pellati et al. Citation2013; Singh & Kumar Citation2013). However, no systematic research has been carried out to examine the antidiarrhoeal activity of Chebulae Fructus extract so far. Especially, its antidiarrhoeal fragment and main constituents have not been reported.
The current study thus was carried out to evaluate the antidiarrhoeal effect of aqueous extract of Chebulae Fructus using various validated models and to determine if the traditional medicinal use has a scientifically justified basis. Moreover, the active fragment was further determined and its main constituents were identified by HPLC-ESI-MS.
Materials and methods
Plant materials and chemicals
The Chebulae Fructus was purchased from a local TCM apothecary in Harbin, China. It was identified and authenticated by Yan-bing Li, Technical Specialist (Heilongjiang University of Chinese Medicine). The voucher specimen (voucher no. 1009011ch) has been deposited in the College of Veterinary Medicine, Northeast Agricultural University.
All solvents used for HPLC analysis were of HPLC grade; methanol for HPLC was purchased from Merck (Darmstadt, Germany). Formic acid (85% v/v) was provided by Carlo Erba (Milan, Italy). Water was purified by a Milli-Qplus system from Millipore (Milford, MA). PTFE membrane filter (0.45 mm) was purchased from Waters Co. (Milford, MA). For the qualitative analysis, the following standards were used: gallic acid, 3,4,6-3-O-galloyl-β-d-Glc, corilagin and ellagic acid (purity 98%).
Extraction and fractionation of the Chebulae Fructus
The air-dried fruits were pulverized using a mechanical grinder and passed through 40-mesh sieve. To obtain the Chebulae Fructus aqueous extract (CFAE), the powdered fruits (100 g) were boiled twice in 1000 mL of distilled water for 60 min with continuous stirring. The combined resultant solution was filtered through a filter paper. The filtrate was completely evaporated under reduced pressure at 60 °C. An equivalent of 8.3 g of powder was obtained from 100 g of dried fruits. Solutions were prepared by dissolving the resultant powder in physiologic salt solution (PSS).
The aqueous extract (6 g) was suspended in distilled water and then partitioned with two different solvents (ethyl acetate and n-butanol) to yield ethyl acetate, n-butanol and aqueous residue fractions, respectively. Each fraction was concentrated under reduced pressure at a temperature less than 60 °C. The ethyl acetate fraction (1.26 g) and the n-butanol fraction (1.81 g) were dissolved in 5% DMSO, while the aqueous fraction (2.75 g) was dissolved in distilled water.
Animals
Wister rats (200 ± 20 g weight) and BALB/c mice (20 ± 2 g weight) of either sex were obtained from the Laboratory Animal Center of Heilongjiang University of Traditional Chinese Medicine (Harbin, China). All the experiments were approved by the Institutional Animal Ethical Committee of Northeast Agricultural University (No. SRM-08).
Acute toxicity
Different doses (1000, 2000 and 4000 mg/kg) of CFAE were administered to groups of mice (10 in each group). Mortality in each group within 48 h was recorded. LD50 value was estimated by the method of Lorke (Citation1983).
Castor oil-induced diarrhoea in mice
The methods reported by Qnais et al. (Citation2007) and Mehmood et al. (Citation2011) were used in this study. Thirty mice were fasted for 18 h before beginning the experiments and randomly divided into five groups with six in each. The first group served as the control and was administered orally PSS (10 mL/kg). The second group received loperamide (10 mg/kg) orally as suspension (positive control). The last three groups received different doses of CFAE (200, 400 and 800 mg/kg). Sixty minutes later, the mice received 0.2 mL of castor oil. They were then housed singly in cages lined with white blotting paper. The number of wet droppings was counted every hour for a period of 4 h. The total of wet droppings after 4 h was counted and averaged.
Castor oil-induced intestinal transit in mice
The effect of the CFAE on intestinal propulsion in mice was tested using the charcoal method (Mbagwu & Adeyemi Citation2008). Mice were fasted for 18 h but allowed free access to water. The mice were randomly allotted into five groups of six mice per group. Treatment was carried out in different groups with PSS (10 mL/kg), CFAE (200, 400 and 800 mg/kg) and loperamide (10 mg/kg). Thirty minutes later, each mouse was given 0.2 mL of charcoal meal (5% deactivated charcoal in 0.5% CMC-Na). After 30 min, each mouse was sacrificed and the small intestine was rapidly removed for inspection. The distance travelled by the charcoal meal from the pylorus to the caecum was measured and expressed as the percent of the total length.
Castor oil-induced enteropooling in rats
Intraluminal fluid accumulation was determined by the method of Robert et al. (Citation1976). Fasted rats were divided into five groups of six animals each. Treatment was carried out in different groups with PSS (10 mL/kg, p.o.), CFAE (200, 400 and 800 mg/kg) and loperamide (10 mg/kg). The above treatments were given 1 h before administration of 1 mL of castor oil orally. Two hours later, the rats were sacrificed, and the small intestine was ligated both at the pyloric sphincter and at the ileocecal junctions and dissected out. The small intestine was weighed. The intestinal contents were collected by milking into a graduated tube and the volume was measured. The intestines were reweighed and the differences between full and empty intestines were calculated.
Histopathological analysis
Eighteen mice were separated in three groups of six animals. Mice in groups A, B and C were carried out with PSS (10 mL/kg), ethyl acetate fraction (400 mg/kg) and PSS (10 mL/kg), respectively. Thirty minutes later, each mouse of the model group (A) and treatment group (B) was given 0.2 mL castor oil, and the mouse of the control group (C) was given 0.2 mL PSS. Four hours later, all animals were submitted to laparotomy for the removal of the small intestine and liver. These tissues were fixed in 10% neutral buffered formalin. The fixed samples were dehydrated in an ascending series of ethanol, cleared in methyl benzoate and embedded in paraffin wax. Sections of 4 μm thick were obtained using a microtome and stained with haematoxylin and eosin. The photomicrographs were taken by digital camera (AxioCam MRc 5, Carl Zeiss, Jena, Germany) driven by software Axio Vision 4.6.3. (Carl Zeiss, Jena, Germany) coupled with an optical microscope (Carl Zeiss, Jena, Germany). Tissue lesion in the intestine was classified by the occurrence of villi alterations and ulceration or inflammatory process. Tissue lesion of the liver was determined by the presence of congestion, hydropic and fatty degeneration, inflammatory infiltrate and necrosis.
Screening and analysis of antidiarrhoeal fraction
Screening of antidiarrhoeal fraction
Twenty-four mice were fasted for 18 h before beginning the experiments and randomly divided into four groups with six in each. The first group served as the control and received PSS (10 mL/kg). According to the yield of the fractions, the dose of different fractions were adapt to the CFAE equivalent (200, 400 and 800 mg/kg). So other three groups received ethyl acetate fraction (41.7, 83.4 and 166.8 mg/kg), n-butanol (60.3, 120.6 and 241.2 mg/kg), and aqueous residue fractions (91.7, 183.4 and 366.8 mg/kg), respectively. Sixty minutes later, the mice received 0.2 mL of castor oil. The total of wet droppings after 4 h was counted and averaged.
Analysis of the main compounds in ethyl acetate fraction
HPLC–ESI-MS was conducted on an Agilent 1100 HPLC, coupled to an Agilent single-quadrupole, mass-selective detector (HP 1101; Agilent Technologies, Waldbronn, Germany). Chromatographic separation of the ethyl acetate fraction was conducted using a reversed-phase Inertsil ODS-SP column (250 mm× 4.6 mm, 5 μm, GL Sciences Inc., Tokyo, Japan). The mobile phase consisted of 0.1% formic acid in water (solvent A) and methanol (solvent B) with a gradient program of 5–100% (B) in 0–60 min. The main compounds were detected by their UV absorbance (A) at 254 nm at room temperature. Mass spectra in the negative and positive ionisation mode were generated under the following conditions: drying gas temperature, 300 °C; drying gas flow, nitrogen at 10 L/min; nebuliser pressure, 30 psi; sheath gas temperature, 250 °C; sheath gas flow, nitrogen at 7 L/min; fragmenter voltage, 100 V; capillary voltage, 2500 V; mass range, 100–1500 D. Data acquisitions and qualitative analyses were controlled via the Agilent MassHunter Workstation software (Agilent Technologies, Waldbronn, Germany).
Statistical analysis
The data were expressed as mean ± standard deviation (SD). Statistical analysis was assessed using one-way analysis of variance (ANOVA) followed by the Newman–Keuls test. Differences were considered statistically significant when p < 0.05.
Results
Acute toxicity study
The CFAE did not produce any mortality up to a dose level of 4000 mg/kg. Hence, 1/20th (200 mg/kg), 1/10th (400 mg/kg) and 1/5th (800 mg/kg) of this dose were used for further investigations.
Inhibition of castor oil-induced diarrhoea
Sixty minutes after administration of castor oil, diarrhoea was apparent in all the animals of the control group for the next 4 h. When the 400 or 800 mg/kg of CFAE was administered orally 1 h before the administration of castor oil, the time in the excretion of the first diarrhoeal faeces was significantly increased compared with the control group (p < 0.05). It also significantly reduced the number of wet faeces excreted in the 4 h after administration of castor oil (p < 0.05). Treatment with the reference compound, loperamide (10 mg/kg), also exhibited significant antidiarrhoeal activity in castor oil induced diarrhoea (p < 0.05) ().
Table 1. Effect of the CFAE on castor oil-induced diarrhoea in mice.
Castor oil-induced transit in mice
In the control group of mice, the charcoal meal transited 78.1% of the total length of the small intestine. The CFAE (200 and 800 mg/kg) significantly reduced the gastrointestinal distance travelled by the charcoal meal in mice, compared with the control (p < 0.05) (). Interestingly, there is no significant inhibited role in the extract (400 mg/kg) group. Comprehensive analysis of the castor oil-induced diarrhoea and transit in mice, the inhibition of intestinal motility should not be the main mechanism of antidiarrhoeal activity of the CFAE. Loperamide (10 mg/kg) caused an intestinal inhibition of 69.9% which was significantly greater than that produced by the highest dose of CFAE (800 mg/kg, 50.2%).
Table 2. Effect of the CFAE on gastrointestinal motility in mice.
Effect on castor oil-induced enteropooling
Pretreatment with CFAE (200, 400 and 800 mg/kg) dose dependently inhibited (47.1–64.7%) castor oil-induced fluid accumulation when compared with control (p < 0.05) (). The standard drug, loperamide (10 mg/kg), also inhibited the intestinal fluid accumulation (p < 0.05) and this inhibition (76.5%) was greater than that of the highest dose of the extract (800 mg/kg, 64.7%). The secretions obtained in animals pretreated with CFAE and loperamide were more viscous than those of the control.
Table 3. Effect of the CFAE on castor oil-induced enteropooling in rats.
Histopathological analysis
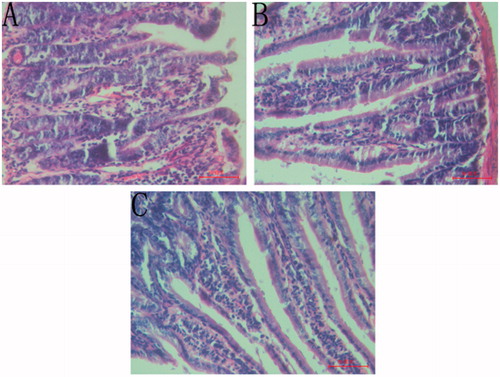
The histopathological analysis of the animals, after 4 h of administration of castor oil, showed significant alterations in intestine and liver tissue. In the model group, the histological examination showed oedema of the villus, intense capillary congestion of the lamina propria, polymorphonuclear infiltration in enteric cavity and shedding of the epithelial layer partly (). However, animals that received CFAE showed milder changes, destruction in the morphology of the villi and regularly arranged mucosal epithelial cells (). Additionally, the number of lymphocytes also obviously raised in the lamina propria. It could be shown that animals that received PSS did not show any modification ().
Figure 1. Small intestine sections of mice treated with PSS + castor oil (A), CFAE + castor oil (B) and PSS + PSS (C).
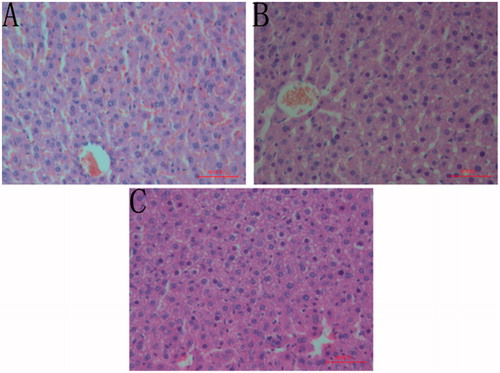
Histopathological analysis of the liver, after administration of castor oil, revealed that the sample caused congestion in the centrolobular veins, expansion in sinusoid capillary and increase in the numbers of red blood cells. Moreover, the arrangement of hepatocyte was more irregular and the boundary of the most hepatocyte was not clear. It could also be seen that variable sizes of cavity in the cytoplasm and some hepatocyte completely dissolved (). However, animals that received CFAE showed milder congestion in the centrolobular veins, cavity and nucleus concentration in small part of the cytoplasm and few hepatocyte dissolved (). It could be shown that animals that received PSS did not show noticeable modification ().
Screening of antidiarrhoeal fraction
Sixty minutes after administration of castor oil, diarrhoea was produced in all the animals of the different groups for the next 4 h. From , it can be seen that the ethyl acetate fraction was the mainly active fraction of the CFAE. It significantly (p < 0.05) delayed in onset of diarrhoea and decreased in the frequency of watery stooling. However, the n-butanol fraction and aqueous residue fraction did not express potent antidiarrhoeal activity.
Table 4. Effect of the CFAE on castor oil-induced diarrhoea in mice.
Identification of constituents in the active fraction of the CFAE by HPLC-ESI-MS
In this study, four constituents in the ethyl acetate fraction were identified by their UV and MS spectral data (). These compounds are mainly hydrolysable tannins, including gallic acid, 3,4,6-3-O-galloyl-β-d-Glc, corilagin and ellagic acid. Data concerning identification of the peaks were shown in , where the retention time, UV–vis absorptions and electrospray ionisation mass spectrometry (in negative ion and positive model) of all the compounds detected are reported along with the identification mode. The four compounds were further identified according to their UV and retention times compared with authentic reference compounds ().
Figure 3. The MS spectra of four representative compounds in the ethyl acetate fraction of CFAE: (1A and 1B) gallic acid; (2A and 2B) 3,4,6-tri-O-galloyl-β-d-Glc; (3A and 3B) corilagin; (4A and 4B) ellagic acid.
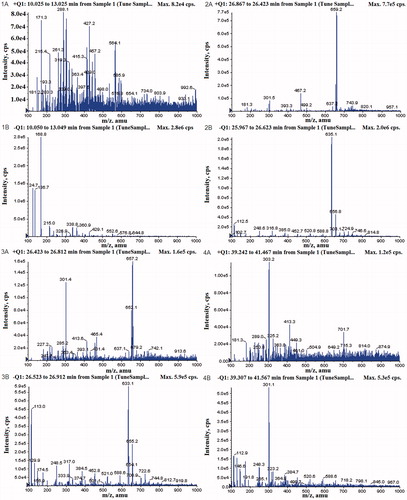
Figure 4. Representative HPLC chromatograms of (A) the ethyl acetate fraction of the CFAE; (B) mixed standard solutions. (1) Gallic acid; (2) 3,4,6-tri-O-galloyl-β-d-Glc; (3) corilagin; (5) ellagic acid. The HPLC analytical conditions were described in the Analysis of the main compounds in ethyl acetate fraction section.
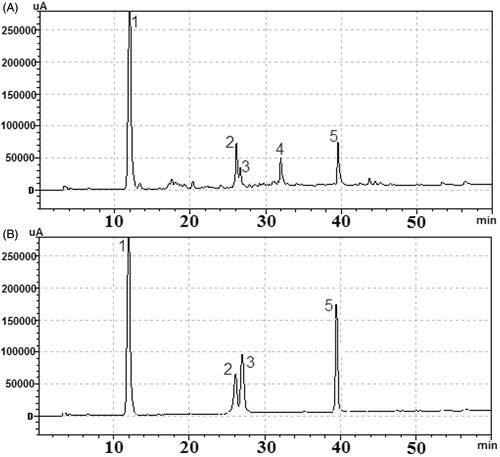
Table 5. HPLC-ESI-MS fragmentation (negative and positive ion modes) and UV–vis absorption data of the compounds detected in the ethyl acetate fraction of the CFAE.
Discussion
Diarrhoea is caused by disturbed bowel function, impaired intestinal absorption, excessive intestinal secretion of water and electrolytes, and a rapid bowel transit (Mulla et al. Citation2011). Therefore, in this study, antidiarrhoeal activity of the CFAE was studied using various parameters, including onset of diarrhoeal stools, inhibition level of watery stools, intestinal motility and enteropooling.
It was shown that the CFAE (200, 400 and 800 mg/kg, p.o.) could reduce diarrhoea by inhibiting intestinal motility and intestinal fluid accumulation. The inhibitory effect justifies the use of the preparation in traditional medicine and its use as a non-specific antidiarrhoeal agent. The extract meets some of the criteria for acceptance of a drug as an antidiarrhoeal (Akah et al. Citation1999). These criteria include inhibition of the production of wet or unformed faeces in animals and inhibition of gastrointestinal propulsive action.
To determine the antidiarrhoeal fraction, a non-specific diarrhoea model should be selected. It is well known that the active component (ricinoleic acid) of castor oil irritates the small intestine leading to increased secretion of fluid and electrolytes and speed intestinal transit (Qnais et al. Citation2007). Hence, for initial identification of active fractions, castor oil-induced diarrhoea model was used. In our present study, we observed that the ethyl acetate fraction produced good antidiarrhoeal activity, whereas other fractions could not produce the antidiarrhoeal effect. This may be due to the main active constituents that have been enriched in the ethyl acetate fraction.
The diarrhoeal effect of castor oil has been attributed to several possible mechanisms. On the one hand, castor oil liberates ricinoleic acid, which results in irritation and inflammation of the intestinal mucosa. This could produce prostaglandins and other autacoids, which stimulate motility and secretion, two mainly suspected factors that may cause diarrhoea. The effect of the aqueous extract was partly similar to loperamide, which is an efficacious antidiarrhoeal drug. The antidiarrhoeal activity of loperamide was due to antimotility and antisecretory properties (Gong et al. Citation2012). Therefore, the extract may mediate its effects through antisecretory activity, which was supported by observation that enteropooling was significantly reduced in treated animals compared with control. On the other hand, castor oil and its active principle reduce active Na+ and K+ absorption and decrease Na+, K+ -ATPase activity in the small intestine and colon (Ezeja et al. Citation2012). This will lead to decreased absorption and cause diarrhoea. Previous reports have demonstrated that gallic acid and chebulinic acid have anti-secretory activity by inhibiting H+ K+-ATPase activity (Chen et al. Citation2006; Mishra et al. Citation2013). In this study, phytochemical analysis of the antidiarrhoeal fraction revealed that the presence of the hydrolysable tannins, including gallic acid, 3,4,6-tri-O-galloyl-β-d-Glc, corilagin and ellagic acid. These constituents may mediate the antidiarrhoeal property of the CFAE.
Conclusion
The results indicate that the extract of Chebulae Fructus possesses significant antidiarrhoeal activity due to inhibiting the gastrointestinal propulsion and fluid secretion. The ethyl acetate fraction is the most active fraction of Chebulae Fructus that decreases the frequency of stooling. The major components of the active fraction were identified by HPLC-ESI-MS, which mainly contains gallic acid, 3,4,6-tri-O-galloyl-β-d-Glc, corilagin and ellagic acid. These findings provide a scientific support for the use of this herb as a non-specific antidiarrhoeal agent in the treatment of diarrhoeal disease.
Funding information
This research was supported by National Natural Science Foundation of China (Grant no. 31201944), and Science and Technology Project Founded by the Education Department of Heilongjiang Province (Grant no. 12531047).
Disclosure statement
The authors report that they have no conflicts of interest.
References
- Agbor GA, Longo F, Makong EA, Tarkang PA. 2014. Evaluation of the antidiarrheal and antioxidant properties of Justicia hypocrateriformis. Pharm Biol. 52:1128–1133.
- Akah PA, Aguwa CN, Agu RU. 1999. Studies on the antidiarrhoeal properties of Pentaclethra macrophylla leaf extracts. Phytother Res. 13:292–295.
- Chang CL, Lin CS. 2012. Phytochemical composition, antioxidant activity, and neuroprotective effect of Terminalia chebula Retzius extracts. Evid. Based Complement Alternat Med. [Online]. [cited 2011 May 10]. Available from: http://dx.doi.org/10.1155/2012/125247.
- Chen JC, Ho TY, Chang YS, Wu SL, Hsiang CY. 2006. Anti-diarrheal effect of Galla Chinensis on the Escherichia coli heat-labile enterotoxin and ganglioside interaction. J Ethnopharmacol. 103:385–391.
- Committee CP. 2010. Pharmacopoeia of the People's Republic of China, vol. 1. Beijing, China: Chemical Industry Press.
- Ezeja IM, Ezeigbo II, Madubuike KG, Udeh NE, Ukweni IA, Akomas SC, Ifenkwe DC. 2012. Antidiarrheal activity of Pterocarpus erinaceus methanol leaf extract in experimentally-induced diarrhea. Asian Pac J Trop Med. 5:147–150.
- Gong Y, Hou ZQ, Gao YX, Xue YS, Liu X, Liu GM. 2012. Optimization of extraction parameters of bioactive components from defatted marigold (Tagetes erecta L.) residue using response surface methodology. Food Bioprod Process. 90:9–16.
- Huang YN, Zhao DD, Gao B, Zhong K, Zhu RX, Zhang Y, Xie Wj, Jia LR, Gao H. 2012. Anti-hyperglycemic effect of chebulagic acid from the fruits of Terminalia chebula Retz. Int J Mol Sci. 13:6320–6333.
- Li SK, Cui DA, Wang SY, Wang H, Huang MZ, Qi ZM, Liu YM. 2015. Efficacy of an herbal granule as treatment option for neonatal Tibetan Lamb diarrhea under field conditions. Livest Sci. 172:79–84.
- Lorke D. 1983. A new approach to practical acute toxicity testing. Arch Toxicol. 54:275–287.
- Mbagwu HOC, Adeyemi OO. 2008. Anti-diarrhoeal activity of the aqueous extract of Mezoneuron benthamianum Baill (Caesalpiniaceae). J Ethnopharmacol. 116:16–20.
- Mehmood MH, Siddiqi HS, Gilani AH. 2011. The antidiarrheal and spasmolytic activities of Phyllanthus emblica are mediated through dual blockade of muscarinic receptors and Ca2+ channels. J Ethnopharmacol. 133:856–865.
- Mishra V, Agrawal M, Onasanwo SA, Madhur G, Rastpgo P, Pandey HP, Palit G, Narender T. 2013. Anti-secretory and cyto-protective effects of chebulinic acid isolated from the fruits of Terminalia chebula on gastric ulcers. Phytomedicine. 20:506–511.
- Mulla WA, Chopade AR, Bhise SB, Burade KB, Khanwelkar CC. 2011. Evaluation of antidiarrheal and in vitro antiprotozoal activities of extracts of leaves of Alocasia indica. Pharm Biol. 49:354–361.
- Pellati F, Bruni R, Righi D, Grandini A, Tognolini M, Pio PF, Poli F, Benvenuti S, Del RD, Rossi D. 2013. Metabolite profiling of polyphenols in a Terminalia chebula Retzius ayurvedic decoction and evaluation of its chemopreventive activity. J Ethnopharmacol. 147:277–285.
- Pfundstein B, El Desouky SK, Hull WE, Haubner R, Erben G, Owen RW. 2010. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): characterization, quantitation and determination of antioxidant capacities. Phytochemistry. 71:1132–1148.
- Qnais EY, Elokda AS, Abu Ghalyun YY, Abdulla FA. 2007. Antidiarrheal activity of the aqueous extract of Punica granatum. (Pomegranate) Peels. Pharm Biol. 45:715–720.
- Rao NK, Nammi S. 2006. Antidiabetic and renoprotective effects of the chloroform extract of Terminalia chebula Retz. Seeds in streptozotocin-induced diabetic rats. BMC Complem Altern Med. [Online]. [cited 2006 May 7]. Available from: http://www.biomedcentral.com/1472-6882/6/17.
- Robert A, Nezamis JE, Lancaster C, Hanchar AJ, Klepper MS. 1976. Enteropooling assay: a test for diarrhea produced by prostaglandins. Prostaglandins. 11:809–828.
- Saleem A, Husheem M, Harkonen P, Pihlaja K. 2002. Inhibition of cancer cell growth by crude extract and the phenolics of Terminalia chebula retz. fruit. J Ethnopharmacol. 81:327–336.
- Sarkar R, Hazra B, Mandal N. 2012. Reducing power and iron chelating property of Terminalia chebula (Retz.) alleviates iron induced liver toxicity in mice. BMC Complement Altern Med. [Online]. [cited 2012 Aug 12]. Available from: http://www.biomedcentral.com/1472-6882/12/144.
- Sasidharan I, Sundaresan A, Nisha VM, Kirishna MS, Raghu KG, Jayamurthy P. 2012. Inhibitory effect of Terminalia chebula Retz. fruit extracts on digestive enzyme related to diabetes and oxidative stress. J Enzyme Inhib Med Chem. 27:578–586.
- Shafi A, Farooq U, Akram K, Jaskani M, Siddique F, Tanveer A. 2014. Antidiarrheal effect of food fermented by various strains of Lactobacillus. Compr Rev Food Sci F. 13:229–239.
- Singh G, Kumar P. 2013. Extraction, gas chromatography-mass spectrometry analysis and screening of fruits of Terminalia chebula Retz. for its antimicrobial potential. Pharmacognosy Res. 5:162–168.
- Tadesse WT, Hailu AE, Gurmu AE, Gurmu AE, Mechesso AF. 2014. Experimental assessment of antidiarrheal and antisecretory activity of 80% methanolic leaf extract of Zehneria scabra in mice. BMC Complem Altern Med. [Online]. [cited 2014 Nov 27]. Available from: http://www.biomedcentral.com/1472-6882/14/460.
- Wansi SL, Nguelefack-Mbuyo EP, Nchouwet ML, Miaffo D, Nyadjeu P, Wabo JP, Mbiantcha M, Nkeng-Efouet PA, Nguelefack TB, Kamanyi A. 2014. Antidiarrheal activity of aqueous extract of the stem bark of Sapium ellipticum (Euphorbiaceae). Trop J Pharm Res. 13:929–935.
- Xu QQ, Shen ZQ, Wang YB, Guo SJ, Li F, Wang YP, Zhou CF. 2013. Anti-diarrhoeal and anti-microbial activity of Flos populi (male inflorescence of Populus tomentosa Carrière) aqueous extracts. J Ethnopharmacol. 148:640–646.