Abstract
Context Alchornea floribunda Müll. Arg. (Euphorbiaceae) leaves are widely used in ethnomedicine for the management of rheumatism, arthritis and toothache.
Objective In this study, flavonoid glycosides isolated from Alchornea floribunda were screened for their effect on the intracellular expression of interferon-gamma (IFNγ) and interleukin-2 (IL-2) type-1 cytokines.
Materials and methods Chromatographic purification of the ethyl acetate fraction of the methanol leaf extract led to the isolation of seven flavonoid glycosides (1–7). Their structures were elucidated by 1D and 2D nuclear magnetic resonance and mass spectrometry. Splenocytes were treated with graded concentrations of the compounds (6.25–25 μg/mL) and incubated for 24 h. Thereafter, their effect on the expression of IFNγ and IL-2 by CD4+ and CD8+ T-lymphocytes was evaluated using intracellular cytokine staining and FACS analysis.
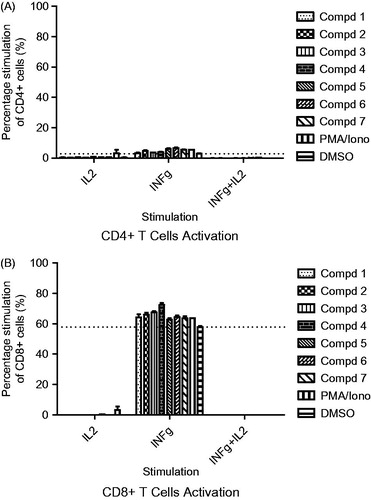
Results Compounds 1–7 (6.25–25 μg/mL) caused the up-regulation of activated CD8+ (57.85–72.45% versus 57.85% for untreated control) and, to a lesser extent, activated CD4+ (3.21–7.21% versus 2.75% for the untreated control) T-lymphocytes that were both largely interferon-gamma-releasing in treated mouse T lymphocytes relative to untreated control. FACS data analysis showed that stimulation with all the compounds increased the proportion of CD8+/IFNγ+ and CD4+/IFNγ+ T lymphocytes up to two-fold when compared with the cells in untreated control wells. Intracellular IL-2 secretion by treated T cells was not detected.
Conclusion This recorded T-lymphocyte-specific immune-modulatory property may contribute to explain in part the dynamics associated with the ethnomedicine of Alchornea floribunda, and may find relevance as a necessary cellular immune response precursor to infection-associated disease management.
Introduction
Cytokines are a large group of soluble extracellular proteins or glycoproteins that function as key intercellular regulators and mobilizers. They are crucial to innate and adaptive inflammatory responses, cell growth and differentiation, cell death, angiogenesis and developmental as well as repair processes (Oppenheim Citation2001). Cytokines are secreted by virtually every nucleated cell type, and usually inducible, in response to injurious and inflammatory stimuli (Oppenheim Citation2001). Importantly, cytokines provide a link between various systems, providing molecular clues for physiological balance and homeostasis (O’Sullivan et al. Citation1998). Modulation of cytokines secretion offers novel approaches in the treatment and management of a variety of disease conditions. The role of cytokines in the onset and pathophysiology of several ailments and health disorders are well documented (Kiecolt-Glaser et al. Citation2002). As a result of the growing recognition of cytokine activities, altering cytokine expression and targeting their receptors may offer therapeutic potential. Current pharmacological strategies include cytokine antagonist, agonist, inhibition and stimulation. One important strategy in the modulation of cytokine expression may be through the use of herbal remedies. Immune-related illnesses have long been treated with herbal medicines. Herbal immunomodulators have been shown to alter the activity of immune function through the dynamic regulation of informational molecules such as cytokines (Spelman et al. Citation2006; Nworu et al. Citation2010, Citation2012). This may offer explanations to some of the wide-range effects of herbs on the immune system and other tissues (Nworu et al. Citation2010).
In this study, we evaluated the modulatory effects of some flavonoid glycosides isolated from Alchornea floribunda Müll. Arg. (Euphorbiaceae) leaves on the expression of interferon-γ and interleukin-2, both of which are type-1 cytokines. Alchornea floribunda has been widely used in ethnomedicine for the management of a variety of ailments. The leaves are used in African Traditional Medicine as remedy for arthritis, rheumatism, pile, muscle pain and other immuno-inflammatory disorders (Neuwinger Citation2000; Duke et al. Citation2002). Although previous reports on this plant (Okoye & Ebi Citation2007; Okoye & Osadebe Citation2009, Citation2010; Okoye et al. Citation2010, Citation2011; Siwe et al. Citation2014) seem to validate some of its ethnomedicinal uses, the chemical potentials and the exact mechanisms through which the active ingredients might be acting appear poorly investigated. This stimulated our interest in carrying out extensive chemical and bioactivity investigation of the polar phytoconstituents with a view to isolating possible new molecules which can be developed into novel therapeutic agents for immuno-inflammatory conditions. Our preliminary investigation revealed that the polar fraction of A. floribunda methanol leaf extract is rich in flavonoid glycosides. It is widely known that flavonoids possess a broad spectrum of biological activity (Robak & Gryglewski Citation1996; López-Lázaro Citation2009). Flavonoids modify the body's reaction to allergens, viruses and carcinogens and also have antiallergic (Kimata et al. Citation2000), anti-inflammatory (Yerra et al. Citation2005), antimicrobial (Gordana et al. Citation2007) and anticancer (Maheep et al. Citation2011) activities. Although it is unclear how flavonoids protect against cancer, it has been suggested that many of their biological actions are attributable to their anti-inflammatory and antioxidant activities (Prasad et al. Citation2010).
In the present study, we report the isolation and characterization of seven flavonoid glycosides and their effects on the expression of type-1 cytokines from activated CD4+ and CD8+ T-lymphocytes. During type-1 immune response, the pro-inflammatory cytokines interferon-γ (IFN-γ) and interleukin-2 (IL-2) are produced by activated T-lymphocytes and natural killer (NK) cells to mount an effective response against pathogens and tumour cells, while type-2 cell responses are characterized by cytokines IL-4, IL-5 and IL-13 (Barth et al. Citation2003; Romagnani Citation2006). In a cross-regulatory manner, type-2 cytokines may suppress type-1 immune response and vice versa (Fiorentino et al. Citation1991; Lucey et al. Citation1996). Generally, type 1 cytokines function predominantly against intracellular pathogens (mycobacteria and selected bacteria, viruses and fungi) and in certain granulomatous or inflammatory diseases (Lucey et al. Citation1996). Therefore, defining the possible roles of the compounds isolated from A. floribunda in this cascade was considered and undertaken in this present study.
Materials and methods
Collection of plants materials
The leaves of Alchornea floribunda were collected in December 2010 from Oba Nsukka Enugu State, Nigeria, with the help of a taxonomist, Mr. Alfred Ozioko of Bioresources Development and Conservation Program, Nsukka, Enugu State, Nigeria, who also authenticated the plant material. A voucher specimen has been deposited at the herbarium of the Department of Pharmacognosy, University of Nigeria, Nsukka, Enugu State, under the herbarium number 06/0085. The leaves were air dried for 10 d and pulverized.
Extraction, isolation and purification of active compounds
About 500 g of pulverized dried leaves of A. floribunda were extracted with 5 L of MeOH for 7 d at room temperature (25 °C) and the extract concentrated in vacuo with a rotary evaporator to obtain a dark green semisolid. The dried extract was reconstituted in 20 mL of MeOH and the dispersion made up to 200 mL with water, sonicated for about 10 min and subsequently partitioned successively against hexane (750 mL × 3), ethyl acetate (750 mL × 3) and n-butanol (500 mL × 2). All fractions were taken to dryness using a rotary evaporator. About 5 g of ethyl acetate fraction was subjected to VLC (silica gel 500 g, sintered funnel 5 L) eluting with 500 mL each of hexane:ethyl acetate (90:10, 70:30, 50:50, 30:70, 10:90 and 0:100) and dichloromethane:MeOH (90:10, 80:20, 60:40, 40:60, 20:80 and 0:100) resulting in 12 pooled fractions EF1–EF12. About 700 mg of the fraction eluted with dichloromethane:MeOH 9:1 (EF7) was subjected to Sephadex LH-20 (3 × 60 cm, internal diameter) separation using dichloromethane:MeOH 1:1 to afford 10 pooled fractions EF7A–EF7J. Fractions EF7F, EF7G and EF7H were purified by semi-preparative reverse phase HPLC to obtain compounds 1 (16.3 mg), 2 (5.0 mg), 3 (4.7 mg), 4 (10.0 mg), 5 (2.5 mg), 6 (60.0 mg) and 7 (2.0 mg).
Structural elucidation of isolated compounds
NMR spectra (1H, 13C, DEPT, HMQC and HMBC) were recorded with Bruker ARX 500 NMR or AVANCE DMX 600 NMR spectrometers (Bruker BioSpin Corporation, Billerica, MA). MS (ESI) was obtained with Finnigan LCQ Deca mass spectrometer (Thermo Electron Corporation, San Jose, CA). Analytical HPLC was carried out with a Dionex P580 HPLC system (Machinio, San Diego, CA) coupled to a photodiode array detector (UVD340S). Routine detection was at 235, 254, 280 and 354 nm. The separation column (125 × 4 mm, length × internal diameter) was prefilled with Eurospher-10 C18 (Knauer, Baden, Germany), and a linear gradient of nanopure water (adjusted to pH 2 by the addition of formic acid) and MeOH was used as an eluent. Semipreparative HPLC was performed with Merck Hitachi L-7100 (Merck/Hitachi, Darmstadt, Germany) coupled to a UV detector (L-7400). A linear gradient of HPLC grade MeOH and nanopure water was used for separation. Vacuum Liquid Chromatography (VLC) was carried out with silica gel (200–400 mesh, Merck, Darmstadt, Germany), Sephadex LH-20 (25–100 μm mesh size, Merck, Darmstadt, Germany) was used for CC while TLC was performed on silica gel 60 F254 (layer thickness 0.2 mm, E. Merck, Darmstadt, Germany) using the following solvent systems: DCM–MeOH (9:1 and 4:1). Compounds were detected under UV absorbance at 254 nm (fluorescence absorption) and 366 nm (fluorescence).
Mice
BALB/c mice obtained from Janvier (Le Genest-ST-Isle, France) were used in the study. The animals were maintained on standard livestock pellets (Altromin Spezialfutter GmbH & Co. KG, Lage, Germany) and were allowed unrestricted access to drinking water. The use and care of laboratory animals in the study were in accordance with ethical guidelines as contained in the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (EEC Directive 86/609/EEC) of 1986.
Preparation of mouse spleen cells for experiments
Female BALB/c mice (6–8 weeks old, 22–28 g) were sacrificed by CO2 asphyxiation and the spleens were removed aseptically into ice-cold Hanks’ Balance Salt Solution (HBSS, Gibco, Darmstadt, Germany). Single-cell suspension was prepared by gentle dispersion of the cells and straining through 70 μM nylon strainer. Red blood cells were lysed by the addition of 3 mL of ACK lysing buffer (Lonza, Walkersville, MD) per mouse spleen for 5 min. The cells were washed and suspended in R-10, consisting of RPMI 1640 medium (Gibco, Darmstadt, Germany) supplemented with 10% heat-inactivated foetal calf serum (FCS), 50 μM 2-mercaptoethanol (Gibco, Darmstadt, Germany), 100 U/mL penicillin and 100 μg/mL streptomycin.
Intracellular cytokine staining in cultures of splenic T-lymphocytes
The effect of treatment with various test compounds on the expression of two cytokines (IFNγ and IL-2) by cultures of splenic T-lymphocytes was investigated by the intracellular cytokine staining technique (ICS). Mice splenocytes (3 × 106 cells/well) were stimulated at 37 °C in CO2 incubator for 16 h with either graded concentration of the isolated compounds (6.25 and 25 μg/mL) or with PMA/ionomycin (5/500 ng/mL) and compared with the unstimulated control wells. Each well also received monensin (2 μM), which inhibited cytokine secretion out of the cells (Jung et al. Citation1993). After stimulation, cells were washed with PBS/bovine serum albumin/azide, and Fc (II/III) receptors were blocked with a mixture of anti-CD16 and anti-CD32 antibody (BD Biosciences, San Jose, CA). Thereafter, cells were labelled with either allophycocyanin (APC)-conjugated anti-CD8 or anti-CD4 antibodies (BD Biosciences, San Jose, CA). Thereafter, cells were washed with PBS and fixed in 2% paraformaldehyde, followed by permeabilization with 0.5% saponin in PBS/bovine serum albumin/azide buffer. Cytokines expressions within the cells were detected by staining for 30 min with anti-IFNγ-PE and anti-IL-2-FITCS (BD Biosciences, San Jose, CA). Cells were analyzed in a FACSCalibur™ using CellQuestPro software (BD Biosciences, San Jose, CA) and reanalyzed with Flowjo™ (TreeStar Inc., Ashland, OR). Data sets are duplicate determinations of two independent experiments.
Statistical analyses
The data are represented as mean ± SEM and analysed by ANOVA using GraphPad Prism 5 software (Graphpad Software Inc., La Jolla, CA). Differences between test treatments and the control treatment were considered significant at p < 0.05.
Results and discussion
Herbal immunomodulators are bioactive phytoconstituents that alter the activities of the immune system through their direct actions on immune cells and informational molecules – cytokines, hormones, neurotransmitters and other peptides. Cytokine-targeted therapies have proven to be beneficial but its wide-spread application is limited by some challenging adverse effects necessitating the idea of using phytotherapy in the modulation of cytokine expression-an idea that has proven useful so far (Barak et al. Citation2002; Spelman et al. Citation2006; Burns et al. Citation2010). This study represents an effort in discovering and harnessing bioactive phyto-compounds as potential agents for the modulation or regulation of cytokine expression and activities.
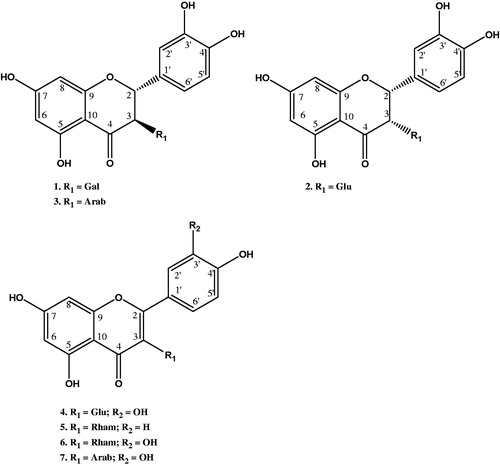
In this study, the ethyl acetate (flavonoid-rich) fraction of the methanol leaf extract of A. floribunda was subjected to vacuum liquid chromatography (VLC) on silica gel, column chromatography on Sephadex LH-20 and finally semi-preparative HPLC on reverse phase column to obtain three flavanones (1–3) and four flavonol (4–7) glycosides (). Our previous bioactivity-guided study revealed that this fraction possesses some useful anti-inflammatory activity (Okoye & Osadebe Citation2010).
Compounds 1–3 were isolated as light brown solids. The compounds showed UV maxima typical of flavanone glycosides (Markham & Mabry Citation1968; Nonaka et al. Citation1987; Sepulveda-Boza et al. Citation1993). The ESI-MS/MS of the compounds also showed a major fragment at m/z 305.0, which is diagnostic of a flavanone nucleus of the 2,3-dihydroquercetin structure. 1H and 1H–1H COSY NMR data showed characteristic aromatic spin systems of the rings A and B of quercetin (). These include the signals of an ABX system of H-2′, H-6′ and H-5′ and the signals of meta-coupled protons of H-6 and H-8. In addition, signals of two vicinal protons were observed and assigned to H-2 and H-3. The sugar moieties were identified based on the characteristic NMR signals. The compounds were thus identified based on detailed analysis of their spectral data and comparison with literature as 2R,3R-dihydroquercetin-3-O-β-d-galactopyranoside (1) (Markham & Mabry Citation1968; Düber et al. Citation1997), 2R,3S-dihydroquercetin-3-O-β-d-glucopyranoside (isoglucodistylin) (2) (Sakurai et al. Citation1982; Düber et al. Citation1997) and 2R,3R-dihydroquercetin-3-O-α-l-arabinopyranoside (3) (Chosson et al. Citation1998) (). This is the first report of the isolation of these compounds from this plant species.
Table 1. NMR spectroscopic data of compounds 1–3.
Compounds 4–7 were isolated as yellow solids and the structures elucidated on the basis of their 1D and 2D NMR and mass spectral analyses. Compounds 4, 6 and 7 exhibited the characteristic spin systems of the quercetin nucleus which include the ABX system in ring B and the two meta-coupled protons of ring A as shown in . Compound 5, however, exhibited the characteristic AA′BB′ COSY spin system of ring B and the two meta-coupled protons of ring A typical of the kaempferol nucleus. The glycosides were identified based on the characteristic NMR signals. The analysis of the NMR data and comparison with those reported in the literature led to the structural assignments of the compounds as quercetin-3-O-β-d-glucopyranoside (4) (Gudej Citation2003; Kim et al. Citation2006), kaempferol-3-O-α-l-rhamnopyranoside (5) (de Almeida et al. Citation1998), quercetin-3-O-α-l-rhamnopyranoside (quercetrin) (6) and quercetin-3-O-β-d-arabinopyranoside (7) (Gudej Citation2003; Kim 2006) (). This is also the first report of the isolation of these compounds from this plant species.
Table 2. NMR spectroscopic data of compounds 4–7.
Previous studies and ethnomedicinal uses suggest that whole extracts and solvent fractions of A. floribunda have some activity and therapeutic benefits on immuno-inflammatory cells and associated disorders (Okoye & Ebi Citation2007; Okoye & Osadebe Citation2009, Citation2010; Okoye et al. Citation2010, Citation2011; Siwe et al. Citation2014). For this reason, all the compounds (1–7) isolated from A. floribunda were screened for immune regulatory activities in relation to the expression of type-1 (IFNγ and IL-2) cytokines by CD4+ and CD8+ T cells using flow cytometry. The splenic T lymphocytes were stimulated with and without three graded concentrations of each of these compounds in the presence of 2 μM monensin which blocked secretion of any expressed cytokines and retains them intracellularly for staining (Jung et al. Citation1993). The procedure allowed staining with specific cell surface antibody markers to define the T cell subtype and the expressed cytokines quantified within the cell after permeabilization, staining, and then the FACS analysis.
FACS data analysis showed that stimulation with all the compounds increased the proportion of CD8+/IFNγ+ and CD4+/IFNγ+ T lymphocytes when compared with the cells in untreated control wells. The CD8+/IFNγ+ population ranged from 57.85 to 72.45% (versus 57.85% for the untreated control) while CD4+/IFNγ+ ranged from 3.21 to 7.21% (versus 2.75% for the untreated control) ( and ). Intracellular IL-2 secretion by treated T cells could barely be detected. Similarly, T-lymphocytes doubly expressing IFNγ/IL-2 were grossly absent ( and ). The IL-2 cytokine together with some other related cytokines family members such as IL-4, IL-7, IL-9, IL-15 and IL-21 demonstrate some unique biological effects including their functionality during different stages of differentiation of specific subsets of lymphocytes where they influence their development, proliferation and function (Floros & Tarhini Citation2015). However, the findings of our present study do not appear to link IL-2 to the up-regulation of our analyzed CD4+ and CD8+ cell population. Moreover, while all the compounds showed stimulatory effect, there were no significant differences between the concentration range (25–6.25 μg/ml) utilized. Our findings taken alone could mean that the type 1 interferon-gamma driven cellular T-lymphocytes response apart from the activation of certain type 1-driven cells of the innate and adaptive immunity such as monocytic, polymorphonucleic, NK and lymphocytic cells, may equally lead to beneficial activation of cytotoxic activity against intracellular infections and tumour, but the picture will be complete when juxtaposed against other type 2 cytokines milieu that are produced in the host at the time of prevalent disease (Lucey et al. Citation1996). However, the activation of type 1 cytokine secreting helper T-lymphocytes could also continue to enhance the activity of the cytotoxic T-lymphocytes which in turn could translate to an improved killing of pathogen-infected cells or various tumour cells.
Conclusions
Although these effects recorded in our study are largely non-specific, the interplay of these antigen-specific and adjuvant-induced immune cells activation are important in the quality and robustness of immune responses that are mounted against infectious threat (Choi et al Citation1997; Lindsey et al. Citation1997). Finally, in any case, the marginal type 1 immune enhancement arising from the activity of compounds isolated from A. floribunda may contribute to the global picture to find explanation to the ethnomedicinal use of A. floribunda.
Acknowledgements
Dr. F. B. C. Okoye acknowledges the Return Fellowship Grant awarded to him by Alexander von Humboldt Foundation, which enabled the completion of the later parts of this work.
Disclosure statement
The authors report that they have no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Barak V, Birkenfeld S, Halperin T, Kalickman I. 2002. The effect of herbal remedies on the production of human inflammatory and anti-inflammatory cytokines. Israel Med Assoc J. 4(11 Suppl.):919–922.
- Barth H, Berg PA, Klein R. 2003. Methods for the in vitro determination of an individual disposition towards TH1 or TH2-reactivity by the application of appropriate stimulatory antigens. Clin Exp Immunol. 134:78–85.
- Burns JJ, Zhao L, Taylor EW, Spelman K. 2010. The influence of traditional herbal formulas on cytokine activity. Toxicology. 278:140–159.
- Chosson E, Chaboud A, Chulia AJ, Raynaud J. 1998. Dihydroflavonol glycosides from Rhododendron ferrugineum. Phytochemistry. 49:1431–1433.
- Choi SY, Yang KM, Jeon SD, Kim JH, Khil LY, Chang TS, Moon CK. 1997. Brazilin modulates immune function mainly by augmenting T cell activity in halothane administered mice. Planta Med. 63:405–408.
- de Almeida AP, Miranda MMFS, Simoni IC, Wigg MD, Lagrota MHC, Costa SS. 1998. Flavonoid monoglycosides from the antiviral fractions of Percia americana (Lauraceae) leaf infusion. Phytother Res. 12:562–567.
- Duke JA, Mary JB, Judi D. 2002. Handbook of medicinal herbs. Boca Raton: CRC Press.
- Düber A, Voltmer G, Gora V, Lunderstädt J, Zeeck A. 1997. Phenols from Fagus sylvatrica and their role in defence against Cryptococcus fagisuga. Phytochemistry. 45:51–57.
- Fiorentino D, Zlotnik F, Mosmann TR. 1991. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 147:3815–3821.
- Floros T, Tarhini AA. 2015. Anticancer cytokines: biology and clinical effects of interferon-α2, interleukin (IL)-2, IL-15, IL-21, and IL-12. Sem Oncolog. 42:539–548.
- Gordana R, Mladen K, Marijana K, Herwig OG. 2007. Quercetin influences response in Nicotiana megalosiphon infected by satellite-associated cucumber mosaic virus. J Plant Dis Protect. 114:145–150.
- Gudej J. 2003. Kaempferol and quercetin glycosides from Rubus idaeus L. leaves. Acta Pol Pharm - Drug Res. 60:313–316.
- Jung TU, Schauer C, Heusser C, Neumann Rieger C. 1993. Detection of intracellular cytokines by flow cytometry. J Immunol Meth. 159:197–207.
- Kiecolt-Glasser JK, McGuire L, Robles TF, Glaser R. 2002. Psychoneuroimmunology and psychosomatic medicine: back to the future. Psychosom Med. 64:15–28.
- Kim M-R, Lee JY, Lee H, Aryal DK, Kim YG, Kim SK, Woo E-R, Kang KW. 2006. Antioxidative effects of quercetin-glycosides isolated from the flower buds of Tussilago farfara L. Food Chem Toxicol. 44:1299–1307.
- Kimata M, Inagaki N, Nagai H. 2000. Effects of luteolin and other flavonoids on IgE-mediated allergic reactions. Planta Med. 66:25–29.
- Lindsey JW, Kerman RH, Wolinsky JS. 1997. T cell–T cell activation in multiple sclerosis. Mult Scler. 3:238–342.
- López-Lázaro M. 2009. Distribution and biological activity of flavonoids. Mini Rev Med Chem. 9:31–59.
- Lucey DR, Clerici M, Shearer GM. 1996. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin Microbiol Rev. 9:532–562.
- Maheep KC, Neelu S, Mahabeer PD, Yogesh CJ. 2011. Flavonoids: a versatile source of anticancer drugs. Pharmacogn Rev. 5:1–12.
- Markham KR, Mabry TJ. 1968. The structure and stereochemistry of two new dihydroflavonol glycosides. Tetrahedron. 24:823–827.
- Neuwinger HD. 2000. African traditional medicine: a dictionary of plants and application. Stuttgard, Germany: Medpharm.
- Nonaka G, Goto Y, Kinjo J, Nohara T, Nishioka I. 1987. Tannins and related compounds. LII. Studies on the constituents of the leaves of Thujopsis dolabrata SIEB. et ZUCC. Chem Pharm Bull. 35:1105–1108.
- Nworu CS, Akah PA, Okoye FBC, Proksch P, Esimone CO. 2010. The effects of Phyllanthus niruri aqueous extract on the activation of murine lymphocytes and bone marrow-derived macrophages. Immunol Invest. 39:245–267.
- Nworu CS, Akah PA, Okoye FBC, Esimone CO. 2012. Supplementation with aqueous leaf extract of Morinda lucida enhances immunorestoration and upregulates the expression of cytokines and immunostimulatory markers. Immunol Invest. 41:799–819.
- Okoye FBC, Ebi GC. 2007. Preliminary antimicrobial and phytochemical investigation of the extracts and column fractions of Alchornea floribunda leaves. J Pharm Allied Sci. 4:395–402.
- Okoye FBC, Osadebe PO. 2009. Studies on the mechanisms of anti-inflammatory activity of the extracts and fractions of Alchornea floribunda leaves. Asian Pac J Trop Med. 2:7–14.
- Okoye FBC, Osadebe PO. 2010. A new anti-inflammatory flavonol glycoside from Alchornea floribunda leaves. Nat Prod Res. 24:266–273.
- Okoye FBC, Osadebe PO, Nworu CS, Omeje EO, Okoye NN, Esimone CO. 2011. Topical anti-inflammatory constituents of lipophilic leaf fractions of Alchornea floribunda and Alchornea cordifolia. Nat Prod Res. 25:1941–1949.
- Okoye FBC, Osadebe PO, Proksch P, Edrada-Ebel RA, Nworu CS, Esimone CO. 2010. Anti-inflammatory and membrane-stabilizing stigmastane steroids from Alchornea floribunda leaves. Planta Med. 76:172–177.
- Oppenheim JJ. 2001. Cytokines: past, present and future. Int J Hematol. 74:3–8.
- O'Sullivan RL, Lipper G, Lernner EA. 1998. The neuro-immunocutaneous endocrine network: relationship of mind and skin. Arch Dermatol. 134:1431–1435.
- Prasad S, Phromnoi K, Yadav VR, Chaturvedi MM, Aggarwal BB. 2010. Targeting inflammatory pathways by flavonoids for prevention and treatment of cancer. Planta Med. 76:1044–1063.
- Robak J, Gryglewski RJ. 1996. Bioactivity of flavonoids. Polish J Pharmacol. 48:555–564.
- Romagnani S. 2006. Regulation of the T cell response. Clin Exp Allergy. 36:1357–1366.
- Sakurai A, Okada K, Okumura Y. 1982. Chemical studies on the mistletoe. IV. The structure of isoglucodistylin, a new flavonoid glycoside isolated from Taxillus kaempferi. Bull Chem Soc Jap. 55:3051–3052.
- Sepulveda-Boza S, Delhvi S, Kassels BK. 1993. Flavonoids from the twigs of Eucyphia glutinosa. Phytochemistry. 32:1301–1303.
- Siwe NX, Krause RWM, van Vuuren SF, Tantoh ND, Oliver DK. 2014. Antibacterial activity of the roots, stems and leaves of Alchornea floribunda. J Ethnopharmacol. 151:1023–1027.
- Spelman K, Burns J, Nichols D, Winters N, Ottersberg S, Tenborg M. 2006. Modulation of cytokine expression by traditional medicines: a review of herbal immunomodulators. Altern Med Rev. 11:128–150.
- Yerra KR, Shih-Hua F, Yew-Min T. 2005. Anti-inflammatory activities of flavonoids isolated from Caesalpinia pulcherrima. J Ethnopharmacol. 100:249–253.