Abstract
Context In a previous study, it has been shown that ellagic acid (EA), a polyphenolic compound found in pomegranate and different berries, prevents cognitive and hippocampal long-term potentiation (LTP) impairments induced by traumatic brain injury in rats through antioxidant and anti-inflammatory mechanisms.
Objective The present study was conducted to assess the potential of EA as a memory enhancer.
Materials and methods The elevated plus maze (EPM) and passive avoidance (PA) paradigm were used to evaluate learning and memory parameters. Three doses (10, 30 and 100 mg/kg, i.p.) of EA were administered to animals. Memory impairment was induced by scopolamine treatment (0.4 mg/kg, i.p.) and/or diazepam (1 mg/kg, i.p.). Acquisition trials were carried out 30 min after scopolamine treatment and retention trials were performed for 5 min 24 h after the acquisition trials.
Results EA at doses 30 and 100 mg/kg significantly reversed the amnesia induced by scopolamine (0.4 mg/kg, i.p.) in the EPM and PA tests in mice. Also, EA at doses 30 and 100 mg/kg significantly antagonized the amnesia induced by diazepam (1 mg/kg, i.p.) in EPM test in rats. Moreover, chronic administration of EA at dose 30 mg/kg ameliorated the memory deficit induced by diazepam (1 mg/kg, i.p.) in rats.
Discussion and conclusion This study demonstrates that ellagic acid is effective in preventing scopolamine- and diazepam-induced cognitive impairments without altering the animals’ locomotion. This suggests the potential of EA application as a useful memory restorative agent in the treatment of dementia seen in elderly persons.
Introduction
Treatment of cognitive disorders such as attention deficit, amnesia, and Alzheimer's disease is still far from being recognized in medicine. Cognitive enhancing drugs such as memantine, aniracetam, piracetam and choline esterase inhibitors like galantamine are being used for improving memory, mood and behaviour, but their side effects have limited the use of these agents. Therefore, other possibilities including plant-derived constituents have been considered and evaluated for memory loss therapy (Park et al. Citation2012).
Phytochemicals are naturally occurring substances found in plants. Dietary bioactive compounds from different functional foods, herbs and nutraceuticals (antioxidant vitamins, ginkgo, soy phytoestrogens, carnitine, curcumin, ginseng, polyphenols, melatonin, etc.) have been shown to ameliorate or even prevent diseases (Reeta et al. Citation2009). Besides, it has been demonstrated that the consumption of flavonoid-rich fruits, such as berries, apple and citrus, throughout life may have the capacity to improve age-dependent deteriorations in memory and cognition (Spencer Citation2010).
Ellagic acid (EA, 2,3,7,8-tetrahydroxybenzopyrano [5,4,3-cde]benzopyran-5-10-dione) is a flavonoid present in fruits and nuts, including blueberry, blackberry, raspberry, strawberry, pomegranate and walnuts (Sellappan et al. Citation2002). It has been reported to show different pharmacological effects, including chemoprevention and antioxidant (Festa et al. Citation2001; Townsend & Tew Citation2003), inhibition of tumorigenesis (Buniatian Citation2003), antinociception and anti-inflammation (Mansouri et al. Citation2013, Citation2014), inhibition of anaphylactic reaction in vivo and in vitro (Choi & Yan Citation2009), inhibition of lipopolysaccharide-induced prostaglandin E2 synthesis in human monocytes (Karlsson et al. Citation2010), antidepressant (Girish et al. Citation2012) and also anxiolytic (Girish et al. Citation2013) activities.
There are also studies that show neuroprotective effects of ellagic acid, such as diminishing the diabetic neuropathy (Liu et al. Citation2011), protecting cerebral ischemic damage (Rafieirad & Ghasemzadeh-Dehkordi Citation2014), improving cognitive impairment caused by 6-hydroxy dopamine (Dolatshahi et al. Citation2015) and preventing cognitive and hippocampal long-term potentiation deficits induced by traumatic brain injury (Farbood et al. Citation2015).
Based on the findings mentioned above, this study was aimed to investigate the beneficial activity of EA against scopolamine and also diazepam-induced cognitive impairments by using elevated plus maze and passive avoidance tests.
Materials and methods
Animals and housing
Male Wistar rats and mice weighing (180 ± 20, 23 ± 3 g, respectively) were obtained from the animal facility of Ahvaz Jundishapur University of Medical Sciences (Ahvaz, Iran). Animals were kept five in each cage, in a temperature (22 ± 2 °C) and light (12 h light/dark cycle) controlled room, with free access to standard laboratory chow and water. All experiments were performed on separate groups of animals (n = 8) between 8.00 am and 1.00 pm, and each animal was used only once. The study was conducted in accordance with the NIH Guide for Care and Use of Laboratory Animals. The Institutional Animal Ethical Committee of Jundishapur University, formed under Committee for Purpose of Control and Supervision of Experiments on Animals (CPCSEA) approved all the pharmacologic protocols.
Chemicals
Ellagic acid, propylene glycol, and scopolamineċHBr (cholinergic receptor antagonist) were obtained from Sigma (St. Louis, MO). Diazepam ampoules (10 mg/2 mL) were kindly gifted from Darupakhsh Pharmaceutical Co, Tehran, Iran. All other materials were of the highest grades commercially obtainable. EA was dissolved in normal saline (containing 10% DMSO) and adjusted to pH of 7.4. All solutions were prepared freshly in test days and administered in a final volume of 0.01 mL/g body weight via the intraperitoneal route. A control treatment consisted of an equal volume of the corresponding vehicles.
Experimental protocols
Animals were divided into six groups of eight animals each. Scopolamine dissolved in sterile saline (NaCl 0.9%) was administered intraperitoneally (i.p.) in different groups at a volume of 0.1 mL/100 g mouse weight. Scopolamine was administered in a dose of 0.4 mg/kg as this dose was found to produce cognitive decline (Gacar et al. Citation2011). EA was given 30 min before the administration of scopolamine in the combination group. The first group served as control and received the vehicle. In the next group, scopolamine was administered and the vehicle was given. In the 3rd, 4th and 5th groups, EA was administered in three doses (10, 30 and 100 mg/kg) along with scopolamine. In the 6th group, the highest dose of EA (100 mg/kg) was administered alone to study any effect of EA on its own on cognitive functions. The animals in this group received vehicle injection i.p. as well.
In a separate experiment, animals were divided into six groups of eight animals each. Diazepam ampoules was diluted in deionized water containing 0.5% propylene glycol and given intraperitoneally (i.p.) to different groups at a volume of 0.1 mL/100 g mouse weight. Diazepam was administered in a dose of 1 mg/kg as this dose was found to produce cognitive decline (Kim et al. Citation2007). EA was given in doses of 10, 30 and 100 mg/kg, 30 min before the administration of diazepam in the combination group. The first group served as control and received the vehicle. In the next group, diazepam was administered and the vehicle was given. In the 3rd, 4th and 5th groups, EA was administered in three doses (10, 30 and 100 mg/kg) along with diazepam. In the 6th group, the highest dose of EA (100 mg/kg) was administered alone to study any effect of EA on its own on cognitive functions. The animals in this group received vehicle injection i.p. as well.
In another experiment, in order to investigate the chronic effects of EA against memory decline induced by diazepam, animals were divided into four groups of eight animals each. Diazepam was given as previously described above (Kim et al. Citation2007). Ellagic acid was administered in groups of mice intraperitoneally (i.p.) at volumes not greater than 1 mL/100 g. EA was given in dose of 30 mg/kg (sub-effective dose of EA in acute administration), once a day for 10 consecutive days and, on the test day, 30 min before the administration of diazepam in the combination group. The first group served as control and was administered the vehicle. In the next group, diazepam was administered in a dose of 1 mg/kg and the vehicle was administered. In the 3rd group, EA was administered at dose 30 mg/kg along with diazepam. In the 4th group, dose of EA (30 mg/kg) was administered alone to study any effect of EA on its own on cognitive functions. The animals in this group received vehicle injection i.p. as well. Doses of ellagic acid were chosen based on earlier reports and they showed neither effect on locomotion when administered alone (Mansouri et al. Citation2013, Citation2014).
The behavioural parameters were performed 30 min after administration of the scopolamine and diazepam.
Elevated plus-maze test
Acquisition and retention of memory were evaluated with the elevated plus maze learning task, which evaluates spatial short-term memory (Reddy & Kulkarni Citation1998). Transfer latency (TL) (the time that the animal moves from the open arm to enclosed arm) was utilized as an index of learning and memory function. The procedure was basically in accordance with the method described by Itoh et al. (Citation1991). The elevated plus maze consisted of two open arms (16 × 5 cm) and two enclosed arms (16 × 5 × 12 cm) with an open roof. The maze was elevated 25 cm above the ground and a video camera was fixed above the maze to record the movements for analysis. The behavioural experiments were conducted in a quiet room illuminated by a dim light (50 lux). For the test, each mouse was placed at the end of either the open arms and the initial transfer latency was noted on the first day. To become habituated with the maze, the animals were allowed to explore the plus maze for 20 s after reaching the enclosed arm. On the second day, 24 h after the first exposure, transfer latency was again noted. A long latency period to reach enclosed arm indicates poor retention compared with significantly shorter latencies.
Passive avoidance test
The passive avoidance task was used for memory retention deficit. The apparatus (Neuroscience, Inc., Herndon, VA) consisted of an illuminated compartment (20 × 10 × 2 cm) with a 6-W tungsten lamp and a dark compartment (30 × 30 × 30 cm) with a grid floor (15 parallel steel rods), separated by a guillotine door (8 × 8 cm). Electroshocks (0.2 mA, 75 V, 50 Hz) were delivered for 3 s through the grid floor in the dark compartment by a shock scrambler (Neuroscience, Inc., Herndon, VA). On the first and second days of testing, each rat was placed on the apparatus and left for 5 min to habituate to the environment. On the third day, an acquisition trial was performed. During the acquisition trial, each rat was placed in the illuminated chamber. After initial habituation period of 60 s, the guillotine door was opened and the time taken by rat to enter the dark chamber was recorded. The latency to step into the dark compartment was recorded as initial transfer or pre-shock latency (ITL). As soon as the rat entered the dark chamber, it was given a mild foot shock of 0.5 mA for 2 s through the grid floor. The rat was allowed to remain in the dark compartment for 5 s and then taken out. After 24 h interval, retention trial was performed and retention transfer or postshock latency (RTL) to step into the dark compartment was noted. The cut-off time was 300 s (Mansouri et al. Citation2013). Short latencies indicated poorer retention.
Spontaneous behaviour in the open-field test
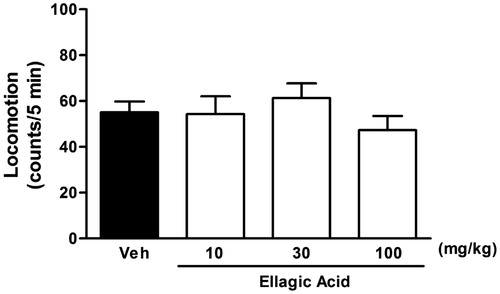
Spontaneous behaviour in the open-field test was conducted in clear black Plexiglas boxes 40 × 40 × 40 cm (length × width × height) equipped with a video-based Ethovision system (Nodulus, Wageningen, The Netherlands) as described previously by Mansouri et al. (Citation2014). The rats were placed in the centre of the apparatus to assess locomotion (the number of line crossing) and frequency of rearing or line crossing 30 min after being treated with the single EA (10, 30 and 100 mg/kg, i.p.), or vehicle (saline). The locomotor activity was recorded for 5 min.
Statistical analysis
The obtained results were presented as means ± SEM. Data were evaluated by one-way analysis of variance (ANOVA) with Dunnett's test for post hoc analysis. All statistical analysis was done by using GraphPad Prism 5.0 (GraphPad Inc., San Diego, CA). Statistical significance was set at p < 0.05.
Results
The effect of ellagic acid on the scopolamine-induced memory impairment in elevated plus maze task
There was no significant difference in the initial transfer latency of the control, scopolamine-treated, ellagic acid (10, 30 and 100 mg/kg) along with scopolamine, and also ellagic acid (100 mg/kg) alone-treated groups [F(5,42) = 0.27, p > 0.05]. In the retention transfer latency, statistical analysis revealed significant difference among the groups [F(5,42) = 6.41, p < 0.001]. Indeed, scopolamine at 0.4 mg/kg caused significantly more retention transfer latency as compared with the control group (). The retention transfer latencies increased from 53 ± 4.7s in control animals to 92.6 ± 7.2 s in scopolamine-treated mice (p < 0.001).
Figure 1. Effect of a single administration of ellagic acid (EA) on scopolamine-induced cognitive impairment using elevated plus maze paradigm. EA (10, 30 and 100 mg/kg, i.p.) or vehicle (Veh) were administered to mice 30 min before the acquisition trials. Memory impairment was induced by scopolamine treatment (0.4 mg/kg, i.p.). Acquisition trials were carried out 30 min after scopolamine treatment. Retention trials were carried out for 5 min 24 h after the acquisition trials. Data represent means ± SEM (n = 8 in each group). *versus the vehicle control group, #versus the scopolamine-treated group (one-way ANOVA followed by Dunnett's test).
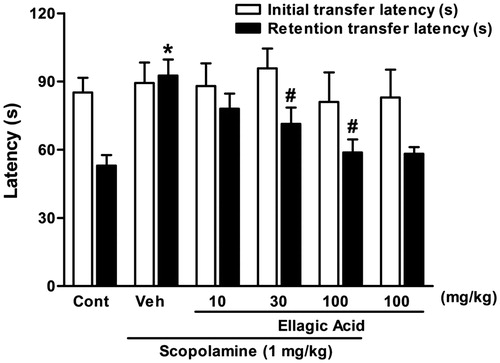
Furthermore, ellagic acid produced dose-dependent reversal of the scopolamine effect, when this agent was administered along with scopolamine. The values decreased from 92.6 ± 7.2s in the scopolamine-treated group to 78 ± 7.8 (p > 0.05), 71.4 ± 7.2 (p < 0.05) and 58.3 ± 2.9 s (p < 0.01) in the ellagic acid 10, 30 and 100 mg/kg co-administered groups, respectively. Besides, ellagic acid alone (100 mg/kg, i.p.) did not produce any significant change in the retention transfer latency as compared with the control group.
The effect of ellagic acid on the diazepam-induced memory impairment in elevated plus maze task
There was no significant difference in the initial transfer latency of the control, diazepam-treated, ellagic acid (10, 30 and 100 mg/kg) along with diazepam and ellagic acid (100 mg/kg) alone-treated groups [F(5,42) = 0.74, p < 0.05]. In the retention transfer latency, statistical analysis revealed significant difference among the groups [F(5,42) = 10.78, p < 0.001]. In fact, diazepam (1 mg/kg, i.p.) caused significantly more retention transfer latency as compared with the control group (). The retention transfer latencies increased from 49 ± 4.7 s in control animals to 75.3 ± 3.3 s in diazepam-treated mice (p < 0.001). Furthermore, ellagic acid produced dose-dependent reversal of the diazepam effect, when this agent was administered along with diazepam. The values decreased from 75.3 ± 3.3 s in the diazepam-treated group to 81.1 ± 6.6 (p > 0.05), 63.8 ± 2.8 (p < 0.05) and 56 ± 2.9 s (p < 0.01) in ellagic acid 10, 30 and 100 mg/kg co-administered groups, respectively. Besides, ellagic acid alone (100 mg/kg, i.p.) did not produce any significant change in the retention transfer latency as compared with the control group.
Figure 2. Effect of a single (panel A) and chronic (panel B) administration of ellagic acid (EA) on diazepam-induced cognitive impairment using elevated plus maze paradigm. For acute experiment, EA (10, 30 and 100 mg/kg, i.p.) or vehicle (Veh) were administered to mice 30 min before the acquisition trials and for chronic experiment, EA (30 mg/kg, i.p.) or vehicle (Veh) were administered to mice for 10 consecutive days and the last dose was given 30 min before the acquisition trials. Memory impairment was induced by scopolamine treatment (0.4 mg/kg, i.p.). Acquisition trials were carried out 30 min after scopolamine treatment. Retention trials were carried out for 5 min 24 h after the acquisition trials. Data represent means ± SEM (n = 8 in each group). *versus the vehicle control group, #versus the scopolamine-treated group (one-way ANOVA followed by Dunnett's test).
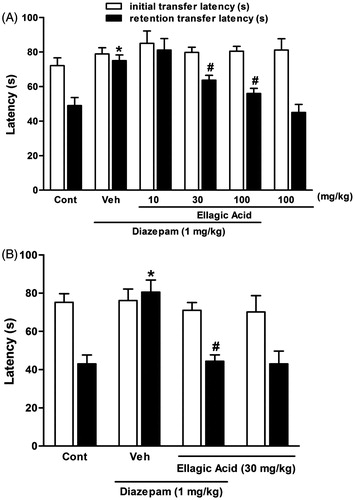
In the chronic study, there was no significant difference in the initial transfer latency of the control, diazepam-treated, ellagic acid (30 mg/kg; i.p. for 10 consecutive days) along with diazepam and also ellagic acid (30 mg/kg) alone-treated groups [F(3,28) = 0.24, p < 0.05]. In the retention transfer latency, statistical analysis revealed significant difference among the groups [F(3,28) = 11.69, p < 0.001]. Indeed, diazepam (1 mg/kg, i.p.) caused significantly more retention transfer latency as compared with the control group (). The retention transfer latencies increased from 43 ± 4.67 s in control animals to 80.5 ± 4.35 s in diazepam-treated mice (p < 0.001). Furthermore, chronic administration of ellagic acid produced a significant reversal of the diazepam effect. The values decreased from 80.5 ± 4.35 s in the diazepam-treated group to 44.4 ± 3.3 (p < 0.05) in ellagic acid 30 mg/kg co-administered group. Besides, ellagic acid alone (30 mg/kg, i.p. for 10 consecutive days) did not produce any significant change in the retention transfer latency as compared with the control group.
The effect of acute administration of ellagic acid on the scopolamine-induced memory impairment in step-through passive avoidance task
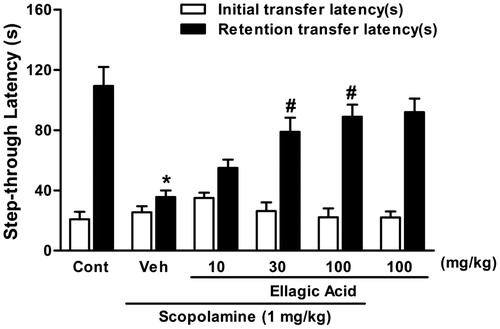
There were no significant differences between mean initial latencies of all treated groups [F(5,42) = 1.2, p < 0.05]. Also, statistical analysis showed a significant difference among the groups [F(5,42) = 9.38, p < 0.001] in the retention transfer latencies. In fact, single dose of scopolamine (0.4 mg/kg, i.p.) caused a significant impairment of memory as indicated by the decrease in retention transfer latency (). The retention latency significantly decreased from 109.5 ± 12.6 s in control animals to 35.6 ± 4.4 s in scopolamine-treated rats (p < 0.001). Ellagic acid produced dose-dependent and significant reversal of the scopolamine effect, when co-administered with together. The retention latency increased from 35.6 ± 4.4 s in scopolamine-treated rats to 55 ± 5.5 (p > 0.05), 78.9 ± 9.3 (p < 0.01) and 88.9 ± 8.1 s (p < 0.001) in the groups treated with EA at 10, 30 and 100 mg/kg, respectively. Moreover, ellagic acid alone at dose (100 mg/kg, i.p.) did not produce any significant change in the retention latency as compared with the control group (109.5 ± 12.6 s in control rats and 92 ± 9.6 s in EA-treated rats).
Figure 3. Effect of a single administration of ellagic acid (EA) on scopolamine-induced memory deficits in the passive avoidance task. EA (10, 30 and 100 mg/kg, i.p.) or vehicle (Veh) were administered to rats 30 min before the acquisition trials. Memory impairment was induced by scopolamine treatment (0.4 mg/kg, i.p.). Acquisition trials were carried out 30 min after scopolamine treatment. Retention trials were carried out for 5 min 24 h after the acquisition trials. Data represent means ± SEM (n = 8 in each group). *versus the vehicle control group, #versus the scopolamine-treated group (one-way ANOVA followed by Dunnett's test).
The effect of ellagic acid (EA) on locomotor activity
A spontaneous locomotor activity test was carried out to determine whether a possible stimulatory effect of ellagic acid could modify the exploratory behaviour. As shown in , a single treatment with ellagic acid (10, 30, and 100 mg/kg) produced no significant changes in line crossing [(F(4,28) = 0.66), p > 0.05] as compared with corresponding controls.
Discussion
The present study was undertaken to evaluate the anti-amnesic effects of ellagic acid in animal model of memory deficits induced by scopolamine or diazepam. The main findings of the study were that single administration of ellagic acid reversed the memory deficits induced by scopolamine in the elevated plus maze and passive avoidance tasks. EA also restored the memory dysfunction induced by diazepam, when given in single and chronic doses in elevated plus maze test. These results suggest the ameliorating effects of EA on cognitive dysfunctions.
In the present study, the memory function has been assessed by using elevated plus maze as well as passive avoidance tests. Although the elevated plus maze is primarily employed for the evaluation of anxiety, it has also been used as a model for assessment of memory in animals (Itoh et al. Citation1991; Reeta et al. Citation2009). It is hypothesized that transfer latency (time in which animal moves from the open arms to the enclosed arms) is shortened when the animal has initially experienced entering the enclosed arms. Thus, the shortened transfer latency is related to retention memory. Elevated plus maze test has, therefore, been considered as a useful tool for discovering memory-enhancing effects (Itoh et al. Citation1991). Scopolamine as well as diazepam administration before the acquisition trial impaired the learning and memory as evidenced by significantly increased retention latencies in elevated plus maze paradigm.
The passive avoidance test was used for the assessment of learning and memory deficit in rats. In this test, the animals upon exposure to the first trial acquire the information that entry into dark chamber results in painful experience of electric shock, and the cognitive ability of the animals was reflected by avoidance of the entry (Mansouri et al. Citation2013). Scopolamine administration before the acquisition trial impaired the learning and memory as evidenced by significantly reduced retention latencies in passive avoidance behaviour.
These results are in agreement with the earlier reports that demonstrated cognitive impairment after systemic administration of scopolamine and diazepam in rodents and humans (Lister Citation1985; Thiebot Citation1985; Blokland Citation1995; Iversen Citation1997). Moreover, in this work, pretreatment with EA caused significant prevention in the latency times in a retention trial of these two tests. Also, chronic administration of ellagic acid to mice restored diazepam-induced impairment of memory retention in elevated plus maze task. However, ellagic acid in normal mice and rats did not cause any change in the acquisition/retention of memory as compared with the controls, thus implying that ellagic acid is not a memory enhancer although it can prevent the memory impairment induced by scopolamine and or diazepam.
Since we administered the drugs just before the acquisition trial, potential drug actions on cognitive function may be confounded by effects of the agent on non-specific processes, such as attention, arousal or sensory motor functions (Hunter et al. Citation1988). To rule out this possibility, we evaluated the locomotor activity to determine any motor disability that might affect inhibitory avoidance performance. Our results indicated that locomotor activities in the control and ellagic acid-treated groups were the same. These data exclude the possibility that locomotion or shock sensitivity may have contributed to the change in step-down latencies during testing.
Ellagic acid, a dietary polyphenolic compound present in many fruits and nuts, has been reported to possess free radical scavenging, iron chelating and anti-inflammatory activities (Majid et al. Citation1991; Sellappan et al. Citation2002). In previous studies, its potential in reversing Ab42 amyloid-associated neurotoxicity in a mouse model of Alzheimer's disease has been determined (Hartman et al. Citation2006; Feng et al. Citation2009). Ellagic acid also inhibited reactive oxygen species (ROS) formation and prevented cell death in PC12 cells (Pavlica & Gebhardt Citation2005), thus supporting its use in the neurodegenerative diseases. These properties suggest a likelihood of ellagic acid to be effective in cognitive impairments induced by chemicals as well.
It has been shown that ellagic acid attenuate memory deficits in 6-OHDA- and TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin)-treated rats (Hassoun et al. Citation2004; Dolatshahi et al. Citation2015). Moreover, this compound has neuroprotective in focal cerebral ischemia, traumatic brain injury and also in brain histopathologic damage induced by streptozotocin in rats (Uzar et al. Citation2012; Rafieirad & Ghasemzadeh-Dehkordi Citation2014; Farbood et al. Citation2015).
Based on the results of the above behavioural studies, we conclude that ellagic acid has the ability to ameliorate memory deficits induced by scopolamine and/or diazepam. These findings suggest that this polyphenolic compound may be useful candidate for anti-amnesic drug development.
Funding information
This research was financially supported by grants (PRC-121) from the Physiology Research Center, funded by the Vice Chancellor of Research, Ahvaz Jundishapur University of Medical Sciences (Iran).
Disclosure statement
The authors report that they have no conflicts of interest.
References
- Blokland A. 1995. Acetylcholine: a neurotransmitter for learning and memory? Brain Res Rev. 21:285–300.
- Buniatian GH. 2003. Stages of activation of hepatic stellate cells: effects of ellagic acid, an inhibiter of liver fibrosis, on their differentiation in culture. Cell Prolif. 36:307–319.
- Choi YH, Yan GH. 2009. Ellagic acid attenuates immunoglobulin E-mediated allergic response in mast cells. Biol Pharm Bull. 32:1118–1121.
- Dolatshahi M, Farbood Y, Sarkaki A, Mansouri MT, Khodadadi A. 2015. Ellagic acid improves hyperalgesia and cognitive deficiency in 6-hydroxidopamine induced rat model of Parkinson's disease. Iran J Basic Med Sci. 18:38–46.
- Farbood Y, Sarkaki A, Dianat M, Khodadadi A, Khaksari Haddad M, Mashhadizadeh S. 2015. Ellagic acid prevents cognitive and hippocampal long-term potentiation deficits and brain inflammation in rat with traumatic brain injury. Life Sci. 124:120–127.
- Feng Y, Yang S, Du X, Zhang X, Sun X, Zhao M, Sun GY, Liu RT. 2009. Ellagic acid promotes Abeta42 fibrillization and inhibits Abeta42-induced neurotoxicity. Biochem Biophys Res Commun. 390:1250–1254.
- Festa F, Aglitti T, Duranti G, Ricordy R, Perticone P, Cozzi R. 2001. Strong antioxidant activity of ellagic acid in mammalian cells in vitro revealed by the comet assay. Anticancer Res. 21:3903–3908.
- Gacar N, Mutlu O, Utkan T, Komsuoglu Celikyurt I, Selcen Gocmez S, Ulak G. 2011. Beneficial effects of resveratrol on scopolamine but not mecamylamine induced memory impairment in the passive avoidance and Morris water maze tests in rats. Pharmacol Biochem Behav. 99:316–323.
- Girish C, Raj V, Arya J, Balakrishnan S. 2012. Evidence for the involvement of the monoaminergic system, but not the opioid system in the antidepressant-like activity of ellagic acid in mice. Eur J Pharmacol. 682:118–125.
- Girish C, Raj V, Arya J, Balakrishnan S. 2013. Involvement of the GABAergic system in the anxiolytic-like effect of the flavonoid ellagic acid in mice. Eur J Pharmacol. 710:49–58.
- Hartman RE, Shah A, Fagan AM, Schwetye KE, Parsadanian M, Beth Finn M, Holtzman DM. 2006. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer's disease. Neurobiol Dis. 24: 506–515.
- Hassoun EA, Vodhanel J, Abushaban A. 2004. The modulatory effects of ellagic acid and vitamin E succinate on TCDD-induced oxidative stress in different brain regions of rats after subchronic exposure. J Biochem Mol Toxicol. 18: 196–203.
- Hunter B, Zornetzer SF, Jarvik ME, McGaugh JL. 1988. Modulation of learning and memory: effects of drugs influencing neurotransmitters. In: Iversen LL, editors. Handbook of psychopharmacology. New York: Plenum Press. p. 531–577.
- Itoh J, Nabeshima T, Kameyama T. 1991. Utility of an elevated plus-maze for dissociation of amnesic and behavioral effects of drugs in mice. Eur J Pharmacol. 194:71–76.
- Iversen SD. 1997. Behavioural evaluation of cholinergic drugs. Life Sci. 60:1145–1152.
- Karlsson S, Nanberg E, Fjaeraa C, Wijkander J. 2010. Ellagic acid inhibits lipopolysaccharide induced expression of enzymes involved in the synthesis of prostaglandin E2 in human monocytes. Br J Nutr. 103:1102–1109.
- Kim DH, Jeon SJ, Jung JW, Lee S, Yoon BH, Shin BY, Son KH, Cheong JH, Kim YS, Kang SS, et al. 2007. Tanshinone congeners improve memory impairments induced by scopolamine on passive avoidance tasks in mice. Eur J Pharmacol. 574: 140–147.
- Lister RG. 1985. The amnesic action of benzodiazepines in man. Neurosci Biobehav Rev. 9:87–94.
- Liu QS, Pang ZR, Liu R, He GR, Cui J, Yin XY. 2011. Effective compounds group of Mongolian prescriptions BAIMAI-SAN protect against peripheral neuropathy in lower limbs of rats through neuroprotective effect. J Ethnopharmacol. 135:786–791.
- Majid S, Khanduja KL, Gandhi RK, Kapur S, Sharma RR. 1991. Influence of ellagic acid on antioxidant defence system and lipid peroxidation in mice. Biochem Pharmacol. 42:1440–1445.
- Mansouri MT, Naghizadeh B, Ghorbanzadeh B. 2014. Involvement of opioid receptors in the systemic and peripheral antinociceptive actions of ellagic acid in the rat formalin test. Pharmacol Biochem Behav. 120:43–49.
- Mansouri MT, Naghizadeh B, Ghorbanzadeh B, Farbood Y. 2013. Central and peripheral antinociceptive effects of ellagic acid in different animal models of pain. Eur J Pharmacol. 707: 46–53.
- Mansouri MT, Farbood Y, Sameri MJ, Sarkaki AR, Naghizadeh B, Rafeirad M. 2013. Neuroprotective effects of oral gallic acid against oxidative stress induced by 6-hydroxydopamine in rats. Food Chem. 138:1028–1033.
- Park SJ, Kim DH, Jung JM, Kim JM, Cai M, Liu X, Hong JG, Lee KR, Ryu JH. 2012. The ameliorating effects of stigmasterol on scopolamine-induced memory impairments in mice. Eur J Pharmacol. 676:64–70.
- Pavlica S, Gebhardt R. 2005. Protective effects of ellagic and chlorogenic acids against oxidative stress in PC12 cells. Free Radic Res. 39:1377–1390.
- Rafieirad M, Ghasemzadeh-Dehkordi K. 2014. Effect of ellagic acid on oxidative stress due to brain ischemia/hypoperfusion in male rat. J Herb Drugs. 5:1–6.
- Reddy DS, Kulkarni SK. 1998. Possible role of nitric oxide in the nootropic and antiamnesic effects of neurosteroids on aging-and dizocilpine-induced learning impairment. Brain Res. 799:215–229.
- Reeta KH, Mehla J, Gupta YK. 2009. Curcumin is protective against phenytoin-induced cognitive impairment and oxidative stress in rats. Brain Res. 1301:52–60.
- Sellappan S, Akoh CC, Krewer G. 2002. Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J Agric Food Chem. 50:2432–2438.
- Spencer JPE. 2010. The impact of fruit flavonoids on memory and cognition. Br J Nutr. 104:40–47.
- Thiebot MH. 1985. Some evidence for amnesic-like effects of benzodiazepines in animals. Neurosci Biobehav Rev. 9:95–100.
- Townsend DM, Tew KD. 2003. The role of glutathione-S-transferase in anticancer drug resistance. Oncogene 22:7369–7375.
- Uzar E, Alp H, Cevik MU, Firat U, Evliyaoglu O, Tufek A, Altun Y. 2012. Ellagic acid attenuates oxidative stress on brain and sciatic nerve and improves histopathology of brain in streptozotocin-induced diabetic rats. Neurol Sci. 33:567–574.