Abstract
Context Psidium guajava L. (Myrtaceae) leaves are used in traditional medicines for the treatment of cancer, inflammation and other ailments.
Objective The current study explores scientific validation for this traditional medication.
Materials and methods We used ferric-reducing antioxidant power (FRAP) and 2,2-diphenyl-1-picryl hydrazil (DPPH) assays to estimate antioxidant activity of P. guajava leaf extracts (methanol, hexane and chloroform). Antitumour and in vivo cytotoxic activities were determined using potato disc assay (PDA) and brine shrimp lethality assay, respectively. Three human carcinoma cell lines (KBM5, SCC4 and U266) were incubated with different doses (10–100 μg/mL) of extracts and the anticancer activity was estimated by MTT assay. NF-κB suppressing activity was determined using electrophoretic mobility shift assay (EMSA). Chemical composition of the three extracts was identified by GC-MS. Total phenolic and flavonoid contents were measured by colorimetric assays.
Results and discussions The order of antioxidant activity of three extracts was methanol > chloroform > hexane. The IC50 values ranged from 22.73 to 51.65 μg/mL for KBM5; 22.82 to 70.25 μg/mL for SCC4 and 20.97 to 89.55 μg/mL for U266 cells. The hexane extract exhibited potent antitumour (IC50 value = 65.02 μg/mL) and cytotoxic (LC50 value = 32.18 μg/mL) activities. This extract also completely inhibited the TNF-α induced NF-κB activation in KBM5 cells. GC-MS results showed that pyrogallol, palmitic acid and vitamin E were the major components of methanol, chloroform and hexane extracts. We observed significant (p < 0.05) difference in total phenolic and flavonoid contents of different solvent extracts.
Conclusion The present study demonstrates that P. guajava leaf extracts play a substantial role against cancer and down-modulate inflammatory nuclear factor kB.
Introduction
Medicinal plants have a key role in combating human health issues since the Stone Age. They act as restorative, defensive and supportive agents for human body. The World Health Organization (WHO) reports revealed that 80% of population in Asian and African countries rely on traditional medicines for primary health care necessities (Kim et al. Citation2012). A pivotal role of plants in the health scenario is attributed to bioactive compounds, which could delay or inhibit the inception of degenerative diseases and increase life expectancy (Jagadish et al. Citation2009). Plant-based products exhibit anticancer potential by apoptosis induction, anti-angiogensis, accumulation of p53, suppression of NF-κB activation, cell-cycle arrest, topoisomerase inhibition, preventing the oxidation of DNA, triggering carcinogen-detoxifying system, hindering carcinogen activation (Chainy et al. Citation2000; Ren et al. Citation2003) and many other pathways. Hence, it is imperative to explore novel natural sources for their anticancer efficacy.
Guava [Psidium guajava L. (Myrtaceae)] is a common fruit tree that grows in several regions around the world. It is cultivated in tropical and subtropical climatic zones of Pakistan and its leaves are used for the treatment of high blood pressure, cancer, inflammation, infection, diabetes, cough and other ailments (Sabeen & Ahmad Citation2009). Antimicrobial, antidiarrhoeal and other biological properties of P. guajava are of special interest for pharmaceutical and food industries (Arima & Danno Citation2002; Mazumdar et al. Citation2015).
Even though some earlier studies are reported on the use of P. guajava for the treatment of cancer (Sulain et al. Citation2012; Ryu et al. Citation2012), no such comprehensive study on different solvent extracts of P. guajava leaves native to Pakistan or elsewhere has been conducted against neoplastic disease causing agent (Agrobacterium tumefaciens), Artemia salina nauplii, and the currently investigated cell lines (KBM5, SCC4 and U266). Moreover, chemical composition, antioxidant and anti-inflammatory activities of P. guajava leaf extracts indigenous to sub-continental region have also not been reported earlier. The ultimate aim of this study was to investigate the pharmaceutical potential of this plant.
Materials and methods
Chemicals and reagents
Gallic acid (GA), quercetin, ascorbic acid, 2,2-diphyenyl-1-picrylhydrazyl and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were obtained from Sigma-Aldrich (St. Louis, MO). Methanol, hexane and chloroform used for extraction purpose were purchased from Merck KGaA (Darmstadt, Germany). Doxorubicin was obtained from Pharmedic, Pakistan. Potassium ferricyanide was provided by DAEJUNG Chemicals and Metals, Gyeonggi-do, Korea. Ferric chloride was attained from BDH Laboratory Supplies, England, UK. Folin–Ciocalteu’s phenol reagent, aluminium chloride hexahydrate and sodium carbonate were acquired from Applichem, GmbH, Darmstadt, Germany. Human recombinant TNF-α purified from bacterial cells to homogeneity with a specific activity of 5 × 107 units/mg was provided by Genentech (South San Francisco, CA).
Plant material
The leaves of P. guajava were collected in August 2011 from Chaudhry Wala, Punjab, Pakistan. They were identified by Dr. Mansoor Hameed (Botanist, Department of Botany, University of Agriculture, Faisalabad, Pakistan) by authentic herbarium techniques. A voucher specimen was deposited in Department of Botany, University of Agriculture, Faisalabad, Pakistan.
Preparation of extract
Fresh leaves of P. guajava were rinsed with distilled water to remove dust and any particulate matter. Leaves were spread separately on paper sheets in a well-ventilated room. Dried leaves were ground with the help of food processor (Singer, FP-500, Singer, New Delhi, India) into fine powder. The powder was sieved through seiver (0.25 mm). Sieved powdered material was stored in tightly packed glass jars. Leaf powder (10 g) was extracted with 100 mL methanol on a rotary shaker at 350 rpm for 6 h (Ashraf, Sarfraz, Anwar, et al. Citation2015). Filtration was carried out with the help of Buchner funnel and Whatman No. 1 filter paper. Filtrate was evaporated in vacuum drying oven at 65 °C to dryness. Dried extract was scratched with the help of sterilized spatula and was transferred to extract vials for storage at −4 °C. A similar procedure was repeated with hexane and chloroform extracts. The temperature of vacuum drying oven was adjusted at 70 °C and 62 °C for hexane and chloroform, respectively.
Total phenolic content
Total phenolics were measured by Folin–Ciocalteu process (Slinkard & Singleton Citation1997; Jagadish et al. Citation2009). Briefly, 1 mL of each extract (methanol, hexane and chloroform) solution was mixed with 7.5 mL of double-deionized water, 500 μL of Folin–Ciocalteu reagent and 1 mL of 5% Na2CO3. Mixture was incubated for 90 min at room temperature. The absorbance of reaction mixture was measured at 760 nm by using UV–Vis spectrophotometer (Lambda EZ 201, Perkin Elmer, Waltham, MA). The phenolic contents were expressed as μg gallic acid equivalents (GAEs) per mg of extract.
Total flavonoid content
Total flavonoids were determined by the AlCl3 method (Ashraf, Sarfraz, Mahmood, et al. Citation2015). Each extract (methanol, hexane and chloroform) solution (2 mL) was mixed with 2 mL of aqueous AlCl3.6H2O (0.1 mol/L). The mixture was incubated at room temperature for 10 min and the absorbance was measured with UV–Vis spectrophotometer at 417 nm. Total flavonoid contents were expressed as μg quercetin equivalents (QEs).
Ferric-reducing antioxidant power (FRAP) assay
FRAP assay (Chan et al. Citation2007) assessed reducing capacities of methanol, hexane and chloroform extracts of P. guajava leaves. Different dilutions (25–800 μg/mL) of each plant extract (methanol, hexane and chloroform) were added to 2.5 mL of phosphate buffer and 2.5 mL potassium ferricyanide (1% W/V). The mixture was kept in an incubator at 50 °C for 25 min followed by addition of trichloro-acetic acid solution (2.5 mL) to stop the reaction. Then 2.5 mL of this mixture was added to 2.5 mL of water and 500 μL of ferric chloride solution. The absorbance was measured at 700 nm after 30 min.
Free radical (2,2-diphenyl-1-picryl hydrazil, DPPH) scavenging assay
DPPH free radical scavenging activity of the subject extracts was determined according to a previous method (Ashraf, Sarfraz, Mahmood, et al. Citation2015). The reaction was carried out in capped glass test tubes that were tightly wrapped with aluminium foil from top to bottom. The DPPH radical stock solution used was freshly prepared. Briefly, methanol solution (4 mL) of DPPH (0.1 mM) was mixed with 1 mL of each of three extracts (methanol, chloroform and hexane) solutions and the reaction mixture was then placed in darkness for 30 min. The absorbance was taken at 515 nm with UV–Vis spectrophotometer (Lambda EZ 201, Perkin Elmer, Waltham, MA). The inhibition percentage of DPPH radicals was calculated as the following:
where Ac is the absorbance of control (reaction mixture excluding the plant extract) and As is the absorbance of sample (plant extract).
Antitumour activity
Antitumour potential of methanol, chloroform and hexane extracts of P. guajava was evaluated by potato disc assay (PDA) (McLaughlin et al. Citation1998). The growth medium was prepared by adding nutrient broth distilled water and then autoclaved. Agrobacterium tumefaciens from storage culture was inoculated in growth medium using aseptic techniques. The culture was vigorously shaken and placed in shaker for 48 h at 28 °C. Red-skinned potatoes were surface sterilized in 10% sodium hypochlorite for 20 min and extensively washed with distilled water and cut into 4 mm thick discs of 8 mm diameter with the help of sterile cork and surgical blades sterilized by gamma irradiation (2.5 M Rads). Agar was prepared by dissolving agar powder in autoclaved-distilled water, poured into sterilized Petri plates and allowed to solidify in laminar airflow. Potato discs (8) were placed on each agar plate with the help of sterilized forceps (Rolzem International, Punjab, Pakistan). Inoculums (50 μL) were placed on the surface of each disc. Plates were wrapped with para film and incubated at 27 °C for 21 d. After 21 d, discs were stained with Lugol solution (10% KI + 5% I2) for 20 min and tumours were counted on each disc by using a dissecting microscope. Inhibition was calculated by the formula of Kanwal et al. (Citation2011).
In vivo cytotoxic activity
The brine shrimp cytotoxicity assay was performed according to the previously described method (Meyer et al. Citation1982). Brine shrimp (A. salina) eggs were hatched in a rectangular tank (19.5 cm length × 19.5 cm width × 20.3 cm height) containing 1 L of artificial sea water (a mixture of commercial salt and distilled water) and subjected to continuous gentle aeration. Brine shrimp eggs were spread in the dark part of container. Mature A. salina nauplii (48 h aged) were collected from other half of container that was exposed to continuous illumination (60 W). In bioassay, different dilutions of each extract (0.5 mL) were taken in graduated vial and subjected to evaporation for overnight. Shrimp were added to each vial and the volume was made up to 5 mL with the help of seawater. Vials were kept at 25 °C under illumination for 24 h. After 24 h, the number of live A. salina nauplii was counted with the help of 5 × magnifying glass. LC50 values were calculated by linear regression analysis (Patil & Magdum Citation2011).
Anticancer activity
Cell lines
Anticancer assays were performed by using KBM5 (human chronic myelogenous leukaemia), SCC4 (human tongue squamous carcinoma cells) and U266 (human multiple myeloma cells). KBM5 and U266 cells were maintained in RPMI-1640, whereas DMEM (Dulbeco’s modification of Eagle’s medium) was used for sustaining SCC4 cells. Both the media were supplemented with 10% foetal bovine serum, and antibiotic. Cultures were maintained in 75 cm2 flasks in a humidified (95% air) incubator at 37 °C with 5% CO2.
In vitro anticancer assay
Anticancer assay was performed as described previously (Prasad et al. Citation2010). Briefly, 5000 cells of KBM5, SCC4 and U266 were seeded individually in Biolite 96-well plates and were incubated (95% humidified air 5% CO2 at 37 °C) with different doses (10, 25, 50 and 100 μg/mL) of methanol, hexane and chloroform extracts of P. guajava for 72 h. Then 20 μL of 5 mg/mL MTT was added to each well. After 2 h incubation, each well was supplemented with 100 μL of lysis buffer. Cells were further incubated for 5 h and optical density values were measured at 570 nm by ELISA.
Assessment of anti-inflammatory potential; electrophoretic mobility shift assay (EMSA)
To assess the anti-inflammatory potential of hexane extract of P. guajava leaves, we determined the NF-κB activation in cancer cells pretreated with hexane extract. We isolated nuclei from treated-, untreated- and induced-cells and performed EMSA as described previously (Chainy et al. Citation2000). In brief, nuclear extracts prepared from cancer cells were incubated with 32P end-labelled 45-mer double-stranded NF-κB oligonucleotide (15 μg of protein with 16 fmol of DNA) from the HIV long-terminal repeat (5′-TTGTTACAAGGGACTTTC CGCTG GGGACTTTC CAGGGA GGCGT GG-3′, with NF-κB-binding sites) for 30 min at 37 °C. The resulting protein–DNA complex was separated from free oligonucleotides on 6.6% native polyacrylamide gels. The dried gels were visualized by Phosphor-Imager imaging device (Molecular Dynamics, Sunnyvale, CA), and radioactive bands were quantified using Image Quant software (General Electric Company – GE Japan Corporation, Tokyo, Japan).
Gas chromatography-mass spectrometry (GC-MS) analysis and identification of compounds
The GC/MS analysis of methanol, chloroform and hexane extracts of P. guajava leaves was performed using GC-MS (Agilent Technologies, Willmington, DE) equipped with HP-5MS capillary column (30 m × 0.25 mm, 0.25 μm). The GC-MS had an electron energy of 70 Ev, and an ion source temperature of 230 °C. Helium was used as a carrier gas at a flow rate of 1.5 mL/min. The injector and the interface temperature were set at 280 °C and 350 °C, respectively. The oven temperature was programmed as 45 °C (5 min) to 325 °C (10 min), at an increasing rate of 15 °C/min. Compounds were identified on the basis of comparison of their relative retention time and mass spectra with those of the NIST library data of GC/MS system.
Statistical analysis
Minitab software version 16 (Minitab Inc., State College, PA) was applied to perform analysis of variance (ANOVA) and to determine significant differences (p < 0.05).
Results and discussion
Total phenolic and flavonoid contents
Phenolic and flavonoid compounds act as antioxidants by scavenging free radicals, chelating metal ions, donating hydrogen and quenching singlet oxygen (Ashraf, Sarfraz, Anwar, et al. Citation2015). In addition, they play a major role against cancer, microbial infections and vast range of degenerative ailments (Jagadish et al. Citation2009). So while investigating pharmaceutical activities of P. guajava leaves, it was imperative to explore the extent of these components.
Total phenolic and flavonoid contents (TPC and TFC) of three extracts determined by Folin-Ciocalteu and aluminium chloride methods were represented as μg gallic acid equivalent/mg of plant extract and μg quercetin equivalents/mg of plant extract, respectively. Significant differences (p < 0.05) were observed among TPC and TFC of three solvent extracts. The results showed that the extraction rate of TPC (μg GAE/mg of plant extract) with three solvents was as methanol (83.34 ± 0.49) > chloroform (71.49 ± 0.48) > hexane (53.24 ± 2.05), while the order of solvents in the case of TFC (μg QE/mg of plant extract) was methanol (53.39 ± 0.89) > chloroform (32.76 ± 1.15) > hexane (21.26 ± 1.49). Difference in the amount of phenolics and flavonoids in different solvents might be due to the difference in the chemical nature of solvent (Bae et al. Citation2012). The total phenolic and flavonoid contents of different P. guajava leaf extracts determined in the current study were noted to be higher than that seen for ethanol extract of P. guajava stem bark (Aminu et al. Citation2012). However, in contrast to our study, Qian and Nihorimbere (Citation2004) investigated remarkably higher extent of total phenolic contents in polar extracts of P. guajava leaves. Such variations in total phenolic contents within the leaves of P. guajava might be linked to variation in agro-climatic conditions, maturity at harvest as well as difference in extraction technique and polarity of extracting solvent.
The results showed that three extracts shared the similar ranking (methanol > chloroform > hexane) for Folin–Ciocalteu and aluminium chloride colorimetric assays. These findings are consistent with Yeboah and Majinda (Citation2009), who observed identical hierarchy of extracting solvents (methanol > chloroform > hexane) for the extraction of total phenolic and total flavonoid contents.
Antioxidant activity
In vitro antioxidant assays are used to imitate the oxidation–reduction reactions commonly occurring in biological systems. Numerous techniques are available to estimate the antioxidant potential of plant extracts or pure chemical compounds, however, due to complex reactive facets of plant extracts; a single procedure cannot elucidate all the possible mechanisms to characterize an antioxidant. Therefore, to establish authenticity, in the present study, two robust in vitro antioxidant assays were used.
The FRAP assay is based on the fact that donation of electron from an antioxidant substance reduces Fe +3 to Fe +2 (Benzie & Strain Citation1996). As the antioxidant activity of an extract is directly linked to its reducing ability, so the FRAP assay is trustworthy method to evaluate antioxidant activity of extracts.
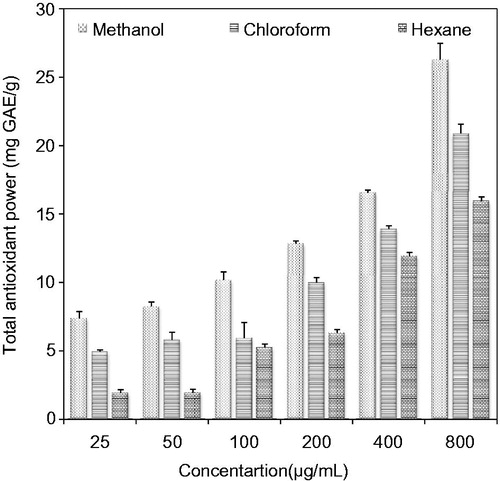
The results () showed that total antioxidant activity of understudy extracts followed the same order (methanol > chloroform > hexane) as was examined for the determination of total phenolic and total flavonoid contents. This observation is in accord with Jagadish et al. (Citation2009), who showed a strong correlation between bioactive components and antioxidant activity. As compared with a study by Ahmed et al. (Citation2013) on P. guajava fruit, we observed higher FRAP values for understudy extracts at the highest tested dose (800 μg/mL). It is interesting to note that another research group (Jimenez-Escriq et al. Citation2001) reported reasonably higher FRAP of P. guajava as compared with our findings. Overall significant differences (p < 0.05) were noticed among total antioxidant activities of three evaluated extracts.
The DPPH assay was also used to determine antioxidant potential of P. guajava leaf extracts (methanol, chloroform and hexane) and pure compounds (such as gallic acid and ascorbic acid). This assay is aimed at measuring the capacity of plant extracts to scavenge purple coloured DPPH (by donating hydrogen atom or electron) and converting it to yellow coloured diphenylpicrylhydrazin (Saha et al. Citation2008). The results () of this assay showed that methanol extract has maximum free radical scavenging activity with an IC50 value of 89.82 μg/mL. Due to the major role of polyphenolic compounds in antioxidant efficacy, high free radical (DPPH) scavenging activity of methanol extract may be a consequence of higher level of polyphenolic (total phenolics and total flavonoids) compounds in it. Moderate free radical (DPPH) scavenging activity was examined in the chloroform extract with an IC50 value of 211.1 μg/mL. Hexane extract (IC50 value = 426.8 μg/mL) exhibited poor scavenging of free radicals. Our results are in accord with Yusri et al. (Citation2012), who examined hexane extract of a Malaysian medicinal plant as poor candidate for scavenging stable free radicals.
Table 1. Free radical (DPPH) scavenging, antitumour, in vivo cytotoxic and in vitro anticancer activities of P. guajava leaf extracts (methanol, chloroform and hexane).
Antitumour activity
PDA is inexpensive, rapid, statistically reliable, animal-sparing and safer technique for screening of antitumour agents (Oran Citation1999; Mazid et al. Citation2011). In the current study, PDA was used to assess potency of different solvent extracts of P. guajava leaves to induce death of neoplastic disease-causing agent (Agrobacterium tumefaciens) in plants. The results of antitumour activity in revealed that all the extracts inhibited viability of A. tumefaciens to varying degrees. Data () exhibited that hexane extract of P. guajava showed higher antitumour activity (IC50 value = 65.02 μg/mL) being the more potent against viability of A. tumefaciens strain. Chloroform extract revealed as the second most active antitumour agent by exhibiting an IC50 value of 160.7 μg/mL. Poor antitumour activity was observed for methanol extract with higher value (337.4 μg/mL) of IC50 value. Overall, hexane extract exhibited significantly (p < 0.05) higher antitumour activity as compared with chloroform and methanol extracts, respectively. Variance in antitumour activity of different extracts might be attributed towards the nature of extractant as well as the availability of chemical compounds extracted (Sahreen et al. Citation2013).
In vivo cytotoxic activity
The in vivo cytotoxic effects of P. guajava leaf extracts (methanol, chloroform and hexane) were evaluated against A. salina nauplii using brine shrimp lethality assay. Artemia salina nauplii are aquatic crustaceans, described by 11 pairs of legs, three eyes, haemoglobin in the blood, but they lack the advance nervous system. Brine shrimp lethality assay is widely used for the assessment of cytotoxic potential of plant extracts (Apu et al. Citation2010; Ullah et al. Citation2013). The results () demonstrate sensitivity of different P. guajava leaf extracts against A. salina naupli. The in vivo cytotoxic effects of methanol, chloroform and hexane extracts of P. guajava leaves were significantly (p < 0.05) different from each other with regard to extraction solvent. Among the different extracts, the hexane extract of P. guajava exhibited significant cytotoxic activity with an LC50 value of 32.18 μg/mL, which was comparable with that of commercial drug, cyclophosphamide (LC50 value = 4.35 μg/mL). Comparison of obtained LC50 data revealed a potent cytotoxic activity for the chloroform extract (LC50 value = 41.05 μg/mL) as compared with methanol extract (LC50 value = 63.81 μg/mL). Our results are consistent with a previous study (Nasrin et al. Citation2012) on Lablab purpureus (L.) leaves, where in, maximum cytotoxic activity against A. salina nauplii was examined for hexane extract followed by the chloroform extract. However, in contrast to our findings, Fasola et al. (Citation2011) reported excellent cytotoxic activity of P. guajava essential oil against A. salina nauplii.
In vitro anticancer activity
Anticancer activity of methanol, hexane and chloroform extracts of P. guajava against three different human cancer cell lines (KBM5, chronic myeloid leukaemia; SCC4, tongue squamous carcinoma; and U266, multiple myeloma) was evaluated by MTT assay. In this assay, mitochondrial dehydrogenase enzyme in metabolically active cells converts yellow tetrazolium salt to dark blue formazan and the intensity of blue colour predicts cell viability (Mena-Rejon et al. Citation2009). The three extracts showed significant cytotoxicity against the investigated (KBM5, SCC4 and U266) cell lines, as represented in and . It was observed that growth of cancer cells declined with an increase in dose of extracts from 10 to 100 μg/mL. A similar relation between dose of plant extract and cell viability was reported by Nisa et al. (Citation2011). Hexane extract exhibited maximum decrease in cell viability (%) with IC50 values of 22.73, 22.82 and 20.97 μg/mL for KBM5, SCC4 and U266 cells, respectively. Maximum cytotoxic activity of hexane extract might be attributed to the presence of tetracosane, α-copaene, γ-sitosterol, vitamin E and squalene in it, as shown in . These vital bioactive components exhibit anticancer potential by different mechanisms including suppression of signalling pathways, apoptosis induction and cell-cycle arrest (Ryu et al. Citation2012; Sundarraj et al. Citation2012). Jurasz et al. (Citation2004) showed that tumour cell-induced aggregation of platelets is necessary for the survival of tumour cells and its successful metastasis. α-Bulnesene present in hexane extract inhibits platelet aggregation (Hsu et al. Citation2006). Squalene is known for its chemo-preventive effects (Smith Citation2000).
Table 2. Chemical components of methanol, chloroform and hexane extracts of P. guajava analyzed by gas chromatography mass spectroscopy (GC-MS).
IC50 values lower than 30 μg/mL enabled the hexane extract to fulfill criteria of American National Cancer Institute, to attain attention for purification (Suffness & Pezzuto Citation1990). Chloroform extract exhibited maximum cytotoxic activity against U266 cells with an IC50 value of 26.72 μg/mL. Anticancer activity of chloroform extract might be due to a significant amount of palmitic acid (30.98%). Harada et al. (Citation2002) reported the apoptotic effect of palmitic acid in human leukaemic cells. Palmitic acid is also known for DNA topoisomerase I inhibition in human lung adenocarcinoma epithelial cells (Karna et al. Citation2012). IC50 values of methanol extract () are in comparison with previously reported values for methanol extract of P. guajava from Malaysia (Sulain et al. Citation2012).
Psidium guajava hexane extract suppresses NF-κB activation in mylegenous leukaemia cells
NF-κB is ubiquitous protein that controls various signalling pathways in different types of cancer (Baud & Karin Citation2009). Chainy et al. (Citation2000) stated that cancer patients have higher levels of proinflammatory cytokines and activated NF-κB. Mounting evidence has indicated that NF-κB-regulated genes are associated with cellular transformation, proliferation, angiogensis, invasion, tumour cell survival and metastasis. Thus, suppression of NF-κB pathway will play a distinctive role in cancer therapy.
We determined in the previous experiment that the three extracts (methanol, hexane and chloroform) of P. guajava had anticancer potential against KBM5 cells, while hexane extract was the top one among the three. In this experiment, we investigated whether P. guajava hexane extract can exhibit anti-inflammation and modulate the NF-κB activation. Our results indicated that TNF-α induced the NF-κB activation, whereas P. guajava hexane extract alone has no effect. The pretreatment of KBM5 cells with this extract suppressed TNF-α-induced NF-κB activation in a dose-dependent manner (). TNF-α-induced NF-κB activation was completely inhibited at 25 μg/mL dose of P. guajava hexane extract. Treatment with hexane extract under these conditions has little effect on cell viability.
Figure 2. Anticancer activity of (A) methanol, (B) chloroform and (C) hexane extracts of P. guajava leaves against KBM5, SCC4 and U266 cell lines. Data are represented as mean ± SD. Mean with different small letters in the same cell line indicate significant (p < 0.05) difference with respect to concentrations tested. Mean with different capital letters at the same concentration indicate significant (p < 0.05) difference with respect to cell line tested. Each cell line (KBM5, SCC4 and U266) was incubated with different doses (10, 25, 50 and 100 μg/mL) of each extract (methanol, chloroform and hexane).
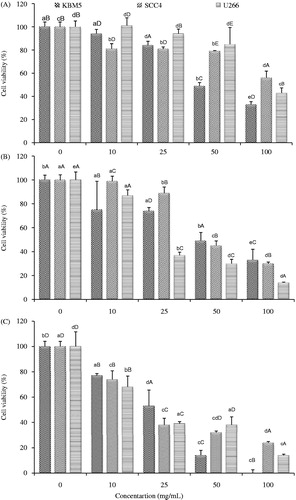
Figure 3. Inhibition of TNF-α induced NF-κB activation by hexane extract of P. guajava leaves. KBM-5 cells were pre incubated with different doses (10, 25 and 50 μg/mL) of hexane extract of P. guajava leaves for 12 h; treated with 0.1 nM TNF-α and subjected to EMSA for NF-κB activation.
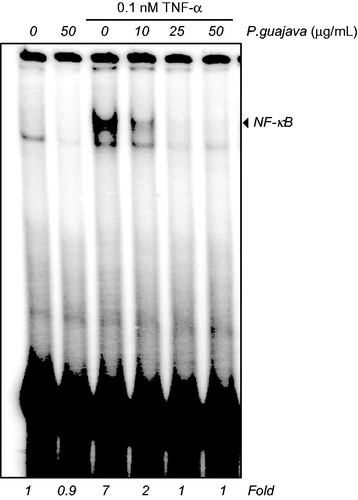
This is the first report to suggest that P. guajava hexane extract can down-modulate the NF-κB activation induced by proinflammatory cytokine (TNF-α) in human mylegenous leukaemia (KBM5) cells. NF-κB suppressing activity of hexane extract of P. guajava might be attributed to innovative spectrum of bioactive components in it (). Vitamin E (30.70%), which was a potent component of hexane extract, plays a significant role against cancer (Constantinou et al. Citation2008). Tahan et al. (Citation2011) reported that vitamin E is potential candidate against inflammation. A significant amount of vitamin E () in hexane extract might be responsible for the inhibition of NF-κB activation (Calfee-Mason et al. Citation2002). Caryophyllene (known as β-caryophyllene) present in significant amount (8.43%) in hexane extract exhibits anti-inflammatory efficacy by inhibiting lipopolysaccharide-stimulated proinflammatory cytokines (TNF-α and IL-1β) in peripheral blood (Gertsch et al. Citation2008).
More than 80% drugs derived from natural products are serving mankind for thousands of years (Kim et al. Citation2012). Garcinol, anethole and many other plant-derived components inhibit NF-κB activity and modulate inflammatory pathways (Chainy et al. Citation2000; Kim et al. Citation2012). These results indicate the requirement of isolation and purification of the major compounds of P. guajava hexane extract.
Identification of anticancer compounds by GC-MS
presents the chemical composition of methanol and hexane extracts. Pyrogallol (35.18%), vitamin E (30.70%) and palmitic acid (30.73%) were the major chemical constituents of methanol, hexane and chloroform extracts, respectively. Pyrogallol has a wide range of pharmacological properties, including inhibition of proinflammatory cytokines (Nicolis et al. Citation2008) and vitamin E is known for its therapeutic potential (Aggarwal et al. Citation2010). Levels of palmitic acid (n-hexadecanoic acid) in methanol and chloroform extracts of P. guajava leaves are higher than the previously reported data for P. guajava essential oil (Khadhri et al. Citation2014).
Other main components in three extracts were caryophyllene oxide, copaene, alloaromadendrene, caryophyllene, sitosterol, α-bulnesene, α-copaene and squalene. The presence of α-copaene in aqueous essence of P. guajava has been reported previously (Jordan et al. Citation2003).
Caryophyllene oxide is used as a preservative in cosmetics, foods and drugs. Antifungal potential of caryophyllene oxide has also been documented (Yang et al. Citation1999). In comparison to our findings, Li et al. (Citation1999) reported a comparatively higher level of copaene and caryophyllene in essential oil from P. guajava leaves. Our results were found different from previously reported results for hexane extract of P. guajava from India (Nisha et al. Citation2011). The current reported difference in phytochemical profile may be attributed to change in variety, climatic condition, genotype and harvesting time. Analysis of methanol, chloroform and hexane extracts of P. guajava leaves by rapid and reliable technique (GC-MS) led to the identification of bioactive components with therapeutic background.
Conclusion
It was concluded that, among all the extracts, the methanol extract of P. guajava leaves exhibited the maximum amount of polyphenolic (total phenolic and total flavonoids) compounds along with the excellent antioxidant activities as revealed by FRAP and DPPH assays. However, the polyphenolic compounds are widely known for anticarcinogenic attributes, but in our study, methanol extract rich in polyphenolic compounds exhibited the poor antitumour, in vivo cytotoxic and in vitro anticancer activities. Excellent antitumour, in vivo cytotoxic and in vitro anticancer activities were observed in hexane extract that might be attributed to the innovative profile of other bioactive components in it as explored by GC-MS study. Our results suggested that chemical nature of the extracting solvent has strong influence on biological attributes. The results of current study would certainly help to ascertain the potency of P. guajava leaves for potential use in neutraceuticals.
Funding information
The authors gratefully acknowledge the Higher Education Commission of Pakistan for financial support.
Acknowledgements
The authors gratefully acknowledge Central Hi-Tech Lab. Staff, Dr. Mazhar Iqbal, National Institute for Biotechnology and Genetic Engineering (NIBGE), Faisalabad, Pakistan and Dr. Bharat B. Aggarwal, Amit Kumar Tyagi and Sahdeo Prasad, Cytokine Research Laboratory, The University of Texas, MD. Anderson Cancer Center, Houston, TX, for their technical and logistic support to accomplish this work.
Disclosure statement
All the authors have no conflict of interest.
References
- Aggarwal BB, Sudaram C, Prasad S, Kannappan R. 2010. Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem Pharmacol. 80:1613–1631.
- Ahmed O, Libsu S, Moges D. 2013. A study of antioxidant activities of guava (Psidium guajava) and mango (Mangifera indica) fruits. Intern J IntegrSci Innov Technol. 2:1–5.
- Aminu M, Bello MS, Abbas O, Aliyu M, Malam BS, Auwalu G, Hafsat, Muhammad A, Shafiu M, Hussaina NN, et al. 2012. Comparative in vitro antioxidant studies of ethanolic extracts of Psidium guajava stem bark and Telfairia occidentalis leaf. Internat J Moder Biochem. 1:18–26.
- Apu AS, Muhit MA, Tareq SM, Pathan AH, Jamaluddin ATM, Ahmed M. 2010. Antimicrobial activity and brine shrimp lethality bioassay of the leaves extract of Dillenia indica Linn. J Young Pharm. 2:50–53.
- Arima H, Danno G. 2002. Isolation of antimicrobial compounds from Guava (Psidium guajava L.) and their structural elucidation. Biosci Biotechnol Biochem. 66:1727–1730.
- Ashraf A, Sarfraz RA, Anwar F, Shahid SA, Alkharfy KM. 2015. Chemical composition and biological activties of leaves of Ziziphus mauritiana L. native to Pakistan. Pak J Bot. 47:367–376.
- Ashraf A, Sarfraz RA, Mahmood A, Din MU. 2015. Chemical composition and in vitro antioxidant and antitumor activities of Eucalyptus camaldulensis Dehn. leaves. Indus Crop Prod. 74:241–248.
- Bae H, Jayaprakasha GK, Crosby K, Jifon JL, Patil BS. 2012. Influence of extraction solvents on antioxidant activity and the content of bioactive compounds in non-pungent peppers. Plant Foods Hum Nutr. 67:120–128.
- Baud V, Karin M. 2009. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 8:33–40.
- Benzie IF, Strain JJ. 1996. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 239:70–76.
- Calfee-Mason KG, Spear BT, Glauert HP. 2002. Vitamin E inhibits hepatic NF-kappaB activation in rats administered the hepatic tumor promoter, phenobarbital. J Nutr. 132:3178–3185.
- Chainy GB, Manna SK, Chaturvedi MM, Aggarwal BB. 2000. Anethole blocks both early and late cellular responses transduced by tumor necrosis factor: effect on NF-kappaB, AP-1, JNK, MAPKK and apoptosis. Oncogene 19:2943–2950.
- Chan EWC, Lim YY, Omar M. 2007. Antioxidant and antibacterial activity of leaves of Etlingera species (Zingerberaceae) in Peninsular Malaysia. Food Chem. 104:1586–1593.
- Constantinou C, Papas A, Constantinou AI. 2008. Vitamin E and cancer: an insight into the anticancer activities of vitamin E isomers and analogs. Int J Cancer. 123:739–752.
- Fasola TR, Oloyede GK, Aponjolosum BS. 2011. Chemical composition, toxicity and antioxidant activities of essential oils of stem bark of Nigerian species of guava (Psidium guajava Linn.). Excli J 10:34–43.
- Gertsch J, Leonti M, Raduner S, Racz I, Chen JZ, Xie XQ, Altmann KH, Karsak M, Zimmer A. 2008. Beta-Caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci USA. 105:9099–9104.
- Harada H, Yamashita U, Kurihara H, Fukushi E, Kawabata J, Kamei Y. 2002. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Res. 22:2587–2590.
- Hsu HC, Yang WC, Tsai WJ, Chen CC, Huang HY, Tsai YC. 2006. Alpha-Bulnesene, a novel PAF receptor antagonist isolated from Pogostemon cablin. Biochem Biophys Res Commun. 345:1033–1038.
- Jagadish LK, Krishnan VV, Shenbhagaraman R, Kaviyarasan V. 2009. Comparative study on the antioxidant, anticancer and antimicrobial property of Agaricus bisporus Imbach before and after boiling. Afr J Biotechnol. 8:654–661.
- Jimenez-Escriq A, Rincon M, Pulido R, Saura-Calixto F. 2001. Guava fruit (Psidium guajava L.) as a new source of antioxidant dietary fiber. J Agric Food Chem. 49:5489–5493.
- Jordan M, Margaria CA, Shaw PE, Goodner KL. 2003. Volatile components and aroma active compounds in aqueous essence and fresh pink guava fruit puree (Psidium guajava L.) by GC-MS and multidimensional GC/GC-O. J Agric Food Chem. 51:1421–1426.
- Jurasz P, Alonso-Escolano D, Radomski MW. 2004. Platelet-cancer interactions: mechanisms and pharmacology of tumour cell-induced platelet aggregation. Br J Pharmacol. 143:819–826.
- Kanwal S, Ullah N, Haq IL, Afzal I, Mirza B. 2011. Antioxidant, antitumor activities and phytochemical investigation of Hedera nepalensis K. Koch, an important medicinal plant from Pakistan. Pak J Bot. 43:85–89.
- Karna S, Lim WB, Kim JS, Kim SW, Zheng H, Bae KH, Cho MS, Oh HK, Kim OS, Choi HR, et al. 2012. C16 saturated fatty acid induced autophagy in A549 cells through topoisomerase I inhibition. Food Nutr Sci. 3:1220–1227.
- Khadhri A, Mokni RE, Almeida C, Nogueira JMF, Eduarda M, Araujo M. 2014. Chemical composition of essential oil of Psidium guajava L. growing in Tunisia. Ind Crop Prod. 52:29–31.
- Kim JH, Gupta SC, Park B, Yadav VR, Aggarwal BB. 2012. Turmeric (Curcuma longa) inhibits inflammatory nuclear factor (NF)-κB and NF-κB-regulated gene products and induces death receptors leading to suppressed proliferation, induced chemo sensitization, and suppressed osteoclastogenesis. Mol Nutr Food Res. 56:454–465.
- Li J, Chen F, Luo J. 1999. GC-MS analysis of essential oil from the leaves of Psidium guajava. Zhong Yao Cai. 22:78–80.
- Mazid MA, Nahar L, Datta BK, Bashar SAMK, Sarker SD. 2011. Potential antitumor activity of two Polygnum species. Arch Biol Sci Belgrade 63:465–468.
- Mazumdar S, Akter R, Talukder D. 2015. Antidiabetic and antidiarrhoeal effects on ethanolic extracts of Psidium guajava (L.) Bat leaves in Wister rats. Asia Pacif J Trop Biomed. 5:10–14.
- Mclaughlin JL, Rogers LL, Anderson JE. 1998. The use of biological assays to evaluate botanicals. Drug Info J. 32:513–524.
- Mena-Rejon G, Caamal-Fuentesa E, Cantillo-Ciaub Z, et al. 2009. In vitro cytotoxic activity of nine plants used in Mayan traditional medicine. J Ethnopharmacol. 121:462–465.
- Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, Mclaughlin JL. 1982. Brine shrimp; a convenient general bioassay for active plant constituents. Planta Med. 45:31–34.
- Nasrin F, Bulbul IJ, Begum Y, Khanum S. 2012. In vitro antimicrobial and cytotoxicity screening of n-hexane, chloroform and ethyl acetate extracts of Lablab purpureus (L.) leaves. Agric Biol J N Am. 3:43–48.
- Nicolis E, Lampronti I, Dechecchi MC, Borgatti M, Tamanini A, Bianchi N, Bezzerri V, Mancini I, Giri MG, Rizzotti P, et al. 2008. Pyrogallol, an active compound from the medicinal plant Emblica officinalis, regulates expression of pro-inflammatory genes in bronchial epithelial cells. Int Immunopharmacol. 10:1672–1680.
- Nisa S, Bibi Y, Waheed A, Zia M, Sarwar S, Ahmed S, Chaudhary MF. 2011. Evaluation of anticancer activity of Debregeasia salicifoliae extract against estrogen receptor positive cell line. Afr J Biotechnol. 10:990–995.
- Nisha K, Darshana M, Madhu G, Bhupendra MK. 2011. GC-MS analysis and anti-microbial activity of Psidium guajava (leaves) grown in Malva region of India. Int J Drug Dev Res. 3:237–245.
- Oran SA. 1999. Potato disc bioassay for some Jordanian medicinal plants. Pharm Biol. 37:296–299.
- Patil SB, Magdum CS. 2011. Determination of LC50 values of extracts of Euphorbia hirta Linn and Euphorbia nerifolia Linn using brine shrimp lethality assay. Asian J Res Pharm. 1:42–43.
- Prasad S, Ravindran J, Sung B, Pandey MK, Aggarwal BB. 2010. Garcinol potentiates TRAIL-induced apoptosis through modulation of death receptors and antiapoptotic proteins. Mol Cancer Ther. 9:856–868.
- Qian H, Nihorimbere V. 2004. Antioxidant power of phytochemicals from Psidium guajava leaf. J Zhejiang Univ Sci. 5:676–683.
- Ren Y, Xiong L, Wu JR. 2003. Induction of mitochondrion-mediated apoptosis of CHO cells by tripchlorolide. Cell Res. 13:295–300.
- Ryu NH, Park KR, Kim SM, et al. 2012. A hexane fraction of guava leaves (Psidium guajava L.) induces anticancer activity by suppressing AKT/mammalian target of rapamycin/ribosomal p70 S6 kinase in human prostate cancer cells. J Med Food. 15:231–241.
- Sabeen M, Ahmad SS. 2009. Exploring the folk medicinal flora of Abbotabad city, Pakistan. Ethnobot Leafl. 13:810–833.
- Saha MR, Hasan SMR, Akter R, Hossain MM, Alam MS, Alam MA, Mazumder MEH. 2008. In vitro free radical scavenging activity of methanol extract of leaves of Mimusops elengi Linn. Bangladesh J Vet Med. 6:197–202.
- Sahreen S, Khan MA, Khan RA, Shah NA. 2013. Estimation of flavoniods, antimicrobial, antitumor and anticancer activity of Carissa opaca fruits. BMC Complement Altern Med. 13:372.
- Slinkard K, Singleton VL. 1997. Total phenol analysis: automation and comparison with manual methods. Am J Enol Vitic. 28:49–55.
- Smith TJ. 2000. Squalene: potential chemopreventive agent. Expert Opin Investig Drugs. 9:1841–1848.
- Suffness M, Pezzuto JM. 1990. Assays related to cancer drug discovery. In: Hostettmann K, editor. Methods in plant biochemistry: assays for bioactivity, 6. London: Academic Press. p. 71–133.
- Sulain MD, Zalzali KE, Ahmad N. 2012. Screening on antiproliferative activity of Psidium guajava leaves extract towards selected cell lines. J US-China Med Sci. 9:30–37.
- Sundarraj S, Thangam R, Sreevani V, Kaveri K, Gunasekaran P, Achiraman S, Kannan S. 2012. γ-Sitosterol from Acacia nilotica L. induces G2/M cell cycle arrest and apoptosis through c-Myc suppression in MCF-7 and A549 cells. J Ethnopharmacol. 14:803–809.
- Tahan G, Aytac E, Aytekin H, Gunduz F, Dogusoy G, Aydin S, Tahan V, Uzun H. 2011. Vitamin E has a dual effect of anti-inflammatory and antioxidant activities in acetic acid-induced ulcerative colitis in rats. Can J Surg. 54:333–338.
- Ullah MO, Haque M, Urmi KF, Zulfiker AHM, Anita ES, Begum M, Hamid K. 2013. Antibacterial activity and brine shrimp lethality bioassay of methanolic extracts of fourteen different edible vegetables from Bangladesh. Asian Pac J Trop Biomed. 3:1–7.
- Yang D, Michel L, Chaumont JP, Millet-Clerc J. 1999. Use of caryophyllene oxide as an antifungal agent in an in vitro experimental model of onychomycosis. Mycopathologia 148:79–82.
- Yeboah EM, Majinda RR. 2009. Radical scavenging activity and total phenolic content of extracts of the root bark of Osyris lanceolata. Nat Prod Commun. 4:89–94.
- Yusri NM, Chan KW, Iqbal S, Ismail M. 2012. Phenolic content and antioxidant activity of Hibiscus cannabinus L. seed extracts after sequential solvent extraction. Molecule 17:12612–12621.