Abstract
Content Our team has identified Labrador tea [Rhododendron groenlandicum L. (Ericaceae)] as a potential antidiabetic plant from the traditional pharmacopoeia of the Eastern James Bay Cree. In a previous in vivo study, the plant extract was tested in a high-fat diet (HFD)-induced obese model using C57BL/6 mice and it improved glycaemia, insulinaemia and glucose tolerance.
Objective In the present study, we assessed the plant’s potential renoprotective effects.
Materials and methods Rhododendron groenlandicum was administered at 250 mg/kg/d to mice fed HFD for 8 weeks to induce obesity and mild diabetes. Histological (periodic acid–Schiff (PAS), Masson and Oil Red O staining), immunohistochemical (IHC) and biochemical parameters were assessed to evaluate the renoprotective potential of R. groenlandicum treatment for an additional 8 weeks.
Results Microalbuminuria and renal fibrosis were developed in HFD-fed mice. Meanwhile, there was a tendency for R. groenlandicum to improve microalbuminuria, with the values of albumin-creatinine ratio (ACR) reducing from 0.69 to 0.53. Renal fibrosis value was originally 4.85 arbitrary units (AU) in HFD-fed mice, dropped to 3.27 AU after receiving R. groenlandicum treatment. Rhododendron groenlandicum reduced renal steatosis by nearly one-half, whereas the expression of Bcl-2-modifying factor (BMF) diminished from 13.96 AU to 9.43 AU.
Discussion and conclusions Taken altogether, the results suggest that R. groenlandicum treatment can improve renal function impaired by HFD.
Keywords:
Introduction
Diabetes mellitus (DM) is a group of metabolic diseases in which a person has high blood glucose. Several symptoms are associated with diabetes, including hyperglycaemia, polyuria, polydipsia and hyperphagia (Coffman et al. Citation2012). Diabetes is due to either the pancreas not producing enough insulin (Type 1 diabetes) or cells of the body do not respond properly to the insulin that is produced (Type 2 diabetes, T2D) (Forbes & Cooper Citation2013).
If untreated, diabetes can cause many complications, which can be attributed to two main categories: microvascular disease (damage to small blood vessels) and macrovascular disease (damage to larger arteries). Acute complications include diabetic ketoacidosis and non-ketotic hyperosmolar coma, whereas serious long-term complications include heart disease, kidney failure and damage to the eyes.
T2D is characterized by insulin resistance in major target organs such as liver, muscle and adipose tissues; however, decreased insulin secretion by the β pancreatic cells is also involved (Cheng et al. Citation2009). So far, around 370 million people in the world have been suffering from diabetes, among which more than 9 million are Canadian. More importantly, the risk of suffering T2D diabetes in Aboriginal population is three to five times higher than the general Canadian population (Ekoe et al. Citation1990; Nachar et al. Citation2013). For instance, data from 2009 indicated that the age-adjusted prevalence of T2D in the Cree populations of Eeyou Istchee (Eastern James Bay area of Quebec, Canada) was 29% on an average (Kuzmina et al. Citation2010). These communities also suffer from higher prevalence of diabetic complications, notably nephropathy. This is due, in part, to the cultural inappropriateness of modern drug treatments (Hanley et al. Citation2005; Garriguet Citation2008).
In an attempt to find culturally relevant complementary and alternative treatments, our team has been testing the antidiabetic potential of selected plants used as traditional medication for several diabetic symptoms in these Cree communities (Haddad et al. Citation2012). Previous studies have focused on 17 promising plant extracts identified through ethnobotanical surveys (Leduc et al. Citation2006; Fraser et al. Citation2007). These plants have been tested using a comprehensive platform of bioassays and animal models of obesity and diabetes in order to identify the plants’ capacity to improve glycaemic control (Spoor et al. Citation2006; Fraser et al. Citation2007; Harbilas et al. Citation2009; Haddad et al. Citation2012; Nachar et al. Citation2013).
Rhododendron groenlandicum L. (Ericaceae), commonly known as Labrador tea, is one of the most promising antidiabetic plants used traditionally by the Eastern James Bay Cree (Eid & Haddad Citation2014). In vitro, bioassays suggested that R. groenlandicum acts like metformin in skeletal muscle (Martineau et al. Citation2010) and as thiazolidinedione drugs (also known as glitazones) in adipose tissue (Spoor et al. Citation2006). In a recent in vivo study, the plant extract was beneficial in restoring glucose homeostatic mechanisms in mice fed with a high-fat diet (HFD) (Ouchfoun et al. Citation2015). Notably, R. groenlandicum reduced blood glucose and insulin while improving the response to an oral glucose tolerance test (OGTT). In the current study, the effects of the HFD on renal integrity were assessed in the presence or absence of the plant. We report that R. groenlandicum treatment tends to improve microalbuminuria and significantly reduces renal fibrosis, renal steatosis and Bcl-2-modifying factor (BMF) staining.
Materials and methods
Plant materials
The leaves of R. groenlandicum were harvested in 2006 in the Eeyou Istchee territory, QC, Canada, according to instructions of Cree Elders and healer. They were dried and kept in dry cool conditions until use. The botanical identity of this plant was confirmed by taxonomist Dr. A. Cuerrier and voucher specimens were deposited at the Marie-Victorin Herbarium of the Montreal Botanic Garden (Spoor et al. Citation2006). The 80% ethanol extract was prepared according to previously published articles (Spoor et al. Citation2006; Harbilas et al. Citation2009). The yield was 31%, total phenolics amounted to 188 mg/g dry weight and major compounds identified included chlorogenic acid, cathechins, procyanidins and quercetin glycosides (Harbilas et al. Citation2009).
Animals
Male non-diabetic C57BL/6 mice on a pure genetic background were obtained from Charles River Laboratories (Saint-Constant, QC, Canada). All mice were housed individually in cages with hardwood chip bedding and maintained on a 12 h light/dark cycle in a temperature-controlled animal room (22 °C). All animals were allowed ad libitum access to solid food and water. The animal experimentation ethics committee of the University of Montreal approved all experimental protocols that were carried out in full respect of the guidelines from the Canadian Council for the Protection of Animals.
Evaluation of antidiabetic potential of R. groenlandicum
Four-week-old C57BL/6 mice were randomly divided into three groups: mice fed with Charles River chow (Charles River rodent chow #5075) that served as non-obese control group (n = 12); mice fed with HFD (Bio-Serv, Flemington, NJ, #F3282) that served as the obese control group (n = 10); mice fed with HFD into which 250 mg/kg/d R. groenlandicum was incorporated for the last 8 weeks of the 16-week HFD regimen that served as the treatment group (n = 12) (Ouchfoun et al. Citation2015). Fresh test diet was prepared every 2 d (frequency of food renewal for the animals) by incorporating R. groenlandicum powder into the HFD and the preparation kept in the fridge (4 °C) to ensure stability. Baseline measurements of fed blood glucose levels, body weight, food intake and water consumption were taken every 2 d at 11 am. The non-fasting blood glucose concentration was measured using an Accu-Chek glucose meter (Roche, Montreal, QC, Canada) by collecting blood from the tip of the tail vein. At the 8th and 15th week of experiments, an oral glucose tolerance test (OGTT) was performed. Briefly, mice were fasted for 5.5 h and then given 2 g/kg glucose by gavage through a gastric tube. Tail vein glucose readings were taken at 0, 15, 30, 60 and 120 min after glucose administration. After the 16th week, the mice were anaesthetized with an intraperitoneal injection of 50 mg/kg pentobarbital, killed by exsanguination, and samples such as the liver, muscle (right femoral muscle), kidney, epididymal fat pad, abdominal fat pad and dorsal fat pad were immediately removed and weighed (Vuong et al. Citation2009; Harbilas et al. Citation2013). Urine was also collected. All samples were stored at −80 °C.
Biochemical assays
At the end of experiments, blood samples were collected from renal artery during exsanguinations carried out under anaesthesia using heparinized tools. The samples were centrifuged at 3000 g for 10 min at 41 °C. The plasma samples were used for the analyses of biochemical parameters of glucose homeostasis.
Physiological studies
Urine and kidneys were immediately weighted and stored at −80 °C. Urine was assayed for albumin and creatinine (ELISA; Albuwell and Creatinine Companion; Exocell, Philadelphia, PA) (Sachetelli et al. Citation2006; Brezniceanu et al. Citation2008Citation; Liu et al. Citation2008, Citation2009; Godin et al. Citation2010). The kidneys were removed, decapsulated and weighed. The kidneys were processed for histology and immunostaining study as described (Godin et al. Citation2010).
Histology
Kidneys collected in Tissue-Tek cassettes (VWR Canlab, Montreal, Quebec, Canada) were dipped immediately in ice-cold 4% paraformaldehyde, fixed for 24 h at 4 °C and then processed by the Center Hospitalier de l’Universite de Montreal Pathology Department. Tissue samples counterstained with periodic acid-Schiff (PAS) or Masson’s Trichrome staining (Sachetelli et al. Citation2006; Brezniceanu et al. Citation2008; Liu et al. Citation2008, Citation2009; Godin et al. Citation2010) were examined visually under a light microscope by an observer unaware of the treatments. The collected images were analyzed and quantified using NIH ImageJ software (NIH, Bethesda, MD).
Oil red O stain
Kidneys collected in Tissue-Tek cassettes (VWR Canlab, Montreal, Quebec, Canada) pre-embedded with optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA), were cut into 8-μm sections using a cryostat microtome (model HM505N; Microm International, Walldorf, Germany) at −20 °C. Sections were placed on gelatin-coated slides and air-dried overnight at 37 °C, then stained by Oil Red O.
Immunohistochemistry
Immunohistochemical staining for BMF was performed by the standard avidin–biotin–peroxidase complex method (ABC Staining System, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) as previously described (Brezniceanu et al. Citation2008; Liu et al. Citation2008; Godin et al. Citation2010).
Statistical analysis
Statistical significance between the experimental groups was analyzed by one-way ANOVA and the Bonferroni or the Tukey test as appropriate. The data are expressed as means ± SEM. The p values < 0.05 were considered to be statistically significant.
Results
Diet-induced-obesity (DIO) and pre-diabetic model
In order to create obesity and a pre-diabetic state, mice were fed with HFD for 16 weeks. As described in , HFD mice had a significantly greater body weight as compared with the chow-fed group (49.1 ± 1.85 g versus 34.7 ± 1.41 g, respectively; p < 0.001). Similarly, significant increases in liver, WAT (retroperitoneal fat pad, p < 0.001) and brown adipose tissue weights (p < 0.001) were noticed. In parallel, average of both kidneys’ weight remained unchanged among the three experimental groups. These results confirmed those obtained in a previous study where increased baseline glycaemia and insulin resistance were also observed (Ouchfoun et al. Citation2015). Such phenomenon occurred without any change in food intake between the HFD and chow groups (data not illustrated).
Table 1. Body and organ weights at the end of treatment.
Effects of HFD on renal parameters in the presence or absence of R. groenlandicum. Microalbuminuria
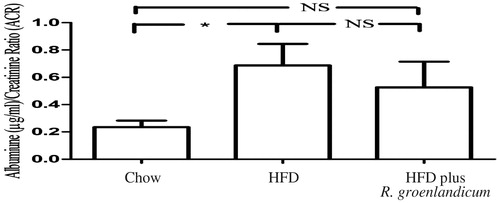
Microalbuminuria (urine albumin) occurs when there is an abnormally high permeability for albumin in the renal glomerulus (Parving et al. Citation2015). Clinically, the albumin/creatinine ratio (ACR) is used to evaluate microalbuminuria and renal function (Bakker Citation1999; Tesar Citation2008; Currie et al. Citation2014). Statistically significant increases in urinary ACR were detectable in mice fed HFD for 16 weeks as compared with chow-fed mice (HFD-fed mice: ACR = 0.69; chow-fed mice = 0.23; p < 0.05, ), which revealed serious renal functional damage. In animals treated with R. groenlandicum, the ACR value at week 16 was reduced by 23% when compared with HFD controls, albeit not in a statistically significant manner (p = 0.70). However, this ACR was also statistically similar to that of chow controls (p = 0.31). These results indicate that R. groenlandicum may possess the potential to prevent the progression of albuminuria in our experimental mice.
Renal fibrosis
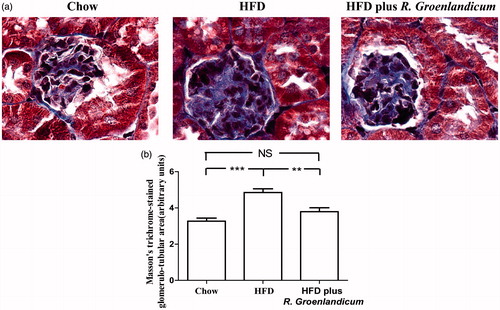
Histological analysis was then carried out to further investigate the damage to renal tissue. We first applied Masson's trichrome stain to detect collagen deposits as a sign of fibrosis. Enhanced expression of collagenous components (stained with blue) was detected in the glomerulotubular area of renal tissues of HFD-fed mice compared with that in chow-fed mice (). This visual distinction was confirmed by quantification of Masson stain (HFD-fed mice 4.85 AU compared with 3.27 AU for chow-fed mice; p < 0.001, ). Meanwhile, results of quantification also indicated that fibrosis was normalized after R. groenlandicum treatment (N.S. versus the chow group; p < 0.05 versus the HFD group; ). This result clearly revealed that R. groenlandicum effectively prevented interstitial fibrosis.
Morphological abnormalities in PAS stain
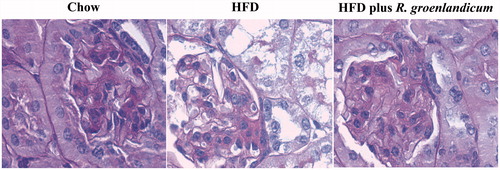
Unlike chow controls, HFD-fed mice exhibited renal structural damage (). Glomeruli and RPTs appeared to be hypertrophic in HFD-fed mice compared with their chow-fed controls (). Rhododendron groenlandicum treatment reversed the glomerular and RPT hypertrophy against HFD ().
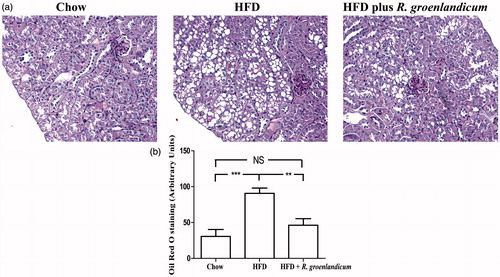
PAS staining also revealed the presence of abundant vacuoles in renal tissues of both HFD-fed mice and HFD plus R. groenlandicum-treated mice (). In order to clarify the nature of those vacuoles, Oil Red O stain was applied and their lipid nature was confirmed (not illustrated). Quantification of these vacuoles suggested that mice receiving R. groenlandicum treatment had significantly improved renal steatosis compared with HFD-fed mice (90.7 AU for HFD versus 46.3 AU for HFD plus R. groenlandicum treatment, p < 0.01; ). Steatosis was limited in the renal tissues of chow-fed mice ().
Figure 4. PAS staining and quantification of vacuoles in mouse kidneys at week 16. (a) PAS stain. Magnification is ×200. (b) Quantification of vacuoles. Values are expressed in arbitrary units (AU) as the mean ± SEM, n = 10–12 for each group. Significantly different from chow controls, **p < 0.01, ***p < 0.001. N.S., not significant.
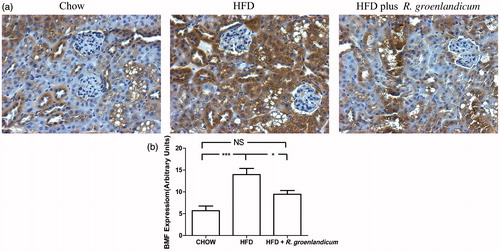
BMF expression in renal tissues
Immunohistochemistry was carried out using an antibody against BMF, a known proapoptotic protein. Results clearly demonstrated elevated BMF staining in renal tissues of HFD-fed mice when compared with chow-fed controls (). In contrast, the difference in BMF expression between chow-fed mice and R. groenlandicum-treated mice was not as important. Quantification confirmed this interpretation. Indeed, HFD induced significant BMF expression as compared with chow-fed mice (p < 0.001, ). Applying R. groenlandicum treatment effectively inhibited HFD-induced increased BMF expression (p < 0.05 versus HFD-fed animals, N.S. versus chow controls; ). This suggests that R. groenlandicum can reduce apoptotic stimuli occurring when mice are fed with HFD.
Figure 5. BMF expression is elevated in renal tissues of HFD-fed mice. (a) BMF immunohistochemical (IHC) staining in kidney sections (original magnification ×200). (b) Quantification of BMF IHC staining. Values are expressed in AU as the mean ± SEM, n = 10–12 for each group. *p < 0.05; ***p < 0.001. N.S., not significant.
Discussion
As previously mentioned, R. groenlandicum is one of the most promising antidiabetic plants used traditionally by the Eastern James Bay Cree (Canada) with several actions observed in in vitro bioassays (Spoor et al. Citation2006; Martineau et al. Citation2010; Nachar et al. Citation2013) as well as in vivo (Ouchfoun et al. Citation2015). In the present studies, we sought to determine if R. groenlandicum could be beneficial against diabetic nephropathy (DN). As done previously (Harbilas et al. Citation2012a,Citationb, Citation2013), we used a diet-induced obesity model in mice to study the effect of R. groenlandicum in a mild diabetic state (Ouchfoun et al. Citation2015). Such studies indeed demonstrated that those 16 weeks of HFD feeding result in a significantly elevated blood glucose level as compared with chow-fed controls (data not illustrated). Under these conditions, R. groenlandicum treatment significantly reduced baseline glycaemia as well as the response to a glucose tolerance test (OGTT) (Ouchfoun et al. Citation2015).
Since elevated blood glucose is the main cause of pathologic manifestations of DN, we hypothesized that HFD-fed mice from this previous study were suffering from DN. A common feature of DN is microalbuminuria whereby the abnormal kidney leaks more serum albumin than normal in the urine (Parving et al. Citation2015). We, therefore, initially examined urine albumin level and HFD-fed mice indeed showed clear signs of microalbuminuria. Aside from such glomerular dysfunction, tubular atrophy and tubulointerstitial fibrosis are associated with the gradual decline of renal function in the later stages of DN (Nangaku Citation2004). Indeed, tubular atrophy and tubulointerstitial fibrosis were proven to be better predictors of late-stage renal disease progression over glomerular pathology (Gilbert & Cooper Citation1999; Marcussen Citation2000; Drummond & Mauer Citation2002; Beyenbach Citation2004). Although mechanisms underlying tubular atrophy are incompletely elucidated, recent findings indicate that renal proximal tubule cell apoptosis may be an initial mechanism for tubular atrophy in T2DM (Lau et al. Citation2012). Next, we thus used renal histology to assess kidney integrity. HFD-fed animals showed signs of hypertrophy of both glomerular and proximal tubular areas as assessed with the PAS stain. Collagen deposition, as assessed with the Masson stain, was significantly augmented in HFD animals, indicating signs of interstitial fibrosis. We also observed a large number of vacuoles that appeared to be lipidic in nature, suggesting the presence of steatosis. Finally, we used immunohistochemistry to quantify BMF, a pro-apoptotic protein. Indeed, BMF has been clearly demonstrated to play an important role in mediating renal proximal tubule cell apoptosis in the diabetic mouse kidney in vivo (Brezniceanu et al. Citation2008; Lau et al. Citation2012). As seen in the db/db mice used in these previous studies, HFD-fed animals also demonstrated a significant increase in BMF expression. All these elements confirmed that HFD-fed animals exhibited signs of kidney damage, albeit not late-stage DN.
We then wanted to determine if R. groenlandicum treatment could be beneficial in such conditions. The results of the present study clearly demonstrate that this is the case. At first, treatment with the plant extract showed a tendency to return microalbuminuria toward values observed in the chow group, albeit not in a statistically significant manner. Similarly, the hypertrophy of both glomerular and proximal tubular areas observed in HFD-fed mice by PAS stain appeared to be improved by R. groenlandicum treatment. Hence, our results suggest that R. groenlandicum extract may have a beneficial impact on glomerular integrity, but the effect is modest. Further studies will be required to address this point.
In contrast, fibrotic collagen deposition was significantly reduced by R. groenlandicum treatment in the face of continuous HFD feeding for 16 weeks. As mentioned above, with PAS staining at low magnification, we observed abundant vacuoles present in renal tissues of HFD-fed mice. Applying Oil Red O stain of triglycerides, we confirmed that these vacuoles represented fat deposits. Quantification of these vacuoles in renal tissues of HFD-fed mice clearly demonstrated that R. groenlandicum treatment could reduce renal steatosis in a potent manner. Such results are consistent with the results of our previous study where the blood lipid profile reflected the high-fat intake. Indeed, LDL, HDL as well as total cholesterol were doubled in HFD-fed animals when compared with chow congeners. Although R. groenlandicum treatment did not significantly modify these blood lipid parameters, it did potently reduce hepatic steatosis (Ouchfoun et al. Citation2015). Our present results demonstrate that R. groenlandicum may also exert its protective role by significantly decreasing renal fibrosis and steatosis.
Finally, we also tested BMF expression in mice consuming Labrador tea extract. Treatment with R. groenlandicum succeeded in normalizing the expression of BMF. It was shown that BMF expression is a precursor of renal proximal tubule cell apoptosis and that this leads to renal dysfunction in diabetes (Lau et al. Citation2012). On the other hand, the significant reduction in BMF induced by Labrador tea treatment occurred in the face of apparently milder improvements in microalbumineria and glomerular integrity. It is thus possible that the duration of the plant treatment was not sufficiently long for the latter effects to be fully expressed. Nonetheless, these results imply that the plant may exert an anti-apoptotic effect, albeit further studies are required to confirm this interpretation.
In summary, our study demonstrates that a prolonged intake of HFD, which is accompanied by disturbed glycaemic control (notably increased insulin resistance), can lead to measurable kidney damage (Boini et al. Citation2010; Lee et al. Citation2012; Zhang et al. Citation2012). Treatment with R. groenlandicum, which improved insulin sensitivity in this diet-induced obesity model (Ouchfoun et al. Citation2015), also improves parameters of kidney integrity. The underlying renal protective mechanisms of R. groenlandicum appear to implicate a milder improvement in microalbumineria and glomerular morphology, a significant reduction in renal fibrosis and steatosis and an important inhibition of BMF expression. Our observations raise the possibility that Labrador tea may possess potential in reversing the pathologic manifestations of DN, particularly tubular atrophy and interstitial fibrosis. The present studies lend further credence to the fact that Labrador tea is a promising antidiabetic plant that should, therefore, be tested clinically in order to be used as a culturally relevant complementary and alternative treatment for Cree diabetics.
Funding information
A Team Grant from the Canadian Institutes of Health Research (CIHR Team in Aboriginal Antidiabetic Medicines; CTP-79855) to Pierre S. Haddad funded these studies.
Acknowledgements
The authors thank the laboratories of Drs. John Chan (notably, Ms. Isabelle Chenier) and Shao Ling Zhang for precious help with the biochemical measures of microalbuminuria and for histological and immunohistochemistry treatment and interpretation. Very special thanks are due to Cree Elders of Eeyou Istchee who kindly agreed to be interviewed. They made this paper possible by allowing us to use, for the purposes of this research, their knowledge relating to medicinal plants transmitted to them by their Elders. Their trust has also enabled a useful exchange between Indigenous knowledge and Western science.
Disclosure statement
The authors report that they have no conflicts of interest.
References
- Bakker AJ. 1999. Detection of microalbuminuria. Receiver operating characteristic curve analysis favors albumin-to-creatinine ratio over albumin concentration. Diabetes Care. 22:307–313.
- Beyenbach KW. 2004. Kidneys sans glomeruli. Am J Physiol Renal Physiol. 286:F811–F827.
- Boini KM, Zhang C, Xia M, Poklis JL, Li PL. 2010. Role of sphingolipid mediator ceramide in obesity and renal injury in mice fed a high-fat diet. J Pharmacol Exp Ther. 334:839–846.
- Brezniceanu ML, Liu F, Wei CC, Chenier I, Godin N, Zhang SL, Filep JG, Ingelfinger JR, Chan JS. 2008. Attenuation of interstitial fibrosis and tubular apoptosis in db/db transgenic mice overexpressing catalase in renal proximal tubular cells. Diabetes. 57:451–459.
- Cheng KK, Iglesias MA, Lam KS, Wang Y, Sweeney G, Zhu W, Vanhoutte PM, Kraegen EW, Xu A. 2009. APPL1 potentiates insulin-mediated inhibition of hepatic glucose production and alleviates diabetes via Akt activation in mice. Cell Metab. 9:417–427.
- Coffman MJ, Norton CK, Beene L. 2012. Diabetes symptoms, health literacy, and health care use in adult Latinos with diabetes risk factors. J Cult Divers. 19:4–9.
- Currie G, McKay G, Delles C. 2014. Biomarkers in diabetic nephropathy: present and future. World J Diabetes. 5:763–776.
- Drummond K, Mauer M. 2002. The early natural history of nephropathy in type 1 diabetes: II. Early renal structural changes in type 1 diabetes. Diabetes. 51:1580–1587.
- Eid HM, Haddad PS. 2014. Mechanisms of action of indigenous antidiabetic plants from the Boreal Forest of Northeastern Canada. Adv Endocrinol. 2014: Article ID 272968, 11 pages.
- Ekoe JM, Thouez JP, Petitclerc C, Foggin PM, Ghadirian P. 1990. Epidemiology of obesity in relationship to some chronic medical conditions among Inuit and Cree Indian populations in new Quebec, Canada. Diabetes Res Clin Pract. 10(Suppl 1):S17–S27.
- Forbes JM, Cooper ME. 2013. Mechanisms of diabetic complications. Physiol Rev. 93:137–188.
- Fraser MH, Cuerrier A, Haddad PS, Arnason JT, Owen PL, Johns T. 2007. Medicinal plants of Cree communities (Québec, Canada): antioxidant activity of plants used to treat type 2 diabetes symptoms. Can J Physiol Pharmacol. 85:1200–1214.
- Garriguet D. 2008. Obesity and the eating habits of the aboriginal population. Health Rep. 19:21–35.
- Gilbert RE, Cooper ME. 1999. The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int. 56:1627–1637.
- Godin N, Liu F, Lau GJ, Brezniceanu ML, Chenier I, Filep JG, Ingelfinger JR, Zhang SL, Chan JS. 2010. Catalase overexpression prevents hypertension and tubular apoptosis in angiotensinogen transgenic mice. Kidney Int. 77:1086–1097.
- Haddad PS, Musallam L, Martineau LC, Harris C, Lavoie L, Arnason JT, Foster B, Bennett S, Johns T, Cuerrier A, et al. 2012. Comprehensive evidence-based assessment and prioritization of potential antidiabetic medicinal plants: a case study from Canadian eastern James Bay Cree traditional medicine. Evid Based. Complement Alternat Med. 2012: Article ID 893426, 14 pages.
- Hanley AJ, Harris SB, Mamakeesick M, Goodwin K, Fiddler E, Hegele RA, Spence JD, House AA, Brown E, Schoales B, et al. 2005. Complications of type 2 diabetes among aboriginal Canadians: prevalence and associated risk factors. Diabetes Care. 28:2054–2057.
- Harbilas D, Brault A, Vallerand D, Martineau LC, Saleem A, Arnason JT, Musallam L, Haddad PS. 2012a. Populus balsamifera L. (Salicaceae) mitigates the development of obesity and improves insulin sensitivity in a diet-induced obese mouse model. J Ethnopharmacol. 141:1012–1020.
- Harbilas D, Martineau LC, Harris CS, Adeyiwola-Spoor DC, Saleem A, Lambert J, Caves D, Johns T, Prentki M, Cuerrier A, et al. 2009. Evaluation of the antidiabetic potential of selected medicinal plant extracts from the Canadian boreal forest used to treat symptoms of diabetes: part II. Can J Physiol Pharmacol. 87:479–492.
- Harbilas D, Vallerand D, Brault A, Saleem A, Arnason JT, Musallam L, Haddad PS. 2012b. Larix laricina, an antidiabetic alternative treatment from the Cree of northern Quebec pharmacopoeia, decreases glycemia and improves insulin sensitivity in vivo. Evid Based Complement Alternat Med. 2012: Article ID 296432, 10 pages.
- Harbilas D, Vallerand D, Brault A, Saleem A, Arnason JT, Musallam L, Haddad PS. 2013. Populus balsamifera extract and its active component salicortin reduce obesity and attenuate insulin resistance in a diet-induced obese mouse model. Evid Based Complement Alternat Med. 2013: Article ID 172537, 13 pages.
- Kuzmina E, Lejeune P, Dannenbaum D, Torrie J. 2010. 2009 CDIS report. Public Health Report Series 3 on Diabetes Cree Board of Health and Social Services of James Bay.
- Lau GJ, Godin N, Maachi H, Lo CS, Wu SJ, Zhu JX, Brezniceanu ML, Chenier I, Fragasso-Marquis J, Lattouf JB, et al. 2012. Bcl-2-modifying factor induces renal proximal tubular cell apoptosis in diabetic mice. Diabetes. 61:474–484.
- Leduc C, Coonishish J, Haddad P, Cuerrier A. 2006. Plants used by the Cree Nation of Eeyou Istchee (Quebec, Canada) for the treatment of diabetes: a novel approach in quantitative ethnobotany. J Ethnopharmacol. 105:55–63.
- Lee W, Eom DW, Jung Y, Yamabe N, Lee S, Jeon Y, Hwang YR, Lee JH, Kim YK, Kang KS, et al. 2012. Dendrobium moniliforme attenuates high-fat diet-induced renal damage in mice through the regulation of lipid-induced oxidative stress. Am J Chin Med. 40:1217–1228.
- Liu F, Brezniceanu ML, Wei CC, Chenier I, Sachetelli S, Zhang SL, Filep JG, Ingelfinger JR, Chan JS. 2008. Overexpression of angiotensinogen increases tubular apoptosis in diabetes. J Am Soc Nephrol. 19:269–280.
- Liu F, Wei CC, Wu SJ, Chenier I, Zhang SL, Filep JG, Ingelfinger JR, Chan JS. 2009. Apocynin attenuates tubular apoptosis and tubulointerstitial fibrosis in transgenic mice independent of hypertension. Kidney Int. 75: 156–166.
- Marcussen N. 2000. Tubulointerstitial damage leads to atubular glomeruli: significance and possible role in progression. Nephrol Dial Transplant. 15:74–75.
- Martineau LC, Adeyiwola-Spoor DC, Vallerand D, Afshar A, Arnason JT, Haddad PS. 2010. Enhancement of muscle cell glucose uptake by medicinal plant species of Canada's native populations is mediated by a common, metformin-like mechanism. J Ethnopharmacol. 127:396–406.
- Nachar A, Vallerand D, Musallam L, Lavoie L, Badawi A, Arnason J, Haddad PS. 2013. The action of antidiabetic plants of the Canadian James Bay Cree traditional pharmacopeia on key enzymes of hepatic glucose homeostasis. Evid Based Complement Alternat Med. 2013: Article ID 189819, 9 pages.
- Nangaku M. 2004. Mechanisms of tubulointerstitial injury in the kidney: final common pathways to end-stage renal failure. Intern Med. 43:9–17.
- Ouchfoun M, Eid HM, Musallam L, Brault A, Li S, Vallerand D, Arnason JT, Haddad PS. 2015. Labrador tea (Rhododendron groenlandicum) attenuates insulin resistance in a diet-induced obesity mouse model. Eur J Nutr. DOI 10.1007/s00394-015-0908-z
- Parving HH, Persson F, Rossing P. 2015. Microalbuminuria: a parameter that has changed diabetes care. Diabetes Res Clin Pract. 107:1–8.
- Sachetelli S, Liu Q, Zhang SL, Liu F, Hsieh TJ, Brezniceanu ML, Guo DF, Filep JG, Ingelfinger JR, Sigmund CD, et al. 2006. RAS blockade decreases blood pressure and proteinuria in transgenic mice overexpressing rat angiotensinogen gene in the kidney. Kidney Int. 69:1016–1023.
- Spoor DC, Martineau LC, Leduc C, Benhaddou-Andaloussi A, Meddah B, Harris C, Burt A, Fraser MH, Coonishish J, Joly E, et al. 2006. Selected plant species from the Cree pharmacopoeia of northern Quebec possess anti-diabetic potential. Can J Physiol Pharmacol. 84:847–858.
- Tesar V. 2008. Examination of the kidneys in a diabetic patient. Vnitr Lek.. 54:494–498.
- Vuong T, Benhaddou-Andaloussi A, Brault A, Harbilas D, Martineau LC, Vallerand D, Ramassamy C, Matar C, Haddad PS. 2009. Antiobesity and antidiabetic effects of biotransformed blueberry juice in KKA(y) mice. Int J Obes (Lond). 33:1166–1173.
- Zhang HM, Dang H, Kamat A, Yeh CK, Zhang BX. 2012. Geldanamycin derivative ameliorates high fat diet-induced renal failure in diabetes. PLoS One. 7:e32746.