Abstract
Context Diabetes is a global health challenge. Although large prospective clinical trials have shown that intensive control of blood glucose or blood pressure reduces the risk for development and progression of vascular complications in diabetes, a substantial number of diabetic patients still experience renal failure and cardiovascular events, which could account for disabilities and high mortality rate in these subjects.
Objective Sulphoraphane is a naturally occurring isothiocyanate found in widely consumed cruciferous vegetables, such as broccoli, cabbage and Brussels sprouts, and an inducer of phase II antioxidant and detoxification enzymes with anticancer properties. We reviewed here the protective role of sulphoraphane against diabetic vascular complications.
Methods In this review, literature searches were undertaken in Medline and in CrossRef. Non-English language articles were excluded. Keywords [sulphoraphane and (diabetes, diabetic nephropathy, diabetic retinopathy, diabetic neuropathy, diabetic complications, vascular, cardiomyocytes, heart or glycation)] have been used to select the articles.
Results There is accumulating evidence that sulphoraphane exerts beneficial effects on vascular damage in both cell culture and diabetic animal models via antioxidative properties. Furthermore, we have recently found that sulphoraphane inhibits in vitro formation of advanced glycation end products (AGEs), suppresses the AGE-induced inflammatory reactions in rat aorta by reducing receptor for AGEs (RAGE) expression and decreases serum levels of AGEs in humans.
Conclusion These findings suggest that blockade of oxidative stress and/or the AGE-RAGE axis by sulphoraphane may be a novel therapeutic strategy for preventing vascular complications in diabetes.
Introduction
Diabetes is a global health challenge. According to the Diabetes Atlas, 6th edition, poster update 2014, an estimated 387 million people worldwide had diabetes (prevalence rate, 8.3%) and one person dies from diabetes every 7 s, reflecting the global trend towards a greater epidemic of diabetes compared with other lifestyle-related diseases [International Diabetes Federation (IDF) Citation2013]. The number of diabetes in China and USA is estimated to be 100 and 24 million, respectively, and Japan has the 10th largest diabetes population in the world (IDF Citation2013). The 2012 National Diabetes Survey of Japan estimated that approximately 9.5 million men and women nationwide were categorized as “those who were strongly suspected of having diabetes”, and approximately 11 million as “those in whom the possibility of having diabetes could not be ruled out”. In keeping with such a trend, although large prospective clinical trials have shown that intensive control of blood glucose and/or blood pressure reduces the risk for the development and progression of vascular complications in diabetes [The Diabetes Control and Complications Trial Research Group Citation1993; UK Prospective Diabetes Study (UKPDS) Group Citation1998], a substantial number of diabetic patients still experience acquired blindness, renal failure and cardiovascular events, which could account for disabilities and high mortality rate in patients with diabetes (Yamagishi & Imaizumi Citation2005; Emerging Risk Factors Collaboration Citation2011). Furthermore, due to diabetes-associated complications, including diabetic angiopathy, average life span is reduced by more than 20 years in middle-aged people with type 1 diabetes and by up to 10 years in middle aged-type 2 diabetic patients compared with non-diabetic subjects (Loukine et al. Citation2012; Rhodes et al. Citation2012; Turin et al. Citation2012). These observations suggest that development of novel therapeutic strategies that specifically target vascular complications in diabetes is urgently needed for patients with diabetes.
Sulphoraphane is a naturally occurring isothiocyanate found in widely consumed cruciferous vegetables such as broccoli, cabbage and Brussels sprouts (Guerrero-Beltrán et al. Citation2012; Kaminski et al. Citation2012; Tortorella et al. Citation2015). Sulphoraphane is an inducer of phase II antioxidant and detoxification enzymes with anticancer properties (Guerrero-Beltrán et al. Citation2012; Kaminski et al. Citation2012). Recently, sulphoraphane has also been shown to play a protective role against oxidative stress-mediated cell and tissue damage (Dinkova-Kostova & Kostov Citation2012; Bhakkiyalakshmi et al. Citation2015). Furthermore, we have recently found that sulphoraphane has an antiglycating capacity, inhibits the formation of advanced glycation end products (AGEs) in vitro and could suppress the receptor for AGEs (RAGE)-mediated vascular injury in diabetes (Maeda et al. Citation2014). Given the pathological role of the AGE-RAGE axis in vascular complications in diabetes (Sourris & Forbes Citation2009; Yamagishi Citation2012; Daffu et al. Citation2013; Fukami et al. Citation2014a; Vlassara & Uribarri Citation2014), it is conceivable that sulphoraphane may be a novel therapeutic strategy for preventing micro- and macrovascular complications in diabetes. However, as far as we know, there are few comprehensive reviews about the role of sulphoraphane in vascular damage in diabetes. Therefore, we reviewed here the protective role of sulphoraphane against diabetic vascular complications. In this review, literature searches were undertaken in Medline and in CrossRef. Non-English language articles were excluded. Keywords [sulphoraphane and (diabetes, diabetic nephropathy, diabetic retinopathy, diabetic neuropathy, diabetic complications, vascular, cardiomyocytes, heart or glycation)] have been used to select the articles.
Nuclear factor (erythroid-derived 2)-related factor-2 (Nrf2)
Nrf2 is a basic leucine zipper transcriptional factor that regulates expression of genes containing an antioxidant response element in the promoter region (Jiménez-Osorio et al. Citation2015). Under basic conditions, Nrf2 is complexed with Kelch-like ECH-associated protein 1 (Keap1), kept in the cytoplasm and ubiquitinated by Cullin 3, which could direct Nrf2 for proteasomal degradation (Jiménez-Osorio et al. Citation2015). Conversely, under oxidative stress conditions, critical cysteine residues in Keap1 are modified, which disrupts the interaction of Keap1 with Nrf2, allowing the translocation of Nrf2 to the nucleus (Jiménez-Osorio et al. Citation2015; Tortorella et al. Citation2015). Sulphoraphane releases Nrf2 from Keap1 by modification of the cysteine residues (Guerrero-Beltrán et al. Citation2012; Jiménez-Osorio et al. Citation2015; Tortorella et al. Citation2015). Then Nrf2 in the nucleus forms heterodimers with small Maf transcriptional factor, which activates antioxidant response element-driven gene expression, subsequently inducing phase II antioxidant and detoxification enzymes (Guerrero-Beltrán et al. Citation2012; Jiménez-Osorio et al. Citation2015; Tortorella et al. Citation2015).
Beneficial effects of sulphoraphane on endothelial cell (EC) damage
There is an accumulating body of evidence, ranging from cell culture experiments to pathologic analysis to epidemiologic clinical studies, that atherosclerosis is intrinsically an inflammatory disease (Tahara et al. Citation2014; Bäck & Hansson Citation2015). The classic pro-inflammatory cytokines, such as tumour necrosis factor-α (TNF-α), induce pro-thrombotic and pro-inflammatory reactions in ECs, contributing to the development and progression of atherosclerotic cardiovascular disease (CVD) (Yamagishi et al. Citation2008; Bäck & Hansson Citation2015). Under inflammatory conditions, ECs have undergone characteristic functional changes that include production of chemokines, cytokines and pro-coagulant factors, induction of adhesion molecules, whose process is mainly mediated via NADPH oxidase-mediated reactive oxygen species (ROS) generation (Kunsch & Medford Citation1999; Griendling et al. Citation2000; Yamagishi & Matsui Citation2010a).
Sulphoraphane at 0.5–1 μM suppresses TNF-α-induced expression of monocyte chemoattractant protein-1 (MCP-1) and adhesion molecules including vascular adhesion molecule-1 (VCAM-1) and intracellular adhesion molecule-1 (ICAM-1) in ECs (Chen et al. Citation2009; Hung et al. Citation2014; Nallasamy et al. Citation2014). Since sulphoraphane inhibits the TNF-α-induced nuclear factor-κB (NF-κB) transcriptional activity in ECs by preventing phosphorylation of IκB kinase and subsequent degradation of IκBα (Hung et al. Citation2014; Nallasamy et al. Citation2014), sulphoraphane could inhibit inflammatory reactions in TNF-α-exposed ECs by suppressing the NF-κB signalling. Sulphoraphane (300 ppm in a mouse diet) also reduced the TNF-α-induced VCAM-1 expression and macrophage infiltration in aorta of C57BL/6 mice and prevented the eruption of endothelial lining in the intima layer of the aorta (Nallasamy et al. Citation2014). Furthermore, as the case of overexpression of mutated form of IκB as well as silencing of NF-κB subunit p65, sulphoraphane inhibited TNF-α-mediated induction of endothelial lipase, a member of triacylglycerol lipase family via inhibition of NF-κB, which may lead to increase in high-density lipoprotein cholesterol levels (Kivelä et al. Citation2010). In addition, isothiocyanates, including 10 μM sulphoraphane, have been shown to inhibit oxidized low-density lipoprotein (oxLDL)-induced ROS generation, NF-κB transcriptional activation and ICAM-1 and VCAM-1 expression in, and monocyte adhesion to, ECs (Chuang et al. Citation2013). Isothiocyanates induced haeme oxygenase-1 (HO-1), glutamate cysteine ligase catalytic and modifier subunit expression, intracellular glutathione content, and antioxidant response element-dependent promoter activity, whereas knockdown of HO-1 and Nrf2 attenuated anti-oxidative and anti-inflammatory effects of isothiocyanates on oxLDL-exposed ECs. Sulphoraphane at 0.5–2.5 μM restored the expressions of superoxide dismutase, glutathione-S-transferase, glutathione peroxidase and thioredoxin reductase and reduced ROS generation in lipopolysaccharide (LPS)-exposed ECs (Li et al. Citation2015). Taken together, these findings suggest that sulphoraphane could block inflammatory insult to ECs, an initial trigger of atherosclerosis by inhibiting NF-κB transcriptional activation via Nrf2-dependent phase II enzymes. Sulphoraphane activated Nrf2 and suppressed p38-VCAM-1 signalling at the susceptible site of atherosclerosis in wild-type but not Nrf2-deficient animals as well (Zakkar et al. Citation2009). Sulphoraphane suppressed the increases in wall thickness and structural derangement, fibrosis, inflammation, oxidative/nitrative stress and apoptosis in aorta of type 2 diabetic mice in association with up-regulation of Nrf2 (Wang et al. Citation2014).
Sulphoraphane at 10–25 μM down-regulated Toll-like receptor (TLR)-4, a receptor of LPS and MyD88, an effector downstream TLR-4 signal pathway in ECs, and resultantly suppressed the LPS-induced ICAM-1 and VCAM-1 expression by blocking the NF-κB activity via inhibition of Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) (Liu et al. Citation2008; Shan et al. Citation2010, Citation2012). Sulphoraphane also inhibited phorbol-12-myristate 13-acetate-, TNF-α- or interleukin-1β-induced EC protein C receptor (EPCR) shedding by suppressing extracellular-regulated kinase (ERK), JNK and p38MAPK, thereby blocking the binding of soluble of EPCR to activate protein C and restoring the anti-coagulant activity of protein C (Ku et al. Citation2014).
However, there is some controversy about the effects of sulphoraphane on NF-κB-signalling pathway in ECs. Indeed, Chen et al. (Citation2009) reported that although sulphoraphane reduced the TNF-α-induced MCP-1 and VCAM-1 mRNA and protein levels in ECs, it had no effect on NF-κB nuclear binding activity, IκBα degradation or activation of NF-κB-driven transcriptional activity. They also showed that dominant negative Nrf2 overexpression did not affect anti-inflammatory effects of sulphoraphane in ECs exposed to TNF-α.
We have previously found that high glucose-induced mitochondrial overproduction of superoxide serves as a causal link between elevated glucose and hyperglycaemic vascular damage in ECs (Nishikawa et al. Citation2000). Indeed, normalizing levels of mitochondrial ROS prevent glucose-induced formation of advanced glycation end products (AGEs), activation of protein kinase C, sorbitol accumulation, hexosamine pathway and NF-κB activation, thus suggesting that five main mechanisms implicated in the pathogenesis of diabetic vascular complications might reflect a single hyperglycaemia-induced process (Du et al. Citation2000). Furthermore, Hammes et al. (Citation2003) have reported that lipid-soluble thiamine-derivative benfotiamine can inhibit these major biochemical pathways as well as hyperglycaemia-associated NF-κB activation by activating a pentose phosphate pathway enzyme transketolase in the retinas, which converts glyceraldehyde-3-phosphate and fructose-6-phosphate into pentose-5-phosphates and other sugars, and resultantly prevents experimental diabetic retinopathy. Sulphoraphane at 4 μM inhibited the ROS generation linked to mitochondrial dysfunction in high glucose-exposed ECs by stimulating nuclear translocation of Nrf2 and subsequently increasing expression of transketolase and glutathione reductase (Xue et al. Citation2008). Since sulphoraphane inhibited hyperglycaemia-induced activation of the hexosamine and protein kinase C pathways and prevented increased cellular accumulation of methylglyoxal, a precursor of AGEs in ECs, blockade of mitochondria-derived ROS by sulphoraphane may be a novel therapeutic target for vascular complications in diabetes.
Pathological role of AGEs in diabetic vascular complications
Recent clinical researches demonstrated the existence of a phenomenon that could be called “metabolic memory” in the mechanisms underlying the development and progression of diabetic vascular complications in humans, similar to what has been observed in experimental diabetic animals (Nathan et al. Citation2014; Writing Group for the DCCT/EDIC Research Group Citation2015). Reducing sugars, such as glucose, can react non-enzymatically with the amino groups of proteins, lipids and nucleic acids to form Schiff bases and Amadori products, followed by repeated cycles of irreversible dehydration and condensation over time to give rise to fluorescent, yellow-brown macromolecules called AGEs (Sourris & Forbes Citation2009; Yamagishi Citation2012; Daffu et al. Citation2013; Fukami et al. Citation2014a; Vlassara & Uribarri Citation2014). Depending on the level and duration of glycaemic exposure, AGEs are irreversibly produced and accumulate in the body, but they are metabolized at a very slow rate once formed, leading to the suggestion that these molecules can best represent the phenomenon of “metabolic memory”. Moreover, AGEs have been reported to increase the expression of their own receptor, RAGE (Yamagishi & Matsui Citation2010b). Based on this, it is hypothesized that the formation of “metabolic memory” is the result of the interaction, or vicious circle, between (1) AGEs, which are slow-turnover molecules that remain in tissues for a prolonged period once they have formed in the body, and (2) RAGE, whose expression is continuously induced by AGEs.
There is a growing body of evidence that interaction of AGEs with the receptor RAGE elicits oxidative stress generation and resultantly evokes proliferative, inflammatory, thrombotic and fibrotic reactions in a variety of cells and organs, thus being involved in diabetic nephropathy, retinopathy, neuropathy, atherosclerotic CVD, cancer growth and metastasis, Alzheimer’s disease and osteoporosis (Sourris & Forbes Citation2009; Takeuchi & Yamagishi Citation2009; Yamagishi Citation2011, Citation2012; Daffu et al. Citation2013; Fukami et al. Citation2014a; Vlassara & Uribarri Citation2014; Yamagishi et al. Citation2015a).
Effects of sulphoraphane on AGE formation
We have recently found that that sulphoraphane at 0.4–100 μM inhibits the formation of AGEs in vitro (Fukami et al. Citation2014b). This compound not only reduced the fluorescence that arose predominantly from AGE-modified proteins but also specifically inhibited the formation of glyceraldehyde-derived AGEs, as measured by enzyme-linked immunosorbent assay (ELISA) (Fukami et al. Citation2014b). Furthermore, daily intake of sulphoraphane precursor-rich broccoli sprouts (25 g/d) for 2 months significantly decreased body weight, body mass index and waist circumference, and reduced HbA1c, diastolic blood pressure and total cholesterol levels in apparently healthy subjects (male 11, female 14, mean age; 49.5 years old) (Yamagishi et al. Citation2015b). Oral consumption of broccoli sprouts also decreased serum levels of AGEs, ratio of AGEs to soluble form of RAGE (sRAGE) and raised consciousness of eating healthy food in these subjects. We have very recently found that (1) the ratio of serum levels of AGEs to sRAGE was an independent predictor of endothelial dysfunction, an initial step of atherosclerosis in humans, and (2) while serum levels of AGEs and sRAGE were positively and inversely associated with endothelial dysfunction, respectively, the significance was lost after adjusting for various confounders (Kajikawa et al. Citation2015). Since sRAGE is mainly generated from the cleavage of cell surface full-length RAGE, the process of which is promoted by the engagement of RAGE with ligands such as AGEs (Raucci et al. Citation2008; Yamagishi & Matsui Citation2010b), a low sRAGE level might be a consequence of impaired shedding of RAGE by AGEs. In these circumstances, the AGE-RAGE axis could be further augmented. This is one possible reason why the ratio of AGEs to sRAGE is a more sensitive biomarker of endothelial dysfunction than each parameter alone. These observations suggests that intake of sulphoraphane precursor-rich broccoli sprouts may suppress the formation of AGEs as well as the activation of AGE-RAGE axis in humans. Consumption of broccoli sprouts has been shown to decrease excretion of biomarkers linked to inflammation and thrombosis in healthy humans as well (Medina et al. Citation2015).
We previously demonstrated that (1) circulating AGE levels determined by the ELISA procedure were elevated under inflammatory and/or diabetic conditions, (2) were correlated with levels of plasminogen activator inhibitor-1 and fibrinogen, both of which are thrombotic markers, as well as those of MCP-1, a marker of vascular damage and inflammation, in both a general population and patients with type 2 diabetes, (3) were served as a marker of disease progression in diabetic nephropathy and retinopathy, (4) were acted as an independent determinant of the reduction in number and migratory capacity of endothelial progenitor cells, (5) had the potential to act as an independent risk factor determining atherosclerotic plaque inflammation and progression, (6) were served as a useful marker for distinguishing non-alcoholic steatohepatitis from simple steatosis, (7) had a strong correlation with an index of insulin resistance in non-diabetic subjects, and (8) were a marker of visceral fat inflammation (Jinnouchi et al. Citation2006; Hyogo et al. Citation2007; Yamagishi et al. Citation2007; Nakamura et al. Citation2008; Ueda et al. Citation2012; Tahara et al. Citation2012a,Citationb; Fukushima et al. Citation2013; Tahara et al. Citation2015). These findings suggest that sulphoraphane plays a protective role against the development and progression of diabetic organ complications by inhibiting the formation of AGEs and suppressing the AGE-RAGE axis, which are closely involved in the pathology of vascular complications in diabetes, insulin resistance and non-alcoholic steatohepatitis.
Anti-thrombotic effects of sulphoraphane
Acute coronary syndromes (ACS), such as myocardial infarction and unstable angina, are leading causes of death in developed countries (Takenaka et al. Citation2006). Atherothrombosis, characterized by atherosclerotic lesion disruption with superimposed thrombus formation, is thought to be the major cause of ACS (Takenaka et al. Citation2006). Sulphoraphane at 50–100 μM inhibited human platelet aggregation caused by different receptor agonists, including collagen, a thromboxane A2 mimic, protease-activated receptor 1 (PAR1) agonist peptide and an ADP P2Y12 receptor agonist, and reduced thrombus formation by preventing phosphatidylinositol 3-kinase (PI3K)/Akt signalling via ubiquitination and degradation of phosphoinositide-dependent kinase 1 as well as regulatory p85 subunit of PI3K (Chuang et al. Citation2013). Sulphoraphane at 15–75 μM has also been shown to suppress platelet activation and aggregation both in vitro and in vivo by inhibiting intracellular signals, such as the PI3-kinase/Akt and phospholipase Cγ2-protein kinase C cascades, through elevation of cyclic AMP (Jayakumar et al. Citation2013). Further, sulphoraphane significantly inhibited P-selectin and glycoprotein IIb–IIIa expression and platelet–neutrophil aggregation as well (Konić-Ristić et al. Citation2013; Oh et al. Citation2013). In addition, treatment with 5–50 μM sulphoraphane prolonged activated partial thromboplastin time and prothrombin time, inhibited activities and production of thrombin and factor Xa in ECs, and intravenous administration of sulphoraphane (3.55–17.33 μg) suppressed thrombin-catalyzed fibrin polymerization and platelet aggregation with anti-coagulant properties in mice (Ku & Bae Citation2014).
We have previously shown that citrated plasma could induce oxidative and inflammatory reactions in ECs via the activation of thrombin-PAR-1 system, which was potentiated by AGEs (Ishibashi et al. Citation2014). Moreover, beyond the direct effects on platelets, AGEs not only inhibited prostacyclin production in ECs through the interaction with RAGE but also induced PAI-1, further promoting platelet aggregation and fibrin stabilization, thereby resulting in a predisposition to thrombogenesis in diabetes (Yamagishi et al. Citation1998; Takenaka et al. Citation2006; Yamagishi et al. Citation2009). Since cyclic AMP-elevating agents could block the AGE-RAGE signalling pathways (Yamagishi et al. Citation1998; Takenaka et al. Citation2006; Ishibashi et al. Citation2010; Ojima et al. Citation2013), sulphoraphane may ameliorate thrombogenic abnormalities in diabetes partly via increase in cyclic AMP levels in platelets and ECs.
Protection of vascular smooth muscle cell (VSMC) injury by sulphoraphane
Sulphoraphane at 0.25–5 μM protected aortic SMCs from oxidative stress injury by inducing total cellular as well as mitochondrial antioxidants and phase 2 enzymes, including superoxide dismutase, catalase, reduced form of glutathione, glutathione peroxidase, glutathione reductase, glutathione-S-transferase and NAD(P) H:quinone oxidoreductase 1 (Zhu et al. Citation2008). As the case in ECs, sulphoraphane at 1–3 μg/mL suppressed TNF-α-induced ICAM-1 and VCAM-1 expression in VSMCs by suppressing activation of p38, ERK and JNK via inhibition of intracellular ROS generation-evoked NF-κB and AP-1-signalling pathways (Kim et al. Citation2012). Sulphoraphane was also shown to suppress cytokine production by oxyhaemoglobin-exposed VSMCs via activation of the Nrf2 (Zhao et al. Citation2013).
Oxidative stress plays a role in SMC proliferation, migration and neointimal hyperplasia after balloon angioplasty (Nakamura et al. Citation2007). Sulphoraphane inhibited platelet-derived growth factor (PDGF)-induced proliferation of rat aortic SMCs by the up-regulation of p53 leading to G1/S cell-cycle arrest and subsequently decreased neointima formation after balloon angioplasty (Yoo et al. Citation2013). Sulphoraphane has been reported to inhibit neointimal hyperplasia via targeting of adhesion molecule expression through the suppression of NF-κB pathway (Kwon et al. Citation2012).
Sulphoraphane at 0.5 μM increased Nrf2, promoted its nuclear localization, increased glutathione levels and lowered ROS generation, which led to the reduction of augmented myogenic tone of mesenteric arterioles in diabetic mice (Velmurugan et al. Citation2013).
Protective role of sulphoraphane against diabetic nephropathy
Diabetic nephropathy is characterized by functional and structural changes in the glomerulus such as glomerular hyperfiltration, thickening of glomerular basement membrane, podocyte loss and an expansion of extracellular matrix in the mesangial areas (Yamagishi & Imaizumi Citation2005; Yamagishi & Matsui Citation2015). It ultimately progresses glomerular sclerosis associated with an increased urinary excretion rate of albumin and renal dysfunction. Although the characteristic histological changes of diabetic nephropathy were supposed to be diffuse and nodular glomerulosclerosis, it has been recognized that changes within tubulointerstitium are more important than glomerulopathy in terms of renal dysfunction in diabetic nephropathy (Yamagishi & Imaizumi Citation2005; Yamagishi & Matsui Citation2015).
Oral or subcutaneous administration of sulphoraphane (12.5 or 0.5 mg/kg, respectively) prevented the increase in urine albumin excretion, glomerular basement membrane thickness, matrix expansion and sclerosis, and reduced oxidative stress generation, transforming growth factor-β1 (TGF-β1) expression, fibronectin and type IV collagen deposition in the kidney of streptozotocin-induced diabetic rats or mice (Zheng et al. Citation2011; Cui et al. Citation2012). Nrf2 activation reduced oxidative damage and suppressed the expression of TGF-β1 and extracellular matrix proteins both in the diabetic kidney and in cultured mesangial cells (Zheng et al. Citation2011). Blockade of Nrf2 expression completely abolished the anti-fibrotic effects of sulphoraphane in high glucose-exposed tubular cells (Cui et al. Citation2012) Intraperitoneal injection of sulphoraphane at 5 mg/kg ameliorated experimental diabetic nephropathy, at least in part, through suppression of glycogen synthase kinase 3beta/Fyn-signalling pathway via activation of Nrf2 (Shang et al. Citation2015). Furthermore, we have previously shown that hyperglycaemia increases oxidative and nitrosative stress and accelerates renal injury in the Nrf2-deficient diabetic mice and that Nrf2 serves as a defence factor against experimental diabetic nephropathy (Yoh et al. Citation2008).
Protective role of sulphoraphane against diabetic retinopathy and neuropathy
Diabetic retinopathy is one of the leading causes of acquired blindness among the people of occupational age (Yamagishi & Imaizumi Citation2005). The earliest histopathological hallmark of diabetic retinopathy is loss of pericytes (Orlidge & D'Amore Citation1987; Yamagishi et al. Citation1993). In parallel with loss of pericytes, several characteristic changes, including thickening of the basement membrane of retinas, retinal vascular hyperpermeability and microaneurysm formation, are observed (Orlidge & D’Amore Citation1987; Yamagishi et al. Citation1993, Citation2002; Yamagishi & Imaizumi Citation2005; Higashimoto et al. Citation2013). Diabetic retinopathy ultimately progresses to proliferative changes associated with neovascularization (Yamagishi & Imaizumi Citation2005; Higashimoto et al. Citation2013). Since pericytes have been shown to play a central role in the maintenance of microvascular homeostasis, it has been postulated that worsening of diabetic retinopathy and ultimately proliferative changes associated with retinal neovascularization are the consequent of loss of pericytes (Yamagishi et al. Citation1993, Citation2002; Yamagishi & Imaizumi Citation2005; Higashimoto et al. Citation2013).
We found that AGE stimulated superoxide generation and RAGE gene and protein expression in bovine-cultured retinal pericytes, which were prevented by the treatment with sulphoraphane (Maeda et al. Citation2014). Sulphoraphane or antibodies raised against RAGE significantly inhibited the AGE-induced decrease in DNA synthesis, apoptotic cell death and up-regulation of MCP-1 mRNA levels in pericytes. These findings suggest that sulphoraphane could inhibit DNA synthesis, apoptotic cell death and inflammatory reactions in AGE-exposed pericytes partly by suppressing RAGE expression via its anti-oxidative properties. Blockade of the AGE-RAGE axis in pericytes by sulphoraphane might be a novel therapeutic target for the treatment of diabetic retinopathy.
Sulphoraphane at 10–80 μM inhibited EC proliferation, migration and tubulogenesis, the key steps of angiogenesis via induction of apoptotic cell death of ECs or endothelial progenitor cells as well as suppression of matrix metalloproteinase-9 activity (Asakage et al. Citation2006; Annabi et al. Citation2008; Nishikawa et al. Citation2009, Citation2010). Inhibition of ERK and PI3K/AKT pathways synergistically inhibited cell migration and capillary tube formation by ECs and further enhanced the anti-angiogenic effects of sulphoraphane, which were associated with the activation of FOXO transcriptional factor (Davis et al. Citation2009). Phosphorylation-deficient mutants of FOXO enhanced anti-angiogenic effects of sulphoraphane by activating the FOXO transcription factor, thus suggesting that sulphoraphane inhibits angiogenesis via FOXO transcriptional activity.
Sulphoraphane at 1–10 μM activated antioxidant response via Nrf2 in high glucose-exposed dorsal root ganglion and protected against oxidative stress-induced injury (Vincent et al. Citation2009). Motor nerve conduction velocity, nerve blood flow and pain behaviour in streptozotocin-induced diabetic rats were improved and malondialdehyde level was reduced by 0.5–1 mg/kg sulphoraphane, which were associated with increased expression of Nrf2 and downstream targets haemeoxygenase-1 and NAD(P)H:quinone oxidoreductase 1 (Negi et al. Citation2011). Sulphoraphane at 2.5 μM has also been reported to inhibit methylglyoxal-induced neuronal cell damage by blocking activation of the caspase-3 enzyme, reducing the phosphorylation of ERK, JNK and p38 and ROS production, and increasing intracellular glutathione levels (Angeloni et al. Citation2015). Furthermore, sulphoraphane enhanced the detoxifying system of methylglyoxal, a precursor of AGEs by increasing the expression and activity of glyoxalase 1. These observations suggest a potential clinical utility of sulphoraphane in preventing diabetic neuropathy.
Effects of sulphoraphane on diabetic cardiomyopathy
Sulphoraphane subcutaneously given at 0.5 mg/kg significantly prevented diabetes-induced cardiac dysfunction, hypertrophy and fibrosis in streptozotocin-induced diabetic mice via Nrf2 activation, which were in association with decreased accumulation of collagen as well as oxidative stress markers and reduced expression of connective tissue growth factor, TGF-β (Bai et al. Citation2013). Moreover, the same dose of sulphoraphane has been shown to attenuate cardiac remodelling, dysfunction and fibrosis and prevent cardiac lipid accumulation, inflammation and oxidative stress reactions in type 2 diabetic mice through Nrf2-5′-AMP-activated protein kinase-signalling pathway (Zhang et al. Citation2014).
Metabolic effects of sulphoraphane in diabetic state
There is some controversy about the metabolic effects of sulphoraphane in diabetic state. A randomized double-blind clinical trial showed that consumption of 10 g broccoli sprouts powder per day for 4 weeks resulted in a significant decrease in serum insulin concentration and homoeostasis model assessment of insulin resistance in type 2 diabetic patients (Bahadoran et al. Citation2012). However, although oral administration of 0.1, 0.25 or 0.5 mg/kg sulphoraphane protected against hypertriglyceridemia in streptozotocin-induced diabetic rats, impairment of hepatic function and cholesterol levels were aggravated after treatment with the compound (De Souza et al. Citation2012). Sulphoraphane at 10 μM has also been shown to suppress hormone-induced differentiation and decreased peroxisome proliferator-activated receptor-γ and fatty acid-binding protein 4 expression in mouse embryonic fibroblasts (Xu et al. Citation2012).
Conclusions
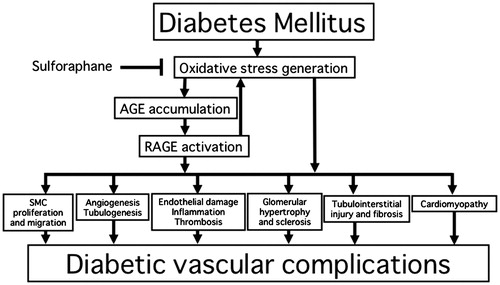
We can posit an overall scheme for the protective role of sulphoraphane against vascular complications in diabetes (). Further longitudinal randomized study is needed to clarify whether sulphoraphane or sulphoraphane-rich food could alleviate pro-inflammatory and pro-oxidative reactions and resultantly prevent vascular complications in diabetes.
Funding information
This study was supported in part by Grants-in-Aid for Scientific Research (B) (Grant no. 25293127) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to S. Y.).
Disclosure statement
The authors report that they have no conflicts of interests in this review.
References
- Angeloni C, Malaguti M, Rizzo B, Barbalace MC, Fabbri D, Hrelia S. 2015. Neuroprotective effect of sulforaphane against methylglyoxal cytotoxicity. Chem Res Toxicol. 28:1234–1245.
- Asakage M, Tsuno NH, Kitayama J, Tsuchiya T, Yoneyama S, Yamada J, Okaji Y, Kaisaki S, Osada T, Takahashi K, et al.. 2006. Sulforaphane induces inhibition of human umbilical vein endothelial cells proliferation by apoptosis. Angiogenesis 9:83–91.
- Annabi B, Rojas-Sutterlin S, Laroche M, Lachambre MP, Moumdjian R, Béliveau R. 2008. The diet-derived sulforaphane inhibits matrix metalloproteinase-9-activated human brain microvascular endothelial cell migration and tubulogenesis. Mol Nutr Food Res. 52:692–700.
- Bäck M, Hansson GK. 2015. Anti-inflammatory therapies for atherosclerosis. Nat Rev Cardiol. 12:199–211.
- Bahadoran Z, Tohidi M, Nazeri P, Mehran M, Azizi F, Mirmiran P. 2012. Effect of broccoli sprouts on insulin resistance in type 2 diabetic patients: a randomized double-blind clinical trial. Int J Food Sci Nutr. 63:767–771.
- Bai Y, Cui W, Xin Y, Miao X, Barati MT, Zhang C, Chen Q, Tan Y, Cui T, Zheng Y, et al, et al. 2013. Prevention by sulforaphane of diabetic cardiomyopathy is associated with up-regulation of Nrf2 expression and transcription activation. J Mol Cell Cardiol. 57:82–95.
- Bhakkiyalakshmi E, Sireesh D, Rajaguru P, Paulmurugan R, Ramkumar KM. 2015. The emerging role of redox-sensitive Nrf2-Keap1 pathway in diabetes. Pharmacol Res. 91:104–114.
- Chen XL, Dodd G, Kunsch C. 2009. Sulforaphane inhibits TNF-alpha-induced activation of p38 MAP kinase and VCAM-1 and MCP-1 expression in endothelial cells. Inflamm Res. 58:513–521.
- Chuang WY, Kung PH, Kuo CY, Wu CC. 2013. Sulforaphane prevents human platelet aggregation through inhibiting the phosphatidylinositol 3-kinase/Akt pathway. Thromb Haemost. 109:1120–1130.
- Cui W, Bai Y, Miao X, Luo P, Chen Q, Tan Y, Rane MJ, Miao L, Cai L. 2012. Prevention of diabetic nephropathy by sulforaphane: possible role of Nrf2 upregulation and activation. Oxid Med Cell Longev. 2012:821936.
- Daffu G, del Pozo CH, O'Shea KM, Ananthakrishnan R, Ramasamy R, Schmidt AM. 2013. Radical roles for RAGE in the pathogenesis of oxidative stress in cardiovascular diseases and beyond. Int J Mol Sci. 14:19891–19910.
- Davis R, Singh KP, Kurzrock R, Shankar S. 2009. Sulforaphane inhibits angiogenesis through activation of FOXO transcription factors. Oncol Rep. 22:1473–1478.
- De Souza CG, Sattler JA, De Assis AM, Rech A, Perry ML, Souza DO. 2012. Metabolic effects of sulforaphane oral treatment in streptozotocin-diabetic rats. J Med Food. 15:795–801.
- Dinkova-Kostova AT, Kostov RV. 2012. Glucosinolates and isothiocyanates in health and disease. Trends Mol Med. 18:337–347.
- Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. 2000. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci USA. 97:12222–12226.
- Emerging Risk Factors Collaboration, Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I. 2011, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med.. 364:829–841.
- Fukami K, Matsui T, Yamagishi S. 2014b. Sulforaphane inhibits formation of advanced glycation end products in vitro. Diabetes Frontier Online 1:e1–e001.
- Fukami K, Yamagishi S, Okuda S. 2014a. Role of AGEs-RAGE system in cardiovascular disease. Curr Pharm Des. 20:2395–2402.
- Fukushima Y, Daida H, Morimoto T, Kasai T, Miyauchi K, Yamagishi S, Takeuchi M, Hiro T, Kimura T, Nakagawa Y, et al. 2013. Relationship between advanced glycation end products and plaque progression in patients with acute coronary syndrome: the JAPAN-ACS sub-study. Cardiovasc Diabetol. 12:5.
- Griendling KK, Sorescu D, Ushio-Fukai M. 2000. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 86:494–501.
- Guerrero-Beltrán CE, Calderón-Oliver M, Pedraza-Chaverri J, Chirino YI. 2012. Protective effect of sulforaphane against oxidative stress: recent advances. Exp Toxicol Pathol. 64:503–508.
- Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, Lin J, Bierhaus A, Nawroth P, Hannak D, et al. 2003. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 9:294–299.
- Higashimoto Y, Matsui T, Nishino Y, Taira J, Inoue H, Takeuchi M, Yamagishi S. 2013. Blockade by phosphorothioate aptamers of advanced glycation end products-induced damage in cultured pericytes and endothelial cells. Microvasc Res. 90:64–70.
- Hung CN, Huang HP, Wang CJ, Liu KL, Lii CK. 2014. Sulforaphane inhibits TNF-α-induced adhesion molecule expression through the Rho A/ROCK/NF-κB signaling pathway. J Med Food. 17:1095–1102.
- Hyogo H, Yamagishi S, Iwamoto K, Arihiro K, Takeuchi M, Sato T, Ochi H, Nonaka M, Nabeshima Y, Inoue M, et al. 2007. Elevated levels of serum advanced glycation end products in patients with non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 22:1112–1119.
- International Diabetes Federation. 2013. IDF diabetes atlas. 6th ed. Brussels, Belgium: International Diabetes Federation.
- Ishibashi Y, Matsui T, Takeuchi M, Yamagishi S. 2010. Glucagon-like peptide-1 (GLP-1) inhibits advanced glycation end product (AGE)-induced up-regulation of VCAM-1 mRNA levels in endothelial cells by suppressing AGE receptor (RAGE) expression. Biochem Biophys Res Commun. 391:1405–1408.
- Ishibashi Y, Matsui T, Ueda S, Fukami K, Yamagishi S. 2014. Advanced glycation end products potentiate citrated plasma-evoked oxidative and inflammatory reactions in endothelial cells by upregulating protease-activated receptor-1 expression. Cardiovasc Diabetol. 13:60.
- Jayakumar T, Chen WF, Lu WJ, Chou DS, Hsiao G, Hsu CY, Sheu JR, Hsieh CY. 2013. A novel antithrombotic effect of sulforaphane via activation of platelet adenylate cyclase: ex vivo and in vivo studies. J Nutr Biochem. 24:1086–1095.
- Jiménez-Osorio AS, González-Reyes S, Pedraza-Chaverri J. 2015. Natural Nrf2 activators in diabetes. Clin Chim Acta. 448:182–192.
- Jinnouchi Y, Yamagishi S, Takeuchi M, Ishida S, Jinnouchi Y, Jinnouchi J, Imaizumi T. 2006. Atorvastatin decreases serum levels of advanced glycation end products (AGEs) in patients with type 2 diabetes. Clin Exp Med. 6:191–193.
- Kajikawa M, Nakashima A, Fujimura N, Maruhashi T, Iwamoto Y, Iwamoto A, Matsumoto T, Oda N, Hidaka T, Kihara Y, et al. 2015. Ratio of serum levels of AGEs to soluble form of RAGE is a predictor of endothelial function. Diabetes Care 38:119–125.
- Kaminski BM, Steinhilber D, Stein JM, Stein JM, Ulrich S. 2012. Phytochemicals resveratrol and sulforaphane as potential agents for enhancing the anti-tumor activities of conventional cancer therapies. Curr Pharm Biotechnol. 13:137–146.
- Kim JY, Park HJ, Um SH, Sohn EH, Kim BO, Moon EY, Rhee DK, Pyo S. 2012. Sulforaphane suppresses vascular adhesion molecule-1 expression in TNF-α-stimulated mouse vascular smooth muscle cells: involvement of the MAPK, NF-κB and AP-1 signaling pathways. Vascul Pharmacol. 56:131–141.
- Kivelä AM, Mäkinen PI, Jyrkkänen HK, Mella-Aho E, Xia Y, Kansanen E, Leinonen H, Verma IM, Ylä-Herttuala S, Levonen AL. 2010. Sulforaphane inhibits endothelial lipase expression through NF-κB in endothelial cells. Atherosclerosis 213:122–128.
- Konić-Ristić A, Srdić-Rajić T, Kardum N, Aleksić-Veličković V, Kroon PA, Hollands WJ, Needs PW, Boyko N, Hayran O, Jorjadze M, et al. 2013. Effects of bioactive-rich extracts of pomegranate, persimmon, nettle, dill, kale and Sideritis and isolated bioactives on arachidonic acid induced markers of platelet activation and aggregation. J Sci Food Agric. 93:3581–3587.
- Ku SK, Bae JS. 2014. Antithrombotic activities of sulforaphane via inhibiting platelet aggregation and FIIa/FXa. Arch Pharm Res. 37:1454–1463.
- Ku SK, Han MS, Bae JS. 2014. Sulforaphane inhibits endothelial protein C receptor shedding in vitro and in vivo. Vascul Pharmacol. 63:13–18.
- Kunsch C, Medford RM. 1999. Oxidative stress as a regulator of gene expression in the vasculature. Circ Res. 85:753–766.
- Kwon JS, Joung H, Kim YS, Shim YS, Ahn Y, Jeong MH, Kee HJ. 2012. Sulforaphane inhibits restenosis by suppressing inflammation and the proliferation of vascular smooth muscle cells. Atherosclerosis 225:41–49.
- Li B, Tian S, Liu X, He C, Ding Z, Shan Y. 2015. Sulforaphane protected the injury of human vascular endothelial cell induced by LPC through up-regulating endogenous antioxidants and phase II enzymes. Food Funct. 6:1984–1991.
- Liu YC, Hsieh CW, Weng YC, Chuang SH, Hsieh CY, Wung BS. 2008. Sulforaphane inhibition of monocyte adhesion via the suppression of ICAM-1 and NF-kappaB is dependent upon glutathione depletion in endothelial cells. Vascul Pharmacol. 48:54–61.
- Loukine L, Waters C, Choi BC, Ellison J. 2012. Impact of diabetes mellitus on life expectancy and health-adjusted life expectancy in Canada. Popul Health Metr. 10:7.
- Maeda S, Matsui T, Ojima A, Takeuchi M, Yamagishi S. 2014. Sulforaphane inhibits advanced glycation end product-induced pericyte damage by reducing expression of receptor for advanced glycation end products. Nutr Res. 34:807–813.
- Medina S, Domínguez-Perles R, Moreno DA, García-Viguera C, Ferreres F, Gil JI, Gil-Izquierdo Á. 2015. The intake of broccoli sprouts modulates the inflammatory and vascular prostanoids but not the oxidative stress-related isoprostanes in healthy humans. Food Chem. 173:1187–1194.
- Nakamura K, Yamagishi S, Adachi H, Matsui T, Kurita-Nakamura Y, Takeuchi M, Inoue H, Imaizumi T. 2008. Circulating advanced glycation end products (AGEs) and soluble form of receptor for AGEs (sRAGE) are independent determinants of serum monocyte chemoattractant protein-1 (MCP-1) levels in patients with type 2 diabetes. Diabetes Metab Res Rev. 24:109–114.
- Nakamura K, Yamagishi S, Matsui T, Yoshida T, Takenaka K, Jinnouchi Y, Yoshida Y, Ueda S, Adachi H, Imaizumi T. 2007. Pigment epithelium-derived factor inhibits neointimal hyperplasia after vascular injury by blocking NADPH oxidase-mediated reactive oxygen species generation. Am J Pathol. 170:2159–2170.
- Nallasamy P, Si H, Babu PV, Pan D, Fu Y, Brooke EA, Shah H, Zhen W, Zhu H, Liu D, et al. 2014. Sulforaphane reduces vascular inflammation in mice and prevents TNF-α-induced monocyte adhesion to primary endothelial cells through interfering with the NF-κB pathway. J Nutr Biochem. 25:824–833.
- Nathan DM, DCCT/EDIC Research Group. 2014. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 37:9–16.
- Negi G, Kumar A, Sharma SS. 2011. Nrf2 and NF-κB modulation by sulforaphane counteracts multiple manifestations of diabetic neuropathy in rats and high glucose-induced changes. Curr Neurovasc Res. 8:294–304.
- Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, et al. 2000. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404:787–790.
- Nishikawa T, Tsuno NH, Okaji Y, Sunami E, Shuno Y, Sasaki K, Hongo K, Kaneko M, Hiyoshi M, Kawai K, et al. 2010. The inhibition of autophagy potentiates anti-angiogenic effects of sulforaphane by inducing apoptosis. Angiogenesis 13:227–238.
- Nishikawa T, Tsuno NH, Tsuchiya T, Yoneyama S, Yamada J, Shuno Y, Okaji Y, Tanaka J, Kitayama J, Takahashi K, et al. 2009. Sulforaphane stimulates activation of proapoptotic protein bax leading to apoptosis of endothelial progenitor cells. Ann Surg Oncol. 16:534–543.
- Oh CH, Shin JI, Mo SJ, Yun SJ, Kim SH, Rhee YH. 2013. Antiplatelet activity of L-sulforaphane by regulation of platelet activation factors, glycoprotein IIb/IIIa and thromboxane A2. Blood Coagul Fibrinol. 24:498–504.
- Ojima A, Ishibashi Y, Matsui T, Maeda S, Nishino Y, Takeuchi M, Fukami K, Yamagishi S. 2013. Glucagon-like peptide-1 receptor agonist inhibits asymmetric dimethylarginine generation in the kidney of streptozotocin-induced diabetic rats by blocking advanced glycation end product-induced protein arginine methyltranferase-1 expression. Am J Pathol. 182:132–141.
- Orlidge A, D'Amore PA. 1987. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol. 105:1455–1462.
- Raucci A, Cugusi S, Antonelli A, Barabino SM, Monti L, Bierhaus A, Reiss K, Saftig P, Bianchi ME. 2008. Soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). FASEB J. 22:3716–3727.
- Rhodes ET, Prosser LA, Hoerger TJ, Lieu T, Ludwig DS, Laffel LM. 2012. Estimated morbidity and mortality in adolescents and young adults diagnosed with Type 2 diabetes mellitus. Diabet Med. 29:453–463.
- Shan Y, Lin N, Yang X, Tan J, Zhao R, Dong S, Wang S. 2012. Sulphoraphane inhibited the expressions of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 through MyD88-dependent toll-like receptor-4 pathway in cultured endothelial cells. Nutr Metab Cardiovasc Dis. 22:215–222.
- Shan Y, Zhao R, Geng W, Lin N, Wang X, Du X, Wang S. 2010. Protective effect of sulforaphane on human vascular endothelial cells against lipopolysaccharide-induced inflammatory damage. Cardiovasc Toxicol. 10:139–145.
- Shang G, Tang X, Gao P, Guo F, Liu H, Zhao Z, Chen Q, Jiang T, Zhang N, Li H. 2015. Sulforaphane attenuation of experimental diabetic nephropathy involves GSK-3 beta/Fyn/Nrf2 signaling pathway. J Nutr Biochem. 26:596–606.
- Sourris KC, Forbes JM. 2009. Interactions between advanced glycation end-products (AGE) and their receptors in the development and progression of diabetic nephropathy-are these receptors valid therapeutic targets. Curr Drug Targets. 10:42–50.
- Tahara N, Tahara A, Honda A, Nitta Y, Kodama N, Yamagishi S, Imaizumi T. 2014. Molecular imaging of vascular inflammation. Curr Pharm Des. 20:2439–2447.
- Tahara N, Yamagishi S, Matsui T, Takeuchi M, Nitta Y, Kodama N, Mizoguchi M, Imaizumi T. 2012a. Serum levels of advanced glycation end products (AGEs) are independent correlates of insulin resistance in nondiabetic subjects. Cardiovasc Ther. 30:42–48.
- Tahara N, Yamagishi S, Takeuchi M, Honda A, Tahara A, Nitta Y, Kodama N, Mizoguchi M, Kaida H, Ishibashi M, et al. 2012b. Positive association between serum level of glyceraldehyde-derived advanced glycation end products and vascular inflammation evaluated by [(18)F]fluorodeoxyglucose positron emission tomography. Diabetes Care 35:2618–2625.
- Tahara N, Yamagishi SI, Kodama N, Tahara A, Honda A, Nitta Y, Igata S, Matsui T, Takeuchi M, Kaida H, et al. 2015. Clinical and biochemical factors associated with area and metabolic activity in the visceral and subcutaneous adipose tissues by FDG-PET/CT. J Clin Endocrinol Metab. 100:E739–E747.
- Takenaka K, Yamagishi S, Matsui T, Nakamura K, Imaizumi T. 2006. Role of advanced glycation end products (AGEs) in thrombogenic abnormalities in diabetes. Curr Neurovasc Res. 3:73–77.
- Takeuchi M, Yamagishi S. 2009. Involvement of toxic AGEs (TAGE) in the pathogenesis of diabetic vascular complications and Alzheimer's disease. J Alzheimers Dis. 16:845–858.
- The Diabetes Control and Complications Trial Research Group. 1993. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 329:977–986.
- Tortorella SM, Royce SG, Licciardi PV, Karagiannis TC. 2015. Dietary sulforaphane in cancer chemoprevention: the role of epigenetic regulation and HDAC inhibition. Antioxid Redox Signal. 22:1382–1424.
- Turin TC, Murakami Y, Miura K, Rumana N, Kadota A, Ohkubo T, Okamura T, Okayama A, Ueshima H; NIPPON DATA 80 Group. 2012. Diabetes and life expectancy among Japanese-NIPPON DATA80. Diabetes Res Clin Pract. 96:e18–e22.
- Ueda S, Yamagishi S, Matsui T, Noda Y, Ueda S, Jinnouchi Y, Sasaki K, Takeuchi M, Imaizumi T. 2012. Serum levels of advanced glycation end products (AGEs) are inversely associated with the number and migratory activity of circulating endothelial progenitor cells in apparently healthy subjects. Cardiovasc Ther. 30:249–254.
- UK Prospective Diabetes Study (UKPDS) Group. 1998. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–853.
- Velmurugan GV, Sundaresan NR, Gupta MP, Gupta MP, White C. 2013. Defective Nrf2-dependent redox signalling contributes to microvascular dysfunction in type 2 diabetes. Cardiovasc Res. 100:143–150.
- Vincent AM, Kato K, McLean LL, Soules ME, Feldman EL. 2009. Sensory neurons and Schwann cells respond to oxidative stress by increasing antioxidant defense mechanisms. Antioxid Redox Signal. 11:425–438.
- Vlassara H, Uribarri J. 2014. Advanced glycation end products (AGE) and diabetes: cause, effect, or both? Curr Diab Rep. 14:453.
- Wang Y, Zhang Z, Sun W, Tan Y, Liu Y, Zheng Y, Liu Q, Cai L, Sun J. 2014. Sulforaphane attenuation of type 2 diabetes-induced aortic damage was associated with the upregulation of Nrf2 expression and function. Oxid Med Cell Longev. 2014:123963.
- Writing Group for the DCCT/EDIC Research Group. 2015. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA 313:45–53.
- Xu j, Kulkarni SR, Donepudi AC, More VR, Slitt AL. 2012. Enhanced Nrf2 activity worsens insulin resistance, impairs lipid accumulation in adipose tissue, and increases hepatic steatosis in leptin-deficient mice. Diabetes 61:3208–3218.
- Xue M, Qian Q, Adaikalakoteswari A, Rabbani N, Babaei-Jadidi R, Thornalley PJ. 2008. Activation of NF-E2-related factor-2 reverses biochemical dysfunction of endothelial cells induced by hyperglycemia linked to vascular disease. Diabetes 57:2809–2817.
- Yamagishi S. 2011. Role of advanced glycation end products (AGEs) in osteoporosis in diabetes. Curr Drug Targets. 12:2096–2102.
- Yamagishi S. 2012. Potential clinical utility of advanced glycation end product cross-link breakers in age-and diabetes-associated disorders. Rejuvenation Res. 15:564–572.
- Yamagishi S, Adachi H, Takeuchi M, Enomoto M, Furuki K, Matsui T, Nakamura K, Imaizumi T. 2007. Serum level of advanced glycation end-products (AGEs) is an independent determinant of plasminogen activator inhibitor-1 (PAI-1) in nondiabetic general population. Horm Metab Res. 39:845–848.
- Yamagishi S, Amano S, Inagaki Y, Okamoto T, Takeuchi M, Makita Z. 2002. Beraprost sodium, a prostaglandin I2 analogue, protects against advanced gycation end products-induced injury in cultured retinal pericytes. Mol Med. 8:546–550.
- Yamagishi S, Fujimori H, Yonekura H, Yamamoto Y, Yamamoto H. 1998. Advanced glycation endproducts inhibit prostacyclin production and induce plasminogen activator inhibitor-1 in human microvascular endothelial cells. Diabetologia 41:1435–1441.
- Yamagishi S, Imaizumi T. 2005. Diabetic vascular complications: pathophysiology, biochemical basis and potential therapeutic strategy. Curr Pharm Des. 11:2279–2299.
- Yamagishi S, Kobayashi K, Yamamoto H. 1993. Vascular pericytes not only regulate growth, but also preserve prostacyclin-producing ability and protect against lipid peroxide-induced injury of co-cultured endothelial cells. Biochem Biophys Res Commun. 190:418–425.
- Yamagishi SI, Matsui T. 2010a. Anti-atherothrombogenic properties of PEDF. Curr Mol Med. 10:284–291.
- Yamagishi S, Matsui T. 2010b. Soluble form of a receptor for advanced glycation end products (sRAGE) as a biomarker. Front Biosci (Elite Ed). 2:1184–1195.
- Yamagishi S, Matsui T. 2015. Protective role of sodium-glucose cotransporter 2 (SGLT2) inhibition against vascular complications in diabetes. Rejuvenation Res. doi: 10.1089/rej.2015.1738.
- Yamagishi S, Matsui T, Fukami K. 2015a. Role of receptor for advanced glycation end products (RAGE) and its ligands in cancer risk. Rejuvenation Res. 18:48–56.
- Yamagishi S, Matsui T, Takenaka K, Nakamura K, Takeuchi M, Inoue H. 2009. Pigment epithelium-derived factor (PEDF) prevents platelet activation and aggregation in diabetic rats by blocking deleterious effects of advanced glycation end products (AGEs). Diabetes Metab Res Rev. 25:266–271.
- Yamagishi S, Nakamura K, Matsui T. 2008. Role of oxidative stress in the development of vascular injury and its therapeutic intervention by nifedipine. Curr Med Chem. 15:172–177.
- Yamagishi S, Nishino Y, Ojima A, Matsui T, Nishi H. 2015b. Oral consumption of sulforaphane precursor-rich broccoli supersprouts decreases serum levels of advanced glycation end products in humans. Diabetes Frontier Online 2:e1–e011.
- Yoh K, Hirayama A, Ishizaki K, Yamada A, Takeuchi M, Yamagishi S, Morito N, Nakano T, Ojima M, Shimohata H, et al. 2008. Hyperglycemia induces oxidative and nitrosative stress and increases renal functional impairment in Nrf2-deficient mice. Genes Cells 13:1159–1170.
- Yoo SH, Lim Y, Kim SJ, Yoo KD, Yoo HS, Hong JT, Lee MY, Yun YP. 2013. Sulforaphane inhibits PDGF-induced proliferation of rat aortic vascular smooth muscle cell by up-regulation of p53 leading to G1/S cell cycle arrest. Vascul Pharmacol. 59:44–51.
- Zakkar M, Van der Heiden K, Luong le A, Chaudhury H, Cuhlmann S, Hamdulay SS, Krams R, Edirisinghe I, Rahman I, Carlsen H, et al. 2009. Activation of Nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler Thromb Vasc Biol. 29:1851–1857.
- Zhang Z, Wang S, Zhou S, Yan X, Wang Y, Chen J, Mellen N, Kong M, Gu J, Tan Y, et al. 2014. Sulforaphane prevents the development of cardiomyopathy in type 2 diabetic mice probably by reversing oxidative stress-induced inhibition of LKB1/AMPK pathway. J Mol Cell Cardiol. 77:42–52.
- Zhao XD, Zhou YT, Lu XJ. 2013. Sulforaphane enhances the activity of the Nrf2-ARE pathway and attenuates inflammation in OxyHb-induced rat vascular smooth muscle cells. Inflamm Res. 62:857–863.
- Zheng H, Whitman SA, Wu W, Wondrak GT, Wong PK, Fang D, Zhang DD. 2011. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes 60:3055–3066.
- Zhu H, Jia Z, Strobl JS, Ehrich M, Misra HP, Li Y. 2008. Potent induction of total cellular and mitochondrial antioxidants and phase 2 enzymes by cruciferous sulphoraphane in rat aortic smooth muscle cells: cytoprotection against oxidative and electrophilic stress. Cardiovasc Toxicol. 8:115–125.