Abstract
Context The present study deals with new biological properties of the wild edible Diplotaxis simplex (Viv.) Spreng (Brassicaceae).
Objectives The current study evaluates the antioxidant, the anti-inflammatory and the anti-cancer properties of ethyl acetate and ethanol extracts from D. simplex flowers.
Materials and methods The anti-proliferative activity of the extracts (10–70 μg/mL) was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) against human colon cancer cell line Caco-2. The anti-inflammatory potential was evaluated by the inhibitory effect of the extracts (1.5–7.5 mg/mL) on phospholipase A2 activity as well as on carrageenan-induced paw oedema in mice. Extracts (200 mg/kg) or indomethacin (50 mg/kg) as positive control were injected intraperitoneally for albino mice prior to the induction of the oedema by carrageenan. Antioxidant activities were investigated using various complementary methods.
Results Flower extracts contained a high level of polyphenolics (17.10–52.70 mg GAE/g) and flavonoids (74.20–100.60 mg QE/g), which correlate with its appreciable antioxidant potential in β-carotene peroxidation (IC50 value: 12.50–27.10 μg/mL), DPPH• radical-scavenging (IC50 value: 0.20–0.40 mg/mL), Fe3+ reducing (EC50 value: 0.10–0.14 mg/mL) and Fe2+ chelating (IC50 value: 0.20–0.60 mg/mL) assays. These extracts were effective in inhibiting cancer cell growth (IC50 value: 62.0–63.25 μg/mL). Besides, the ethyl acetate extract inhibited phospholipase A2 activity (IC50 value: 2.97 mg/mL) and reduced the paw oedema in mice (from 0.38 ± 0.01 to 0.24 ± 0.01 cm), 4 h post-carrageenan challenge.
Conclusion These data suggest that D. simplex may be useful as a candidate in the treatment of inflammation and the colon cancer.
Introduction
Inflammation is a pathophysiological process of plasma-derived and cellular events in response to infection and tissue injury. Acute inflammation is characterized by oedema, redness, fever, pain and even a loss of function depending on the severity of the aggression. Plasma exudation induced oedema by tissue distension and causes excessive pressure on local nerve endings which explains the feeling of swelling and pain. The increase in the circulatory flow in the inflamed site partially explains the appearance of heat and redness. These symptoms result from the action of several mediators such as serotonin, bradykinin, histamine, arachidonic acid derivatives, platelet-activating factor (PAF) and nitric oxide, which can originate locally or from cells that infiltrate in the site of inflammation (Mequanint et al. Citation2011). Phospholipases A2 (PLA2) are regulatory enzymes that mediate eicosanoids (including prostaglandins and leukotrienes) synthesis and PAF production. Thus, they play an important role in the initiation and amplification of the inflammatory reaction by the release of arachidonic acid from the membrane phospholipids, which are metabolized either by the cyclooxygenase or by the lipoxygenase enzymatic pathway to produce various groups of eicosanoids. Experimental and clinical evidence suggest that PLA2 may serve as primary regulatory role in the development of inflammatory disorders (Anderson et al. Citation1994). To treat acute inflammatory disorders, steroidal anti-inflammatory drugs were used. However, the use of these medicines is frequently related with disorders on gastric mucosa, as well as on the renal and cardiovascular systems (Harirforoosh et al. Citation2013). Non-steroidal anti-inflammatory drugs are also an important group of drugs largely utilized in the treatment of inflammatory diseases, acting by inhibition of the cyclooxygenase and then prevent the conversion of arachidonic acid to prostaglandins (Blackler et al. Citation2014). Nevertheless, these drugs have some limitations and cannot regulate the production of leukotrienes or PAF that continues to cause inflammation. Besides, cyclooxygenase inhibitors could cause thrombosis or renovascular hypertension in patients predisposed to these conditions (FitzGerald Citation2003). It seems reasonable that inhibitors of PLA2 could deplete the sources of arachidonic acid and, therefore, its downstream metabolites and PAF.
Oxidative stress is considered crucial in the initiation and development of many chronic and age-related degenerative diseases including inflammation and cancer. Actually, it is commonly admitted that an imbalance between oxidants and antioxidants in the body results in overproduction of free radicals at the site of inflammation, which leads to oxidative stress and contributes to tissue damage (Ksouri et al. Citation2012). Strong and consistent epidemiology evidence indicates that antioxidant-rich food consumption is effective in reducing the risk of many cancers. Phenolics constitute one of the most numerous and ubiquitous groups of plant metabolites, and are an integral part of the human diet. These compounds have demonstrated a possible role in the prevention of degenerative diseases associated with oxidative stress (Scalbert et al. Citation2005).
The plant kingdom is a very promising and probably inexhaustible source of drugs and nutraceuticals. However, very few plants have been well studied and a large majority expects to be interested. Diplotaxis simplex (Viv.) Spreng (Brassicaceae) is native to the Mediterranean region and represents an important wild edible herb in Tunisia. Many previous studies refer to the genus Diplotaxis as traditionally used plants with therapeutic properties. Moreover, several species of Diplotaxis are reported as food crops in different regions (Guarrera Citation2003). In fact, these herbs are appreciated for their strong pungent flavour and they are consumed raw or cooked, in salads and soups (Zouari Citation2015). Recently, it was shown that this species suppresses postprandial hyperglycaemia in mice by inhibiting key-enzymes linked to type 2 diabetes (Jdir et al. Citation2015).
The objective of the present work was to investigate the anti-proliferative activity of the flower extracts against the human colon cancer Caco-2 cells. The anti-inflammatory potential was assessed by the inhibitory effect of the extracts on phospholipase A2 activity as well as on carrageenan-induced paw oedema in mice. Total polyphenolics and flavonoids content, and antioxidant potential using five complementary different methods were also investigated.
Materials and methods
Chemicals
2,2-Diphenyl-1-picrylhydrazyl (DPPH•), quercetin (QE), gallic acid (GA), linoleic acid, β-carotene, Tween 40, pancreatic phospholipase A2 (PLA2), Egg yolk phosphatidylcholine and sodium taurodeoxycholate (NaTDC) were purchased from Sigma-Aldrich (St. Louis, MO). All other chemicals were of analytical grade.
Plant material
Diplotaxis simplex was collected, in February 2014, from south-eastern Tunisia (Medenine) and identified by Pr. Mohamed Debouba. A voucher specimen is deposited at Higher Institute of Applied Biology of Medenine (Medenine, Tunisia) under the number Ds01. After harvest, flowers were separated and shade-dried for 20 d, then ground into fine powder and stored at ambient temperature in a dry place and in the dark until use.
Animals
Male albino mice with body masses of 20–25 g, obtained from the Veterinary Research Institute (Sfax, Tunisia), were used in this study. The animals were maintained under standard environmental conditions of temperature, relative humidity, a 12 h dark/light cycle, with ad libitum access to food and water. The experimental protocol was approved by the Medical Ethics Committee for the Care and Use of Laboratory Animals of the Pasteur Institute of Tunis (approval number: FST/LNFP/Pro 152012) and performed according to the European convention for the protection of living animals used in scientific investigations (Council of European Communities Citation1986).
Preparation of D. simplex extracts
The dried powder of the D. simplex flowers (25 g) was Soxhlet-extracted with 300 mL ethyl acetate followed by 300 mL ethanol during 6 h for each solvent. Each solvent was then evaporated using a rotary evaporator and the residual solvent was removed by flushing with nitrogen. Finally, the obtained extracts were kept in the dark at 4 °C until further analysis.
Antioxidant potential
Polyphenolic and flavonoid contents
Polyphenolic and flavonoid contents of D. simplex extracts were determined as previously described by Dewanto et al. (Citation2002) and they were expressed as mg gallic acid equivalent (GAE)/g extract and mg quercetin equivalent (QE)/g extract, respectively. Plant extracts were dissolved in dimethyl sulphoxide (DMSO).
Antioxidant activities
DPPH• radical-scavenging, metal (Fe2+) chelating, β-carotene bleaching and reducing power assays of D. simplex extracts were measured as previously described (Dinis et al. Citation1994; Yildirim et al. Citation2001; Zouari et al. Citation2011). Results of DPPH• radical-scavenging, metal (Fe2+) chelating and β-carotene bleaching assays are presented by IC50 values, defined as the extract concentration needed to scavenge 50% of DPPH•, to chelate 50% of Fe2+ and to obtain 50% inhibition of β-carotene peroxidation, respectively. In the reducing power assay, the presence of antioxidants in the sample would result in the reducing of Fe3+ to Fe2+, which can be monitored by measuring the formation of Perl’s Prussian blue (Fe4[Fe(CN)6]3) at 700 nm. The extract concentration (EC50) providing 0.5 of an absorbance at 700 nm was presented. Lower IC50 and EC50 values reflected better antioxidant activities.
DNA nicking assay
The capacity of D. simplex extracts to protect DNA from oxidation by hydroxyl radicals generated by Fenton reaction was performed using pCRII-TOPO plasmid (Invitrogen, Carlsbad, CA) by the method of Lee et al. (Citation2002) with slight modifications. A mixture of 10 μL of plant extract at a concentration of 250 or 500 μg/mL and plasmid DNA (0.5 μg/well) were incubated for 5 min at room temperature followed by the addition of 10 μL of Fenton’s reagent (30 mmol/L H2O2, 50 μmol/L ascorbic acid and 80 μmol/L FeCl3). After 5 min incubation at 37 °C, the DNA was analyzed on an agarose gel (1%).
Carrageenan-induced mice paw oedema
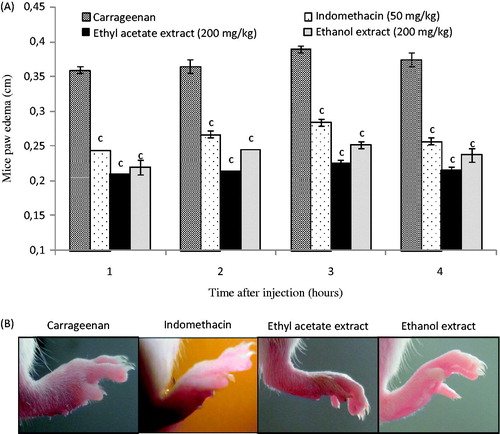
A total of 20 mice were divided into four groups of five (n = 5). Group 1 was served as a negative control and received normal saline [solution of 0.90% (m/v) NaCl]. Prior to the induction of the oedema, groups 2 and 3 were injected intraperitoneally with ethyl acetate and ethanol extracts (200 mg/kg), respectively. Group 4 was administered by an intraperitoneal injection of indomethacin (50 mg/kg) as a positive control. All drugs were suspended in normal saline and were administered 30 min before the carrageenan challenge. In fact, 0.3 mL of 2% carrageenan in normal saline was injected in the right hind paw of all animals under the subplantar region to produce acute inflammation (Rotelli et al. Citation2003). The paw oedema thickness was measured before and after induction of the inflammatory response by using a digital micrometer (MT-045B; Shangai Metal Great Tools Co., Shangai, China). All the assessments were performed by the same investigator in order to reduce any potential inter-operator differences.
Phospholipase A2 inhibition assay
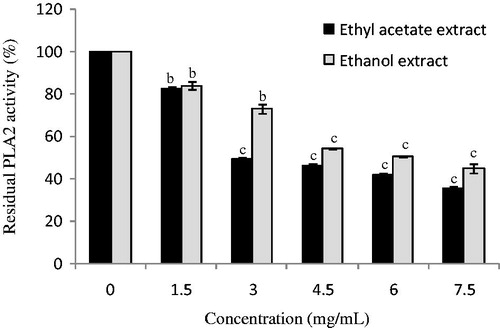
Phospholipase inhibition assay was performed using PLA2/extract pre-incubation method during 1 h at room temperature in the absence of substrate (Kammoun et al. Citation2011). Pre-incubation medium consisted of 10 units of PLA2 and ethyl acetate or ethanol extract dissolved in dimethyl sulphoxide (DMSO) with varying concentrations from 1.5 to 7.5 mg/mL. A control sample was prepared accordingly without plant extract. The residual activity was measured titrimetrically at pH = 8 and 40 °C with a pH-stat (Metrohm, Buchs, Switzerland), using 0.5% (m/v) egg yolk phosphatidylcholine as a substrate in 30 mL of 150 mmol/L NaCl, 4 mmol/L sodium taurodeoxycholate (NaTDC) and 8 mmol/L CaCl2. The results were expressed as residual activity compared with the control. IC50 value, defined as the sample concentration (mg/mL) at which 50% inhibition of the enzyme activity occurs, was calculated from the graph plotting enzyme residual activity against sample concentration. All tests were carried out for three sample replications and the results were averaged.
Cell culture
Human cancer cell line Caco-2 was purchased from the Riken Cell Bank (Tsukuba, Japan). Cell culture was maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) (Sigma-Aldrich, St. Louis, MO) supplemented with 10% heat-inactivated foetal bovine serum (Sigma-Aldrich, St. Louis, MO), 1% non-essential amino acids (Cosmo Bio Co., Ltd., Tokyo, Japan) and 1% penicillin (5000 IU/mL)/streptomycin (5000 μg/mL) solution (ICN Biomedicals, Santa Ana, CA) at 37 °C under 5% CO2 atmosphere.
Anti-proliferative effect by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
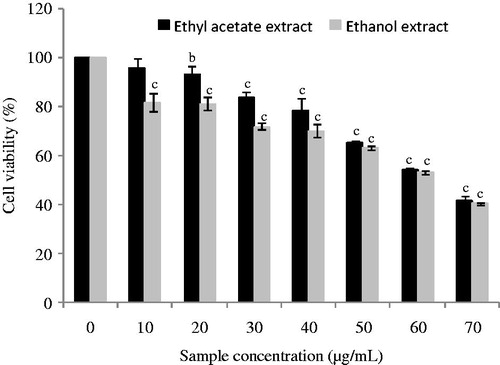
Cell viability was assessed using MTT (Sigma-Aldrich, St. Louis, MO) assay as previously described (Boulaaba et al. Citation2013). Caco-2 cells were seeded in 96-well plates at a concentration of 2 × 104 cells/mL in DMEM medium. Cells were kept at 37 °C under 5% CO2 and treated with different concentrations (10–70 μg/mL) of D. simplex extracts dissolved in 70% ethanol. After 48 h treatment, 10 μL MTT solutions (5 mg/mL) were added to the culture medium. After 24 h of incubation, the formazan product was dissolved using 100 μL of 10% SDS solution (Wako, Osaka, Japan). The absorbance was measured at 570 nm on a multi-detection microplate reader (Thermo Labsystems, Franklin, MA). The results were expressed as a percentage of cell proliferation by using the following formula:
where As is the absorbance of the sample and Ac is the absorbance of the control reaction without plant extract. IC50 value, defined as the sample concentration (mg/mL) that inhibited 50% of cell proliferation, was calculated from the graph plotting cell viability against sample concentration. All tests were carried out for three sample replications and the results were averaged.
Statistical analysis
All analytical determinations were performed at least in triplicate. Values were expressed as the mean ± standard deviation. Analysis of variance was conducted, and differences between variables were tested for significance by one-way analysis of variance using SPSS 11 (Statistical Package for the Social Sciences, The Predictive Analytics Company, Chicago, IL). A difference was considered statistically significant at least when p < 0.05.
Results and discussion
Phenolic compound contents and antioxidant activities of D. simplex flowers
shows that the yield of extractable compounds relative to the mass of dried flowers ranged from 7.40 g/100 g (ethanol extract) to 38.30 g/100 g (ethyl acetate extract). It is well known that phenolic compounds such as flavonoids, phenolic acids and tannins contribute directly to the antioxidant capacity of plants. Indeed, phenolic compounds exhibit considerable free radical-scavenging activities (through their reactivity as hydrogen- or electron-donating agents) and metal ion-chelating properties, preventing metal-induced free radical formation (Rice-Evans et al. Citation1996). Therefore, the amounts of polyphenolics and flavonoids contents in flower extracts were determined (). Results showed that ethanol extract of D. simplex flowers presented the highest content of polyphenolics (52.70 ± 2.1 mg GAE/g extract) than ethyl acetate extract (17.10 ± 0.3 mg GAE/g extract). Nevertheless, the highest flavonoids content was found in ethyl acetate extract (100.60 ± 3.4 mg QE/g extract). Falleh et al. (Citation2013) reported that the amount of phenolics present in Diplotaxis species exceeded those found in other medicinal plants. Besides, they showed that D. simplex flowers exhibited the highest polyphenolics and flavonoids contents with respect to leaves and stems of the plant. HPLC analysis showed that the main phenolic compound identified in D. simplex flowers was caffeic acid. Furthermore, epigallocatechin, chlorogenic, p-coumaric and 3,4-dimethoxybenzoic acids were also identified in the plant flowers (Falleh et al. Citation2013). After that, flower extracts were subjected to their antioxidant activities which were evaluated by complementary methods such as 2,2-diphenyl-1-picrylhydrazyl (DPPH•) radical-scavenging, reducing power, metal (Fe2+) chelating and β-carotene bleaching assays (). Ethanol extract, which contains the highest polyphenolics content, presented the highest DPPH• radical-scavenging (IC50 value: 0.20 ± 0.02 mg/mL), β-carotene bleaching (IC50 value: 12.5 ± 0.02 μg/mL) and Fe3+ reducing (EC50 value: 0.10 ± 0.01 mg/mL) activities than ethyl acetate extract. However, ethyl acetate extract which contains the highest flavonoids content showed stronger metal (Fe2+) chelating activity (IC50 value: 0.20 ± 0.02 mg/mL) than ethanol extract. Many studies have demonstrated that flavonoids not only have anti-radicals activities but also have the ability to chelate transition metal ions, which may afford a better protection against lipid peroxidation (Olennikov et al. Citation2014). The protective effect of D. simplex extracts on DNA damage was also studied. shows the electrophoretic pattern of plasmid DNA after the Fenton’s reagent induced damage both in the presence and in the absence of D. simplex extracts. The Fenton reaction involves the reaction between hydrogen peroxide and Fe2+ to form hydroxyl radicals (OH•), which are known to cause oxidative breaks in DNA strands. Lane 1 shows an untreated plasmid with its two forms: the upper one was the open-circular (nicked) DNA and the faster migrating band corresponds to closed-circular (supercoiled) plasmid. However, lane 6 shows complete degradation of all DNA bands treated with Fenton’s reagent. The action of flower extracts on the DNA damage caused by OH• was indicated from lane 2 to lane 5. In the presence of D. simplex extracts, the DNA forms were protected against the hydrogen peroxide-induced damage. The obtained results suggest that flower extracts from D. simplex protected DNA through antioxidant activity. Oxidative modification of DNA has been suggested to contribute to aging and various diseases including cancer and chronic inflammation (Kumar & Chattopadhyay Citation2007). Interestingly, it was reported that antioxidant compounds such as phenolic acids and flavonoids possess promising anti-inflammatory potential and anti-proliferative activity against various tumour cell lines (Park et al. Citation2008; Weng & Yen Citation2012).
Figure 1. Gel electrophoresis pattern of the DNA plasmid incubated with Fenton’s reagent both in the presence and in the absence of flowers extracts from D. Simplex. Lane 1: untreated control: native DNA plasmid (0.5 μg); lanes 2 and 3: DNA with ethyl acetate extract at 500 and 250 μg/mL, respectively; lanes 4 and 5: DNA with ethanol extract at 500 and 250 μg/mL, respectively; lane 6: DNA sample incubated with Fenton’s reagent.
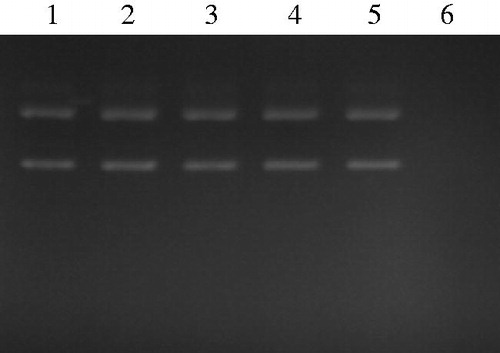
Table 1. Polyphenolic and flavonoid contents, and antioxidant activities of extracts from D. simplex flowers.
Evaluation of the anti-inflammatory effect of D. simplex flowers
Inhibition of phospholipase A2 activity
Ethyl acetate and ethanol extracts from D. simplex flowers were tested to inhibit phospholipase A2 activity, which play an important role in the initiation and amplification of the inflammatory reaction. Flower extracts showed inhibitory activities against PLA2 activity in various percentages depending on the concentrations of the plant extracts (. Ethyl acetate extract inhibited PLA2 with an IC50 value of 2.97 ± 0.03 mg/mL, which is more potent than ethanol extract (IC50 value: 5.0 ± 0.02 mg/mL). Obtained results were comparable with the inhibitory effect of the PLA2 activity by various extracts from Aloe vera L. (Aloeaceae), which possess an interesting anti-inflammatory potential (Habeeb et al. Citation2007; Kammoun et al. Citation2011). Li et al. (Citation2003) studied the effect of ethanol extracts from various plants, used in traditional Chinese medicine to treat inflammatory conditions, against a panel of key enzymes related to inflammation process such as phospholipase A2, cyclooxygenases and lipoxygenases. In fact, all the plant extracts showed inhibitory activities against at least one of these enzymes. The PLA2 inhibition could be explained by the richness of the studied extracts in phenolic compounds, since it was previously reported that phenolic compounds present an important capacity of inhibiting the enzymatic activity of PLA2 (Kammoun et al. Citation2011). Molecular-modelling studies suggested that phenolic hydroxyls are linked to the amino acid Asp 49 of PLA2 and influence the capacity of this residue to participate in the coordination of the calcium atom, that is, essential to the catalytic activity (Da Silva et al. Citation2009).
Effect of D. simplex extracts on carrageenan-induced paw oedema
Oedema is an important sign of inflammation. Carrageenan-induced paw oedema as an in vivo model of inflammation is the most frequently used method to evaluate the anti-oedema effect of natural products. In this study, the oedema was quantified by measuring the hind paw thickness at different time points. Before induction of the inflammatory response, mice paw thickness was found to be 0.20 ± 0.01 cm. As shown in , mice of the negative control group that received carrageenan in the plantar fascia of the hind paw developed an oedema characterized by an increase in paw thickness up to 0.36 ± 0.01 and 0.37 ± 0.01 cm after 1 and 4 h, respectively. The treatment with the standard indomethacin significantly (p < 0.001) inhibited the paw thickness of carrageenan-induced mice, which reached 0.26 ± 0.01 cm after 4 h time course of the experiment. Interestingly, D. simplex extracts presented important anti-oedema activity after carrageenan injection compared with the positive control indomethacin. As was found for the PLA2 inhibition, ethyl acetate extract seems to be more potent than ethanol extract in the reduction of mice paw oedema (. Oueslati et al. (Citation2015) also investigated the effect of flowers extract from D. simplex on nitric oxide (NO•) overproduction in stimulated RAW 264.7 macrophages. More importantly, these researchers reported that flower extract displayed a strong anti-inflammatory activity by inhibiting NO• release in lipopolysaccharide-induced RAW 264.7 cells. All these results suggest that D. simplex flowers had appreciable anti-inflammatory potential. The antioxidant potential observed in this plant may also contribute for reducing inflammation. Thus, the potent anti-inflammatory activity of D. simplex may be related to cumulative effects of different active compounds to reduce the synthesis, release and action of prostaglandins or free radicals.
Figure 3. (A) Effect of ethyl acetate and ethanol extracts from D. simplex flowers, and indomethacin as positive control administered intraperitoneally on carrageenan-induced paw oedema in mice. Each point represents the mean of five animals and different letters above the bars indicate significant differences when compared with the negative control group: c (p < 0.001). (B) Photographs of paw oedema in mice at 4 h after injection of different treatments.
Evaluation of anti-proliferative effect of D. simplex extracts on Caco-2 cancer cells
The strong accumulation of phenolic compounds in the D. simplex flowers as well as their interesting antioxidant potential prompted us to investigate for the first time the anti-proliferative capacity of the plant extracts on human colon cancer Caco-2 cells. shows that flower extracts from D. simplex were significantly (p < 0.001) active against the Caco-2 cells growth. The IC50 values were found to be 62.0 ± 0.5 μg/mL for ethanol extract and 63.25 ± 0.25 μg/mL for ethyl acetate extract. Obtained results suggest that D. simplex flowers had an interesting influence on Caco-2 cancer cells, indicating the presence of powerful cytotoxic compounds in ethyl acetate and ethanol fractions, such as phenolic compounds. In this regard, various studies showed that polyphenolic compounds affect cancer cell growth by inducing apoptosis in many cell lines related to colon cancer (CaCo-2, SW620, HT-29 and HCT-116) (Dai & Mumper Citation2010). In addition, our results could also be explained by the presence of specific compounds, such as glucosinolates that characterize the Brassicaceae family and the genus Diplotaxis, and which are known by their cancer chemoprotective attributes (Fahey et al. Citation2001).
Figure 4. Antiproliferative activity of ethyl acetate and ethanol extracts from D. simplex flowers on Caco-2 cancer cells. Data are presented as mean ± SD of triplicate determinations. Different letters above the bars indicate significant differences when compared with the control: b (p < 0.01) and c (p < 0.001).
One important aspect of carcinogenesis is recognized to be the involvement of inflammation. Pro-inflammatory prostaglandins are assumed to be associated with colon, lung, breast and prostate carcinogenesis. Interestingly, natural phenolics could exhibit anti-inflammatory properties and also a possible role in the inhibition of cancer development through a number of basic cellular mechanisms (Dai & Mumper Citation2010).
Conclusion
The trends toward natural products promoting health is likely to increase. Furthermore, few data about D. simplex are available in the literature. Therefore, improving knowledge on the pharmacological properties of D. simplex would assist in efforts for functional applications of these plants as a new potential health-promoting vegetable. Interestingly, D. simplex flowers showed interesting antioxidant potential associated with important anti-inflammatory capacity and anti-proliferative effect against Caco-2 cancer cells. Further investigations are needed to identify the active constituents responsible for the anti-inflammatory and anti-proliferative properties of D. simplex.
Funding information
This work received financial support from « Ministère de l’Enseignement Supérieur et de la Recherche Scientifique, Tunisia ».
Acknowledgements
This work is part of a doctoral thesis by Hamida Jdir. Special thanks to Miss Amina Gammoudi (ISBAM) for her kind help with English language.
Disclosure statement
The authors report that they have no conflicts of interest.
References
- Anderson BO, Moore EE, Banerjee A. 1994. Phospholipase A2 regulates critical inflammatory mediators of multiple organ failure. J Surg Res. 56:199–195.
- Blackler RW, Gemici B, Manko A, Wallace JL. 2014. NSAID-gastroenteropathy: new aspects of pathogenesis and prevention. Curr Opin Pharmacol. 19:11–16.
- Boulaaba M, Mkadmini K, Tsolmon S, Han J, Smaoui A, Kawada K, Ksouri R, Isoda H, Abdelly C. 2013. In vitro antiproliferative affect of Arthrocnemum indicum extracts on Caco-2 cancer cells through cell cycle control and related phenol LC-TOF-MS identification. Evid Based Complement Alternat Med [Online]. [cited 2013 Aug 16]. Available from: http://dx.doi.org/10.1155/2013/529375.
- Council of European Communities. 1986. Council instructions about the protection of living animals used in scientific investigations. Official J Eur Communities (JO 86/609/CEE). L358:1–18.
- Dai J, Mumper RJ. 2010. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 15:7313–7352.
- Da Silva SL, Calgarotto AK, Maso V, Damico DC, Baldasso P, Veber CL, Villar JA, Oliveira AR, Comar M Jr, Oliveira KM, et al. 2009. Molecular modeling and inhibition of phospholipase A2 by polyhydroxy phenolic compounds. Eur J Med Chem. 44:312–321.
- Dewanto V, Wu X, Adom KK, Liu RH. 2002. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 50:3010–3014.
- Dinis TC, Maderia VM, Almeida LM. 1994. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 315:161–169.
- Fahey JW, Zalcmann AT, Talalay P. 2001. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 56:5–51.
- Falleh H, Msilini N, Oueslati S, Ksouri R, Magne C, Lachaal M, Karray-Bouraoui N. 2013. Diplotaxis harra and Diplotaxis simplex organs: assessment of phenolics and biological activities before and after fractionation. Ind Crop Prod. 45:141–147.
- FitzGerald GA. 2003. COX-2 and beyond: approaches to prostaglandin inhibition in human disease. Nat Rev Drug Discov. 2:879–890.
- Guarrera PM. 2003. Food medicine and minor nourishment in the folk traditions of Central Italy (Marche, Abruzzo and Latium). Fitoterapia. 10:515–544.
- Habeeb F, Stables G, Bradbury F, Nong S, Cameron P, Plevin R, Ferro VA. 2007. The inner gel component of Aloe vera suppresses bacterial-induced proinflammatory cytokines from human immune cells. Methods. 42:388–393.
- Harirforoosh S, Asghar W, Jamali F. 2013. Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci. 16:821–847.
- Jdir H, Khemakham B, Chakroun M, Zouari S, Ben Ali Y, Zouari N. 2015. Diplotaxis simplex suppresses postprandial hyperglycemia in mice by inhibiting key-enzymes linked to type 2 diabetes. Rev Bras Farmacogn. 25:152–157.
- Kammoun M, Miladi S, Ben Ali Y, Damak M, Gargouri Y, Bezzine S. 2011. In vitro study of the PLA2 inhibition and antioxidant activities of Aloe vera leaf skin extracts. Lipids Health Dis. 10:30.
- Ksouri R, Ksouri WM, Jallali I, Debez A, Magné C, Hiroko I, Abdelly C. 2012. Medicinal halophytes: potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit Rev Biotechnol. 32:289–326.
- Kumar A, Chattopadhyay S. 2007. DNA damage protecting activity and antioxidant potential of pudina extract. Food Chem. 100:1377–1384.
- Lee J, Kim H, Kim J, Jang Y. 2002. Antioxidant property of an ethanol extract of the stem of Opuntia ficus-indica var. Saboten. J Agric Food Chem. 50:6490–6496.
- Li RW, Lin GD, Myers SP, Leach DN. 2003. Anti-inflammatory activity of Chinese medicinal vine plants. J Ethnopharmacol. 85:61–67.
- Mequanint W, Makonnen E, Urga K. 2011. In vivo anti-inflammatory activities of leaf extracts of Ocimum lamiifolium in mice model. J Ethnopharmacol. 134:32–36.
- Olennikov DN, Kashchenko NI, Chirikova NK. 2014. A novel HPLC-assisted method for investigation of the Fe2+-chelating activity of flavonoids and plant extracts. Molecules. 19:18296–18316.
- Oueslati S, Ellili A, Legault J, Pichette A, Ksouri R, Lachaal M, Karray-Bouraoui N. 2015. Phenolic content, antioxidant and anti-inflammatory activities of Tunisian Diplotaxis simplex (Brassicaceae). Nat Prod Res. 29:1189–1191.
- Park HH, Lee S, Son HY, Park SB, Kim MS, Choi EJ, Singh TS, Ha JH, Lee MG, Kim JE, et al. 2008. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch Pharm Res. 31:1303–1311.
- Rice-Evans CA, Miller NJ, Paganga G. 1996. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 20:933–956.
- Rotelli AE, Guardia T, Juárez AO, de la Rocha NE, Pelzer LE. 2003. Comparative study of flavonoids in experimental models of inflammation. Pharmacol Res. 48:601.
- Scalbert A, Johnson IT, Saltmarsh M. 2005. Polyphenols: antioxidants and beyond. Am J Clin Nutr. 81:215S–217S.
- Weng CJ, Yen GC. 2012. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat Rev. 38:76–87.
- Yildirim A, Mavi A, Kara AA. 2001. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem. 49:4083–4089.
- Zouari N, Fakhfakh N, Zouari S, Bougatef A, Neffati M, Ayadi MA. 2011. Chemical composition, angiotensin I-converting enzyme inhibitory, antioxidant and antimicrobial activities of essential oil of Tunisian Thymus algeriensis Boiss. et Reut. (Lamiaceae). Food Bioprod Process. 89:257–265.
- Zouari N. 2015. Eating wild edible plants can be a good alternative? Med Aromat Plants [Online]. [cited 2015 Oct 23]. Available from: http://dx.doi.org/10.4172/2167-0412.S1-e001