Abstract
Context The roots of Ilex asprella (Hook. et Arn.) Champ. ex Benth. (Aquifoliaceae) are widely used in Chinese medicine to treat influenza, amygdalitis, pertussis, etc. Their mechanism of action is still unknown, which raises the need to identify new bioactive compounds in this plant.
Objective In this study, we isolated a novel saponin containing sulphonic groups, namely, asprellcoside A (1) and a known phenolic glycoside compound (2) from the roots of Ilex asprella and evaluated their bioactivities.
Materials and methods Molecular structures were elucidated by analysing their spectral and chemical properties. The viability of A549 cells was tested using a MTT assay. Ability of the compounds to inhibit viruses was determined using the neuraminidase activity assay. Their anti-inflammatory effects were tested using the IP-10 activity assay using various concentrations (compound 1: 0.6, 0.2, 0.6, 1.70, 5.00 and 15.00 μM; compound 2: 0.4, 1.2, 3.6, 11.0, 33.0 and 100 μM). Their inhibitory effect on platelet aggregation induced by adenosine diphosphate (ADP) in rabbit plasma was determined at 60 and 80 μM.
Results Both compounds inhibit influenza virus strain A/PuertoRico/8/1934 (H1N1) strongly with EC50 values of 4.1 and 1.7 μM, respectively. Both compounds inhibit the secretion of IP-10 with EC50 values of 6.6 and 2.5 μM, respectively. Compound 1 alone inhibited platelet aggregation significantly, with the rate of suppression being 47 ± 8 and 38 ± 3%, at 60 and 80 μM, respectively.
Conclusions The results suggest that both compounds may be valid therapeutics against influenza virus infection and that compound 1 may be a novel agent for treating thrombosis.
Introduction
Ilex asprella (Hook. et Arn.) Champ. ex Benth. (Aquifoliaceae) originates from southeast China. The plant is widely distributed in the Guangdong, Guangxi and Fujian provinces. The roots of I. asprella are commonly used by famous doctors of the Lingnan style in traditional Chinese medicine. It is also a major ingredient of ‘San-Dong Herbal Tea’ and ‘WangLoKAT’, which have been widely accepted as beneficial beverages by locals for a long time. Previous studies revealed that it is mainly composed of the triterpenoid saponin, phenolic acid and alkaloid compounds (Cai et al. Citation2010; Huang et al. Citation2012). Modern pharmacologic studies demonstrated that the extract of IA has significant anti-inflammatory, anti-influenza, anti-bacterial and anti-tumor activities (Liu et al. Citation2004; Zhu et al. Citation2007; Luo et al. Citation2010). Considerable recent research interests focus on the roots of I. asprella with the target of obtaining new compounds and screening for bioactive molecules. Our previous studies showed that total phenolic acids and total saponins in the IA extract remarkably reduce inflammation caused by H1N1 influenza virus infection and decrease mortality. We also showed that the extract of IA is used to treat acute respiratory distress syndrome (ARDS) potentially due to the attenuation of systemic and pulmonary inflammatory responses and inhibition of viral replication (Dai et al. Citation2014). Nevertheless, investigations of the bioactive chemicals in the IA extract are rarely reported. In this study, a novel saponin containing sulpho groups and a known phenolic glycoside were separated and identified from the roots of I. asprella using chromatographic, spectroscopic and chemical analyses. Some polysaccharides have no or weak antiviral activity at first, but show strong antiviral activity after sulphonation, implying that compounds containing sulphur groups may possess antiviral activity (Huang et al. Citation2007). Furthermore, anticoagulant activity is mainly conferred by the presence of sulphonic acid in the molecular side chains of polysaccharides (Mei et al. Citation2013). Therefore, we evaluated these compounds for their anti-viral and anti-inflammatory effects against influenza A virus infection and inhibitory effect on ADP-induced platelet aggregation in vitro.
Materials and methods
Materials
NMR spectra were obtained using a Bruker BioSpin GmbH AV 400 spectrometer (Karlsruhe, Baden-Württemberg, Germany). MS spectra were obtained using a Thermo LTQ Orbitrap mass spectrometer (Waltham, MA). Column chromatography was performed using X-5 macro-reticular resin (Cangzhou Bon Adsorber Technology Co. Ltd, Cangzhou, Hebei, PR China), RP-C18 silica gel (Merck, Darmstadt, Germany) and Sephadex LH-20 (Sigma, Santa Clara, CA). Thin layer chromatography was performed using silica gel 60 F254 (Merck, Darmstadt, Germany). Preparative HPLC was performed on an Agilent 1200 with a UV detector and a C18 reversed-phase column (Phenomenex, 10 × 250 mm, 5 μm, Torrance, CA) with HPLC solvents (Merck, Darmstadt, Germany).
Plant material
The roots of I. asprella were collected in July 2012 from Conghua City, Guangdong province, PR China. The plant was identified by Prof. XiaoPing Lai. The sample (NO.201,207) was deposited in the School of Chinese Materia Medica, Guangzhou University of Chinese Medicine.
Extraction and isolation
The dry roots of I. asprella (10 kg) were homogenized and extracted with 54% (v/v) ethyl alcohol (4 × 80 L) using the percolation method. The 54% ethyl alcohol extract was condensed under vacuum to obtain the residual extract. Some of the residue (385 g) was depurated using an X-5 macro-reticular resin column (ϕ 25 × 100 cm) and eluted in succession with H2O, 40%, 70% and 95% ethyl alcohol. The concentrated 40% ethyl alcohol fraction (86 g) was further purified using a RP-C18 column (ϕ 3.5 × 60 cm) by eluting with MeOH:0.1%HCOOH (3:7 → 3:2 v/v) and monitored by TLC (CH2Cl2: EtOAc: H2O: HCOOH = 3:22:5:2:0.2 v/v) analysis to yield four combined fractions (Fr.1–Fr.4). Fr.4 was applied to Sephadex LH-20 (ϕ 2.6 × 100 cm), washed with MeOH:0.1%HCOOH (1:1 v/v), and finally purified on a reversed-phase preparative HPLC column using MeOH:0.1%HCOOH (2:3 v/v) as the solvent to yield compound 2 (29 mg). The concentrated 70% ethyl alcohol fraction was separated using Sephadex LH-20 (ϕ 2.6 × 100 cm) by eluting with methanol to obtain four major fractions. Fraction 3 was further separated using an ODS-C18 column (ϕ 2.6 × 70 cm) by eluting with CH3OH:H2O (2:8→8:2 v/v) to obtain seven main fractions (A–G). Fraction D was purified using an ODS-C18 column (ϕ 2.6 × 40 cm) by washing with CH3OH–H2O (2:8→7:3 v/v) and recrystallized to yield compound 1 (47.2 mg).
Biological assay
Cell lines and viral strains
The Human Pulmonary Epithelial (A549) cell line (ATCC CCL-185) was provided by the Wuhan Institute of Virology, Chinese Academy of Sciences (CAS) and maintained in Minimal Essential Medium (MEM). The A/PuertoRico/8/34 (H1N1) (PR8) viral strain developed in Madin–Darby canine kidney (MDCK) cells was obtained from the Wuhan Institute of Virology, CAS. The 50% tissue culture infective dose (TCID50) in MDCK cells was calculated using the Reed and Muench method. The virus was stored at −80 °C.
MTT assay to test cytotoxicity
The MTT [3-(4,5-dimethylthiazol-2-yl)-3,5-diphenyltetrazolium bromide, Sigma] assay procedure for cytotoxicity was performed as previously described (Liao et al. Citation2013). A549 cells were grown in 96-well plates at a density of 2 × 104 cells per well. After 16 h, the culture medium was replaced with a fresh medium containing the compounds at six different concentrations and the cells were incubated for 48 h in an incubator at 37 °C. The medium was removed and replaced with 100 μL MTT solution and incubated for 4 h. The MTT solution was carefully removed, and 150 μL of DMSO was added to each well. The absorbance at a wavelength of 490 nm was measured using a microplate reader.
Neuraminidase activity assay to test viral inhibition
The neuraminidase activity assay procedure for viral inhibition was previously reported (Liao et al. Citation2013). Cells were grown in 96-well plates at a density of 2 × 104 cells per well. After 16 h, cells were infected with 100 TCID50 for 1 h. After attachment, the influenza solution was removed and replaced with different concentrations of compounds (compound 1: 0.6, 0.2, 0.6, 1.70, 5.00 and 15.00 μM; compound 2: 0.4, 1.2, 3.6, 11.0, 33.0 and 100 μM) for 12 h. Then, the solution was replaced with a fresh medium for 36 h. Supernatants were collected, and the influenza virus neuraminidase activity was measured using a standard fluorimetric assay. In brief, all samples were mixed with 70 μL buffer, 10 μL Milli-Q water and 10 μL neuraminidase fluorogenic substrate, and were incubated for 20 min at 37 °C. The fluorescence intensity was measured at 355 nm (excitation) and 485 nm (emission) using a multilabel plate reader (Wallac Envision, PerkinElmer, MA). The effective concentration that induced 50% inhibition of the virus (EC50) was calculated through non-linear regression analysis (sigmoidal dose-response variable slope) using GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA).
IP-10 activity assay to test the anti-inflammatory effect
Cell culture supernatants were collected using the same protocol as the viral inhibition assays. Levels of IFN-γ-inducible Protein 10 (IP-10) in the cell supernatants were determined using an enzyme-linked immunosorbent assay (ELISA, R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. In brief, all samples were mixed with 50 μL enzyme tag solution and 100 μL Milli-Q water, and were incubated for 60 min at 37 °C. Then, the solution was removed and washed five times with the cleaning solution. Fifty microliters each of Chromogenic reagent A and B were added to each well and incubated for 10 min at 20–25 °C on an opaque background. The reaction was terminated by adding 50 μL stop buffer. The absorbance was measured at 490 nm using a multilabel plate reader. The concentrations of the samples were calculated according to the standard curve. The effective concentration that inhibited the virus by 50% (EC50) was calculated through non-linear regression analysis (sigmoidal dose-response variable slope) using the GraphPad Prism 5.0 software.
Animals
Specific pathogen-free (SPF) male New Zealand rabbits, each weighing 2.0 kg, were purchased from the Laboratory Animal Center, Guangzhou University of Chinese Medicine, Guangzhou, China, on 20 December 2014 (Animal permit number: SCKX (Cantonese) 2013–0020). The rabbits were housed individually in stainless steel cages in a climate-controlled animal facility at a temperature of 22 ± 2 °C with a 12 h light cycle (7:00–19:00). All animals had free access to food and water throughout the experiment. All experimental protocols were approved by the Ethics Committee of the Guangzhou University of Chinese Medicine.
Evaluation of platelet aggregation activity
The protocol for evaluation of platelet aggregation activity was previously described (Fan et al. Citation2015). Blood was collected from the carotid arteries of New Zealand white rabbits with 3.8% trisodium citrate (9:1, v/v) as an anticoagulant. Platelet-rich plasma (PRP) and platelet-poor plasma (PPP) were obtained from the supernatants by centrifugation at 1000 rpm for 10 min and 3000 rpm for 20 min, respectively. Platelet aggregation was measured using an aggregometer (SHANDA PA-196, Shanghai Shan Da Industrial Co. LTD, Shanghai, PR China) and calculated by the turbidimetric method. The PRP was pre-incubated with the studied compounds at concentrations of 60 and 80 μM for 10 min at 37 °C, and platelet aggregation was induced by the addition of ADP (final concentration 4 μM). Platelet aggregation inhibition (%) was calculated using the following formula: Inhibition rate (%) = [1 − (D/S)] × 100, where D = maximal aggregation of compounds and S = maximal aggregation of solvent.
Results and discussion
Structure determination and identification of compounds 1 and 2
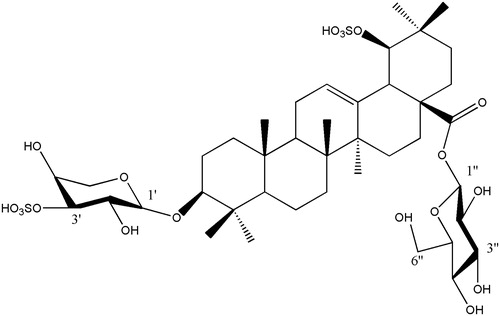
Compound 1 was obtained as colorless needles (MeOH). Preliminary analysis showed that compound 1 was a triterpenoid saponin as both the Liebermann-Burchard and Molish reactions were positive. The HR-ESI-MS spectrum of 1 at m/z 925.3554 [M – H]– and element analysis showing 6.610% sulphur content correspond to a molecular weight of 926.36 and chemical formula of C41H66O19S2. The 13C NMR spectra and DEPT showed 41 carbon signals (), which consisted of 30 carbon resonances attributable to a triterpenoid skeleton and the surplus 11 ascribed to a hexose and a pentose sugar unit.
Table 1. 1H NMR (pyridine-d5, 400 MHz) and 13C NMR (pyridine-d5, 100 MHz) data for Compound 1 (δ values in ppm, J values in Hz are in parentheses).
In the 1H NMR spectrum of 1 (), signals for seven methyl groups appeared at δH 1.21, 0.96, 0.85, 1.13, 1.62, 1.15, 0.98 (each s), signals for two anomeric protons at δH 4.68 (d, J = 7.2 Hz) and 6.37 (d, J = 7.9 Hz), and an olefinic proton at δH 5.50 (br s). These data indicated that compound 1 was an oleanane-type glycoside containing two glycosyls.
In the 13C NMR spectra, five sugar carbons at δC 106.4, 73.9, 82.4, 74.7 and 63.9 were characteristic of a β-arabinose residue except for 82.4, which was a downfield shift signal indicating that one of the sulphur groups was attached at C-3′. Six sugar carbons at δC 95.8, 74.1, 78.8, 71.0, 79.2 and 62.1 were characteristic of a β-glucose residue. The HMBC spectrum (, ) showed correlation peaks between the H-1′ (δH 4.68) of arabinose and C-3 (δC 106.4) of the aglycone, as well as the H-1′′ (δH 6.37) of glucose and C-28 (δC 95.8) of the aglycone, affirming that the glycosidation positions of β-arabinose and β-glucose are located on C-3 and C-28 of the triterpenoid sapogenin, respectively. These data were similar to those of asprellanoside A (Zhou et al. Citation2012). However, the formula weight of 1 was 80 Da greater than that of asprellanoside A, which can be ascribed to a SO3H group. In the 13C NMR spectrum, the C-19 of 1 shifted to the lower field by 7.3 ppm, while the C-18, C-20 and C-22 shifted to the upper field by 10.5, 7.1 and 6.3 ppm, respectively, when compared to asprellanoside A. The above data demonstrated that the hydroxyl group at C-19 was sulphated with another SO3H group in 1. From the above evidence, the structure of compound 1 () was identified as 3-O-β-l-sulphonylarabinopyranosyl-19-O-sulphonylhydroxy-oleanic acid-28-O-β-d-glucopyranosyl ester and we named it asprellcoside A.
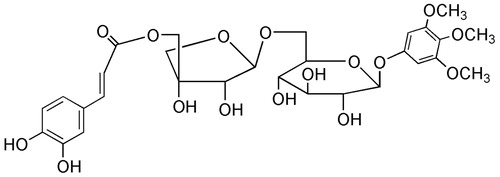
Compound 2 was isolated as a yellowish powder. Its chemical formula was determined as C29H36O16 by HR-ESI-MS at m/z 639.1925 [M − H]− with a molecular weight of 640.19. The 1H NMR spectrum showed signals for a pair of trans-olefinic protons at δH 7.50 (1H, d, J = 15.9 Hz) and 6.27 (1H, d, J = 15.9 Hz) conjugated with a carbonyl group; three aromatic proton signals at δH 7.06 (1H, d, J = 2.2 Hz), 7.01 (1H, d, J = 8.1 Hz) and 6.76 (1H, dd, J = 2.2, 8.1 Hz) attributed to one ABX-bin system; three methoxys at δH 3.73 (6H, s) and 3.57 (3H, s); and two anomeric protons at δH 4.86 (1H, d, J = 2.9 Hz) and δH 4.80 (1H, d, J = 7.6 Hz), respectively.
The 13C NMR spectrum exhibited signals for a caffeoyl group [δC: 125.6 (C-1), 116.0 (C-2), 145.9 (C-3), 148.8 (C-4), 113.8 (C-5), 121.6 (C-6), 145.7 (C-7), 115.1 (C-8), 166.7 (C-9)]; a 3,4,5-trimethoxyphenoxy group [δC: 154.1 (C-1), 94.6 (C-2,6), 153.3 (C-3,5), 132.8 (C-4), 60.3 (4-OCH3), 56.0 (3,5-OCH3)]; and two glycosyl groups assignable to d-glucose [δC: 101.0 (C-1), 75.6 (C-2), 76.9 (C-3), 70.2 (C-4), 76.7 (C-5), 67.9 (C-6)] and d-apiose [δC: 108.8 (C-1), 73.3 (C-2), 77.2 (C-3), 73.5 (C-4), 66.0 (C-5)]. Further comparison between the NMR spectral data with those published in the literature (Fuchino et al. Citation1997) led to its identification as 3,4,5-trimethoxyphenol β-d-5-O-caffeoyl-apiofuranosyl-(1→6)-β-d-glucopyranoside (. It was first isolated by Fuchino et al. (Citation1997) from the roots of I. asprella.
Inhibitory effects of isolated compounds against influenza virus a/PR/8/34 (H1N1) in A549 cells
The inhibitory activities of both compounds against influenza A/PR/8/34 (H1N1) were evaluated using oseltamivir as a positive control. On the basis of the cardiac concentration response and data presented in , and , the 50% inhibitory concentration (EC50) values of the compounds against influenza virus A/PR/8/34(H1N1) were 4.1 and 1.7 μM, respectively. The 50% cytotoxic concentration (CC50) values of both compounds were >100 μM in A549 cells. The selective index (SI) values of the two compounds were 24.3 and 58.8, respectively.
Figure 4. Inhibitory effects of Oseltamivir on the neuraminidase and IP-10 activity. The bar indicates the standard deviation of three different experiments.
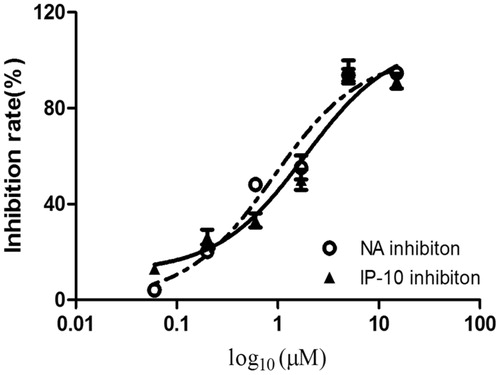
Figure 5. Inhibitory effects of compound 1 on the neuraminidase and IP-10 activity. The bar indicates the standard deviation of three different experiments.
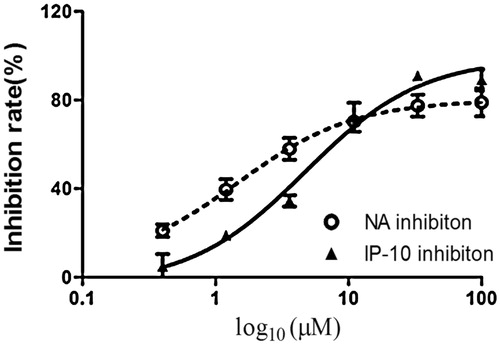
Table 2. Cytotoxicity and inhibitory effects of compounds 1 and 2 on neuraminidase activity.
Suppression of pro-inflammatory cytokines in H1N1-infected cells
Infection with the influenza virus causes a cytokine storm. IP-10 plays an important role in the influenza virus induced inflammation response and can serve as a biomarker for the inflammation mediated by the virus. The inhibitory effects of both compounds against pro-inflammatory cytokines were assessed using oseltamivir as a positive control. As shown in and , both compounds inhibited the secretion of IP-10 in influenza A/PR/8/34 (H1N1)-infected A549 cells in a dose-dependent manner. The EC50 values of the compounds on IP-10 levels were 6.6 and 2.5 μM, respectively. The CC50 values of both compounds were >100 μM. The SI values were 15.5 and 40, respectively.
Table 3. Cytotoxicity and inhibitory effects of compounds 1 and 2 on IP-10 activity.
Inhibitory effect of the compounds on platelet aggregation induced by ADP in rabbits
The inhibition of platelet aggregation by compounds 1 and 2 was analyzed in rabbit plasma (). In this experiment, we used ozagrel as a positive control. Compound 1 inhibited platelet aggregation significantly, while compound 2 did not. The data are shown in .
Figure 6. Inhibitory effects of compound 2 on the neuraminidase and IP-10 activity. The bar indicates the standard deviation of three different experiments.
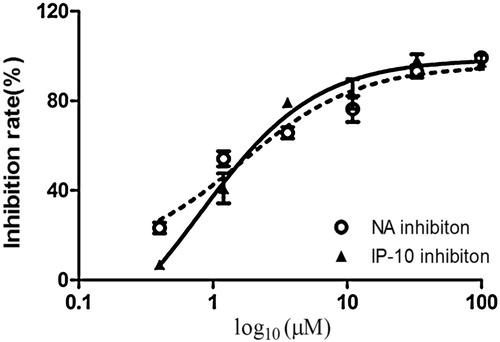
Table 4. Inhibitory effects of compounds 1 and 2 on platelet aggregation.
Conclusions
The incidence of influenza among the normal population and rate of death in the aged and other critically ill patients are considerable. Antiviral therapy is effective when administered within 48 h after the appearance of symptoms, but the curative effect diminishes with time. Research on anti-inflammatory therapy to ameliorate influenza-induced inflammation is important to impact the clinical outcome. Anti-viral and anti-inflammatory drugs with novel mechanisms of action are urgently needed. In the present study, two compounds were isolated from the roots of Ilex asprella which showed strong antiviral activity against the influenza A virus in vitro. The selective indices of compounds 1 and 2 were >24.3 and >58.8, respectively, indicating that these two compounds have potent antiviral activity.
Chemokines are chemotactic cytokines produced by various cells upon influenza virus infection, which recruit monocytes, neutrophils and leukomonocytes to the site of inflammation. Mononuclear cells and macrophages secrete multiple chemokines during influenza A virus infection, including IP-10, which induce the recruitment of inflammatory cells to the infected sites (Baggiolini Citation1998). Clinical and in vivo studies show that pro-inflammatory cytokines are highly induced during an H5N1 infection (Peiris et al. Citation2009). Induction of the pro-inflammatory cytokine IP-10, one of the key factors that contributes to lung inflammation during an H5N1 virus infection, has been shown to be associated with the pathogenesis of H5N1 infection (De Jong et al. Citation2006; Cameron et al. Citation2008; Perrone et al. Citation2010; Ichikawa et al. Citation2013). In this study, we showed that compounds 1 and 2 decreased the levels of IP-10 in H1N1-infected cells. The selective indices are >15.5 and >40, respectively, indicating that these two compounds have potent anti-inflammatory effects against influenza-associated inflammation. Our results strengthen the potential of the two compounds as therapeutic agents in the treatment of influenza virus infection due to a combination of anti-viral and anti-inflammatory effects.
It was indicated that the antiviral mechanism of sulphated polysaccharides may be due to the binding of the negative charge in the sulphur groups with the positive charge in the viral proteins, preventing the adsorption of virus to the cells and sealing off the binding sites between the enveloped virus and its cellular receptors. Although the type of virus is different, the antiviral mechanism is similar (Huang et al. Citation2007). The antiviral activity of compound 1 may be attributed to its sulphur groups.
Little information is available about the pharmacological activity of monomeric chemical substances from the roots of I. asprella. According to a previous report, only two triterpenoids from the roots exhibited anti-HSV-1 activity (Zhou et al. Citation2012). Our results demonstrate that both compound 1 and 2 have antiviral activity against the H1N1 influenza virus in A549 cells. However, the exact mechanisms are not clear. Further studies are needed to ascertain which stages of the viral life cycle are impaired and which targets are inhibited by these two compounds. Compounds 1 and 2 should also be explored as novel anti-influenza drugs, either alone or in combination with other anti-influenza drugs.
In the circulating blood, platelets are usually separate and are not aggregated. When bleeding, platelets and other coagulating substances, such as calcium and thrombin, gather into a mass and seal the injured vessel wall. Hence, the aggregation of platelets leads to the formation of thrombosis (Born & Cross Citation1963). Sulphonic acid has electrostatic repulsive force to electronegatively separate blood constituents, which decreases the absorption of fibrinogen and platelets, leading to its anticoagulant activity (Chen et al. Citation2011). Thus, in our study, only compound 1, which contains sulphur groups, showed a suppressive effect in vitro on ADP-induced rabbit platelet aggregation. This result shows that this triterpenoid glycoside may be developed as a novel antithrombotic agent.
In conclusion, this study examined for the first time the significant antiviral and antithrombotic properties of the principal components from the roots of Ilex asprella. Further studies may focus on the mechanism involved in the antiviral and antithrombotic activities, and on the synthesis and screening of triterpenoid glycoside and phenolic glycoside derivatives to derive inhibitors with greater potency. In addition, histopathologic assessment of the major organs of Swiss Albino mice should be performed to assess the safety profiles of these two compounds (Bhakta et al. Citation2013).
Funding information
This work was funded by the Major Program of the Guangdong Natural Science Foundation of China (No. S20 1230,006598), the innovative program for college graduates of Guangdong Province (No. E1-KFD0,1,5141K01), the State major research program for college graduates of Guangdong Province (No. E1-KFD0,1,5151K02), and the Excellent Young Scholars Research Fund of the Guangzhou University of Chinese Medicine (No. 201, 301).
Disclosure statement
The authors declare that they have no conflicts of interest.
References
- Baggiolini M. 1998. Chemokines and leukocyte traffic. Nature. 392:565–568.
- Bhakta D, Sivaramakrishna A, Siva R. 2013. Bioactive iridoid glycoside isolated from Morinda tinctoria (Roxb.) roots exhibit therapeutic efficacy. Ind Crops Prod. 42:349–356.
- Born GV, Cross MJ. 1963. The aggregation of blood platelets. J Physiol (Lond). 168:178–195.
- Cai Y, Zhang QW, Li ZJ, Fan CL, Wang L, Zhang XQ, Ye WC. 2010. Chemical constituents from roots of Ilex asprella. Chin Traditional Herbal Drugs (in Chinese). 41:1426–1429.
- Cameron CM, Cameron MJ, Bermejo-Martin JF, Ran LS, Xu LL, Turner PV, Ran R, Danesh AL, Fang Y, Chan PKM, et al. 2008. Gene expression analysis of host innate immune responses during lethal H5N1 infection in ferrets. J Virol. 82:11308–11317.
- Chen C, Huang MF, Yan SY, Yan W, Yi CF, Xu ZS. 2011. Development of research on heparin-like polymer. New Chem Mater (in Chinese). 39:18–21.
- Dai WP, Li G, Li X, Hu QP, Liu JX, Zhang FX, Su ZR, Lai XP. 2014. The roots of Ilex asprella extract lessons acuterespiratory distress syndrome in mice induced by influenza virus. J Ethnopharmacol. 155:1572–1582.
- De Jong MD, Simmons C, Thanh TT, Hien VM, Smith GJD, Chau TNB, Hoang DM, Van Vinh Chau N, Khanh TH, Dong VC, et al. 2006. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nature Med. 12:1203–1207.
- Fan Z, Zhou L, Xiong TQ, Zhou JS, Li QG, Tan QL, Zhao ZX, Jin J. 2015. Antiplatelet aggregation triterpene saponins from the barks of Ilex rotunda. Fitoterapia. 101:8–15.
- Fuchino H, Tachibana H, Tanaka N. 1997. Three new hemiterpene glycosides from Ilex macropoda. Pharmaceutical Soc Japan. 45:1533–1535.
- Huang JC, Chen FL, Chen HM, Zeng YE, Xu HH. 2012. Chemical constituents in roots of Ilex asprella. Chin Traditional Herbal Drugs (in Chinese). 43:1475–1478.
- Huang XY, Kong XF, Wang DY, Hu YL. 2007. Research progress on sulfating modification of polysaccharides and sulfated polysaccharides. Nat Prod Res Dev (in Chinese). 19:328–332.
- Ichikawa A, Kuba K, Morita M, Chida S, Tezuka H, Hara H, Sasaki T, Ohteki T, Ranieri VM, Dos Santos CC, et al. 2013.– CXCL10-CXCR3 enhances the development of neutrophil-mediated fulminant lung injury of viral and nonviral origin. CXCR3 of the fulminant injury viral nonviral. Am J Respir Crit Care Med. 187:65–67.
- Liao QJ, Qian ZX, Liu R, An LW, Chen XL. 2013. Germacrone inhibits early stages of influenza virus infection. Antiviral Res. 100:578–588.
- Liu CS, Chen HP, Li WQ, Wang ZR. 2004. Anti-Inflammatory activity of the methanol extract from Roughhaired Holly Root. Chin Traditional Herbal Drugs (in Chinese). 27:519–520.
- Luo YJ, Sun YX, Dai ZQ, Zheng SP, Zeng QW. 2010. Studies on antiInflammatory activity of the methanol extract from Roughhaired Holly Root. Guizhou Anim Sci Vet Med (in Chinese). 34:1–2.
- Mei TX, Wang XY, Wan G, Nie HJ, Bao GM. 2013. Progress in modifications of polysaccharide and its anticoagulant activity. Acta Agric Univ Jiangxiensis (in Chinese). 35:1108–1113.
- Peiris JSM, Cheung CY, Leung CY, Nicholls JM. 2009. Innate immune responses to influenza A H5N1: friend or foe? Trends Immunol. 30:574–584.
- Perrone LA, Szretter KJ, Katz JM, Mizgerd JP, Tumpey TM. 2010. Mice lacking both TNF and IL-1 receptors exhibit reduced lung inflammation and delay in onset of death following infection with a highly virulent H5N1 virus. J Infect Dis. 202:1161–1170.
- Zhou M, Xu M, Ma XX, Zheng K, Yang K, Yang CR, Wang YF, Zhang YJ. 2012. Antiviral triterpenoid saponins from the roots of Ilex asprella. Planta Med. 78:1702–1705.
- Zhu WQ, Liu HS, Yan GH, Li PB. 2007. Anti-viral activity of the water extract from Radix et Caulis Ilicis asprellae. J Trop Med (in Chinese). 7:555–557.