Abstract
Context: Myrtus communis L. (Myrtaceae), myrtle, is an evergreen shrub with strong antibacterial, anti-inflammatory, antihyperglycemic and antioxidant activities. Also, it is used as a sedative-hypnotic plant in Iranian traditional medicine.
Objective: This study evaluates the effect of 80% ethanolic extract of M. communis leaves on sleep and anxiety in mice and rats.
Materials and methods: Male NMRI mice were subjected to open field, righting reflex, grip strength and pentylentetrazole-induced seizure tests. Male Wistar rats were used to evaluate the alterations in rapid eye movement (REM) and non-REM (NREM) sleep. They were treated with 25–400 mg/kg doses of the extract intraperitoneally.
Results: The applied doses (50–200 mg/kg) of M. communis extract increased vertical (ED50 = 40.2 ± 6.6 mg/kg) and vertical and horizontal activity (ED50 = 251 ± 55 mg/kg), while treatment with 200 and 400 mg/kg attenuated muscle tone significantly compared to vehicle treated animals (p < 0.001 for all) in a dose-independent manner. Also, a significant hypnotic and not anticonvulsant effect was observed when animals were treated with 200 mg/kg of the extract (p < 0.01). In this regard, electroencephalography results showed that REM sleep time was decreased (2.4 ± 0.5%), while total and NREM sleep times were increased significantly compared to the control group of mice (82.5 ± 7.6%).
Discussion and conclusion: The data show the anxiolytic and muscle relaxant effect of the extract without anticonvulsant activities. The anxiolytic, myorelaxant and hypnotic effects without effect on seizure threshold are in line with the effect of a alpha 2 GABA receptor agonist.
Introduction
Studies in the USA and Europe have revealed that, on average, 30% of people have difficulty initiating and/or maintaining sleep (Doghramji Citation2006). Hypnotics such as alcohol, benzodiazepines (BNZs) and other antidepressants, as well as over-the-counter medications are used to treat insomnia (Richey & Krystal Citation2011). However, drug dependence, rebound insomnia, muscle relaxation, memory dysfunction and changes in homeostatic regulation of electroencephalography (EEG) are some of the unpleasant side effects associated with the use of such medicines (Borbely & Achermann Citation1991; Dijk et al. Citation1997). Efforts are being made to find an ideal hypnotic, with minimum side effects, including minimal effects on sleep architecture [rapid eye movement (REM) and non-REM (NREM) duration]. REM sleep behaviour disorder, a primary sleep disorder, can occur after withdrawal from alcohol, tricyclic and SSRIs antidepressants. Sleep disorders are characterized by the lack of atonia and REM sleep that leads to dreams involving aggression, and violence (Olson et al. Citation2000). It has been shown that BNZs reduce EEG power in frequencies below 3 Hz within REM sleep. Furthermore, they increase the power of EEG within frequency above 13 Hz during NREM sleep.
Myrtus communis L. (Myrtaceae), myrtle, is an evergreen perennial shrub with numerous stems and ramified white flowers. This plant can be found in humid and semi-humid areas (Messaoud et al. Citation2005). It has been widely used in Iranian traditional medicine. Decocted leaves of myrtle are used for various purposes such as hair conditioning, astringent activity, digestive discords and as a bronchodilator (Pirbalouti Citation2009). Several studies have exposed the strong antibacterial, anti-inflammatory, antihyperglycemic and antioxidant activities in different extracts of this plant (Hayder et al. Citation2004; Feißt et al. Citation2005). Only a few studies have reported an unclear sedative effect of myrtle (Mulas & Cani Citation1999). The major constituents of this plant include monoterpenoids like 1,8-cineole, limonene, linalool, α-terpineol (Rahimmalek et al. Citation2013). Some studies show that linalool (Elisabetsky et al. Citation1995), 1,8-cineole (Santos & Rao Citation2000) and α-terpineol (Buchbauer et al. Citation1993) possess sedative properties. Due to these characteristics, it could be useful in the treatment of insomnia and mood disorders such as anxiety.
In this study, we evaluated the effects of the hydroalcoholic extract of myrtle on sleep disorders and anxiety in male NMRI mice and rats. Behavioural and electrophysiological experiments were used to uncover the efficacy and probable mechanisms of action of the extract.
Materials and methods
Plant and extract preparation
The leaves of M. communis were collected in May 2013 from Khorram Abad (Lorestan), Iran and were identified by Mr. Kamalinejad, a qualified botanist at the Department of Botany of the Medical Sciences, University of Shahid Beheshti. The specimen was deposited in the herbarium of the Department of Pharmacognosy, School of Pharmacy, Shahid Beheshti University of Medical Sciences and Health Services, Tehran, Iran.
Leaves of the plant were dried, powdered (200 g) and macerated with an 80% ethanol solution (700 mL) for 3 days with three changes of the solution. The resulting extract was filtered and evaporated under a vacuum into a dried powder extract. The remaining powder was kept in a refrigerator (4 °C). At the time of the experiment, the dry residue was dissolved in normal saline to produce the required concentrations.
Animals
Male NMRI mice (22–27 g) and male Wistar rats (295–320 g) were purchased from Pasteur Institute of Iran. The animals were maintained at controlled room temperature (25 ± 2 °C) in a 12 h light/dark cycle (light from 07:00 to 19:00), with free access to food and water except at the time of the experiments. All behavioural observations were conducted between 10:00 and 14:00.
Materials
Diazepam (PubChem CID: 3016) was provided from Darupakhsh Co. (Tehran, Iran). Flumazenil (PubChem CID: 3373), pentylentetrazole (PTZ) (PubChem CID: 5917) and pentobarbital sodium (PubChem CID: 4737) were purchased from Sigma-Aldrich (St. Louis, MO).
Experimental set-up
The extract of myrtle and normal saline (control) was injected intraperitoneally (10 mL/kg for mice and 1 mL/kg for rats). All animals were handled before the experiments and were habituated to the lab environment for 30 min before each test. Two different types of experiments, behavioural and EEG evaluations were conducted in this study. Mice were randomly selected for the behavioural tests while rats were randomly selected for the EEG experiment. Each animal was used only once in each behavioural or EEG recording test.
Behavioural experiments
Open field test
An open field arena (40 cm × 40 cm × 40 cm) made of white-opaque Plexiglas was used for evaluation of spontaneous locomotor activity. The arena had a virtual central zone (28 cm × 28 cm). Normal saline (control) and the extract in doses of 25–200 mg/kg were injected into the different groups of mice (n = 10) and after 20 min, the activity of animals was recorded for 10 min. The mice were gently placed at the centre of the arena and their behaviours (velocity, distance moved, number of rears and duration in centre) were recorded by a camera for 10 min. All the records were analysed by Ethovision XT 8 (Noldus, Wageningen, Netherlands) software.
Loss of righting reflex test
The pentobarbital induced loss of righting reflex test was used for evaluating the hypnotic effects of the extract (Erden et al. Citation1997). The mice were pretreated by normal saline (control), diazepam as a reference drug (2 mg/kg) or M. communis extract (25–400 mg/kg). After 30 min, sodium pentobarbital (30 mg/kg, i.p., n = 10) was injected and the duration of sleep (loss of righting reflex) was recorded.
Flumazenil was used in both open field and righting reflex tests in order to investigate the action of the active compound(s) of the extract on GABAA receptor. Flumazenil (10 mg/kg) was administrated 15 min after the injection of normal saline or the extract.
Grip strength test
The grip strength test is a technique used to measure paw strength in mice. Thirty minutes after the administration of normal saline or the extract (100–400 mg/kg), muscle strength of each mouse was recorded using the Bioseb Grip Strength Instrument (In Vivo Research Instruments Co., Tehran, Iran).
PTZ- and MES-induced seizure tests
Pentylenetetrazole (PTZ) induced seizure and maximal electroshock (MES) models were used for assessment of the anticonvulsant effect of the extract. PTZ (100 mg/kg, i.p.) was injected (n = 10 mice in each group) 30 min after pretreatment with normal saline (control) or the extract (100–400 mg/kg). In PTZ model, the percentage of animals showing convulsion and the mortality rate was recorded (Faizi et al. Citation2012).
In MES method, 30 min after the administration of normal saline or the extract (100–400 mg/kg) to the mice (n = 10 in each group), the electrodes were connected to the ears of the animals and they received an electrical stimulus (current intensity 37.2 mA, frequency 60 Hz, duration 0.3 s) to induce maximal seizures. Hind limbs with tonic extension (HLTE) were used as the endpoint of the test. The behaviour of the animals was observed and the percentage of animals showing HLTE was reported (White Citation1997).
Acute toxicity evaluation
Doses of the extract ranging from 1 to 4 g/kg were administrated intraperitoneally to mice. The mortality rate was recorded until 1 week after the administration of extract.
Electroencephalographic evaluations
Surgery method
In order to identify electrophysiological sleep–wake states of rats, we used chronically implanted electrodes. A cocktail of ketamine (90 mg/kg) and xylazine (9 mg/kg) was injected i.p. to induce and maintain anaesthesia and analgesia in animals. Two parietal electrodes, one frontal and one occipital, were pointed in the surface of the skull. While occipital and left parietal electrodes were selected to record EEG, frontal and right parietal were chosen as EEG and EMG references. Stainless-steel screws were threaded into the holes as EEG electrodes. Two subcutaneous wire electrodes were implanted in the area between the neck muscle and the occipital bone to record EMG. Differential recordings from the two electrodes located in the lateral parietal and lateral occipital cortices were used to record slow wave and spindle neocortical EEG activity. The electrode located in the frontal cortex was used as a reference electrode (Pivik et al. Citation1993). Pins of all the electrodes used for both EEG and EMG recordings were connected to small plastic sockets. The sockets were then glued to the skull using dental cement. Animals were housed individually for 7–10 days in order to recover. During the recording of EEG and EMG, rats were allowed to move freely.
Sleep–wake state analysis of rats
Characteristic features of the EEGs and EMGs were analysed simultaneously to differentiate sleep and wakeful state. The awake animals represent high EMG activity, while sleeping rats show very low EMG activity (sometimes close to zero). Therefore, the amount of EMG activity is an indicator of wake/sleep condition. In our experiment, EMG exhibits high tonus and occasional bursts of activity consistent with movements made by the animals. In EEG records, δ (frequency <4.5 Hz) and spindle activities (frequency = 12–14 Hz), which are the distinct features of NREM sleep were reported (Ebrahimi et al. Citation2008). The absence of activity during the above-mentioned frequencies is an indicator of REM sleep.
The signals were recorded throughout the experiment (from 9:00 to 13:00) using ScienceBeam D3111 System of Biological Function (ScienceBeam, Tehran, Iran). The EEG (0–100 Hz) and EMG (20–2000 Hz) signals were amplified and the recorded. The data were analysed using a custom-made program developed in MATLAB software (The Mathworks Inc., Natick, MA), based on sleep scoring with the conventional threshold method. In this program, the recorded signal was down sampled and divided into 10-s episodes. As mentioned before, we attempted to differentiate awake, REM and NREM stages in sleep by using conventional threshold formula and calculating EEG band frequency. Wavelet Daubechies order 2 (db2) was applied to each 10 s segments of EEG signals. Then, EEG band frequencies were calculated from wavelet packet coefficients. Frequency ranges were detected in the written algorithm to categorize different EEG waves consisting of δ: 0.39–3.13 Hz, θ: 3.13–8.46 Hz, α: 8.46–10.93, spindle; 10.93–15.63, β1: 15.63–21.88 Hz and β1: 21.88–37.50 Hz. Finally, classic threshold formulas were applied for detecting different sleep stages (Brankack et al. Citation2010; Sukhorukova et al. Citation2010).
Statistical analysis
The ED50 of the extract was calculated using the method described by Tallarida (Citation2000). The slope deviation of the dose–response curve was analysed using Fieller’s theorem. All the mentioned computations were performed using Microsoft Excel. The statistical analysis was performed using a Student’s t-test or an analysis of variance followed by Tukey’s multiple comparison when appropriate. The probability level of 0.05 was considered as significant.
Results
Open field test
Distance moved
In the locomotor activity test, a significant difference was observed between the groups in the total distance moved [F(6,62) = 18.4, p < 0.001]. The extract as well as diazepam significantly decreased the total distance moved compared to the control group with an ED50 value 251 ± 55 mg/kg (p < 0.001 for 25, 50, 100 and 200 mg/kg; ).
Figure 1. Effects of the M. communis extract on open field parameters: (a) Velocity, (b) distance moved, (c) number of rears. While the anxiety parameters were significantly reduced by all applied doses (**p < 0.01 and ***p < 0.001), flumazenil could not reduce those effects (ns: not significant, p > 0.05). Values represent mean ± SEM (n = 10 mice/group).
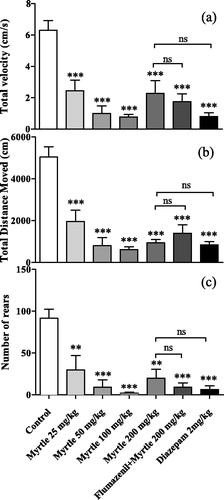
Velocity
The velocity parameter was changed significantly in the open field test between the groups [F(6,68) = 13.8, p < 0.0001]. The extract significantly reduced the velocity of the treated groups compared to the control group (p < 0.001 for 200, 100, 50 and 25 mg/kg, ED50 = 20.3 ± 10 mg/kg; ).
Number of rears
The number of rears was counted by an observer. There was a significant difference between the groups [F(6,68) = 11.2, p < 0.001]. The extract significantly decreased the number of rears in the treated groups compared to the control group (p < 0.01 for 200 and 25 mg/kg and p < 0.001 for 100 and 50 mg/kg, ED50 = 40.2 ± 6.6 mg/kg). Flumazenil (10 mg/kg) failed to have an effect on the measured parameters of the M. communis extract during the open field test (p > 0.05; ).
Hypnotic effects
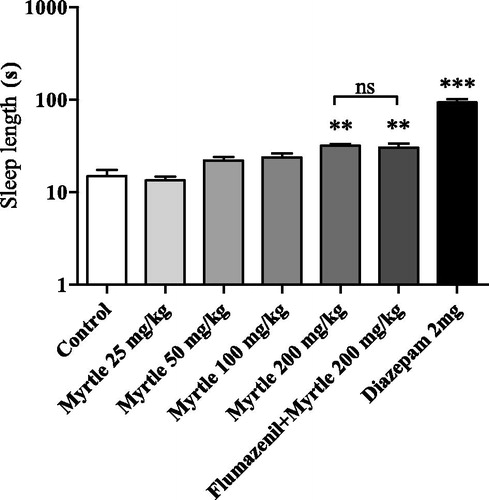
Sleep length was significantly increased in the pentobarbital induced loss of righting reflex test [F(6,68) = 6.61, p < 0.01]. The extract significantly increased sleep length in dose of 200 mg/kg compared to the control group (p < 0.001). Flumazenil (100 mg/kg) was unable to reverse these effects (200 mg/kg; ).
Figure 2. Effects of the M. communis extract in pentobarbital induced loss of righting reflex. The extract induced sleep at dose 200 mg/kg (**p < 0.01 and ***p < 0.001). The response was not reduced by co-injection of flumazenil (ns: not significant, p > 0.05). Values represent the mean ± SEM (n = 10 mice/group). The Y-axis is shown in logarithmic scale.
Muscle relaxant activity
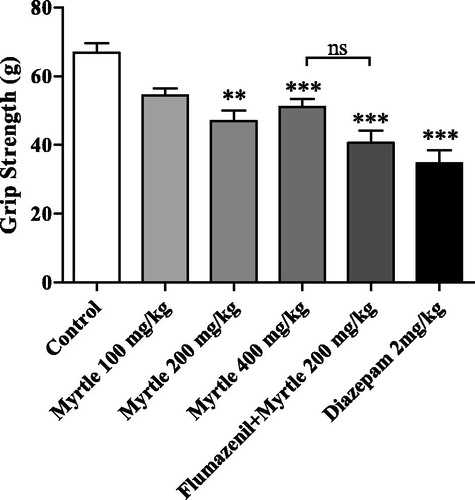
A significant difference between the experimental groups in muscle strength was revealed by the ANOVA test [F(5,54) = 14.2, p < 0.001]. The extract of M. communis significantly decreased grip strength in mice compared to the control group (p < 0.01 for 100 mg/kg, and p < 0.001 for 200 and 400 mg/kg; ). The obtained dose–response curve was U-shaped and the slope deviation of the dose–response curve was not significant compared to zero.
Figure 3. Muscle relaxation effects of diazepam, M. communis extract and their combination with flumazenil in grip strength test. The results showed significant muscle tone reduction by the extract (200 and 400 mg/kg) as well as diazepam (**p < 0.01 and ***p < 0.001) while, flumazenil could not diminish the myorelaxant effects (p > 0.05). Values represent mean ± SEM for the applied force to pull on from the instrument.
Anticonvulsant activity
In both PTZ- and MES-induced seizure tests, we did not observe any significant anticonvulsant effects on tonic and latency to clonic seizure in doses of the extract up to 400 mg/kg compared to the control group.
Effects on sleep parameters
One-way ANOVA indicated a significant difference in the total sleep length [F(2,15) = 58.0, p < 0.001], REM% [F(2,15) = 8.4, p < 0.01] and NREM% [F(2,15) = 17.0, p < 0.001]. Tukey’s post-test showed that the M. communis extract and diazepam significantly increased the duration of sleep (p < 0.001) compared to the control group. An increase in NREM% was observed by both M. communis and diazepam (p < 0.1 and p < 0.001, respectively). Myrtus communis decreased the REM% in comparison to the control group (p < 0.01). However, diazepam had no effect on the REM% ().
Table 1. Effects of M. communis extract (200 mg/kg) and diazepam (2 mg/kg) on EEG spectra.
Acute toxicity analysis
We observed no mortality rate in the subjects even at a high dose (4 g/kg, i.p.) of the extract during the first week after injection (n = 8). Due to the low toxicity, LD50 of the extract could not be estimated.
Discussion
The recorded parameters verified the in vivo activities of the selected M. communis extract on hypnosis, locomotion factors and muscle tone (50–200 mg/kg). The extract significantly potentiated pentobarbital-induced sleep and reduced vertical (rearing) and horizontal activity in mice (distance moved and velocity). These effects are in accordance with its traditional use in humans. On the contrary, the results of the PTZ- and MES-induced seizures indicate that there are differences between the effectiveness and mechanisms of action of the extract and diazepam. The extract lacks anticonvulsant activity even at the highest dose (400 mg/kg), whereas its effects on anxiety, muscle relaxation and sleep are similar to BNZs.
In the present study, the extract showed antianxiety activity but did not have any effect on PTZ- and MES-induced seizures. Since the active compound(s) were not isolated, we cannot deny the presence of active constituents that might have an effect on PTZ- and MES-induced seizures. However, further studies are needed to explore and isolate the active constituents from the extract that have efficacy against seizures.
Many of the sedative-hypnotic target the GABAA receptor to treat anxiety and insomnia (Azad et al. Citation2003). Therefore, the affinity of the active compound(s) in the extract can be attributed by interacting with specific GABAA receptor subtypes. The molecular affinity and selectivity of hypnotics is reflected in its behavioural and clinical responses to treatment. Since receptor subtype selectivity by a drug can be translated to a defined behaviour, GABAA-mediated transmission by a sedative-hypnotic compound is of particular importance. Taking into consideration the above facts, we focused on the effects of the extract on anxiety, sedation-hypnosis and convulsion. The heterogeneity characteristic of the GABA receptor revealed that 95% of the pentameric receptor is composed of α, β and γ subunits. Each subunit is divided into different subtypes (α1–6, β1–3, γ1–3). This heterogeneity characteristic of GABA receptors leads to different homeostasis of sleep. The major type of the GABA receptors is constructed by a single α subunit. The most abundant subtype is α1βγ2 and α2βγ2 has medium abundance present in the most area of the brain (Sieghart & Sperk Citation2002), which has high affinity for BNZs. The receptors of those containing α1 subunits in the brain are responsible for anxiolytic, sedative-hypnotic and anticonvulsant effects. The α2 subtype is also involved in all the mentioned effects but not anticonvulsant in the most behavioural methods. It seems that the myorelaxant response is extensively related to the α2 subunit (Shannon et al. Citation1984). Z-compounds are other allosteric modulators of GABAA receptor included imidazopyridine (e.g., zolpidem), cyclopyrrilone (e.g., zopiclone and eszopiclone) and pirazolopyrimidine (e.g., zaleplon) derivatives. BNZs and Z-compounds are allosteric positive modulators of GABAA, affect through binding to α subunit. So as expected, diazepam, an α subunit non-selective BNZs agonist, produces all the BNZs effects, whereas zolpidem, an α1 selective agonist, does not display myorelaxant effect at the therapeutic anxiolytic and sedative doses. The α1 subunit is primarily responsible for anticonvulsant properties. These findings were confirmed by studies in which α1-selective agonist, β-CCT (β-carboline-3-carboxylate-t-butyl ester) was applied (Griebel et al. Citation1999), and is complemented by another experiments on [α2(H101R)] mice in which histidine was replaced by arginine at position 101 of the α2 subunit (Kopp et al. Citation2004). The pharmacological distinct effects of the extract as a sedative-hypnotic and muscle relaxant with no anticonvulsant activity indicate the low affinity of the active compound(s) in the extract towards the α1 subunit of the GABAA receptor. Drugs with high affinity (∼10-fold) to α1 subunit (e.g., zolpidem) expose a slight myorelaxant effect at the therapeutic anxiolytic and sedative doses. Also, the muscle relaxant effects of diazepam are not diminished by flumazenil (Jung et al. Citation2004). These confirm that muscle relaxation by diazepam is not dependent on activation of α1 subunit of the GABA receptor. The same results were obtained by the injection of diazepam (2 mg/kg) before the grip strength test in the present study. Furthermore, flumazenil failed to reverse sedative and hypnotic effects of the myrtle extract. The present results indicate that these effects were not exhibited by modulation of the α1 subunit. Moreover, administration of the extract showed significant reduction of the muscle tone in the grip strength test (p < 0.01 for 100 mg/kg, and p < 0.001 for 200 and 400 mg/kg). These results are in parallel with seizure experiments data, which authorized the low affinity of the active compounds to α1 subunits of GABA receptors.
The results of the present study indicate that EEG alterations by diazepam are in line with the previous studies. Diazepam slightly reduces REM sleep (Kopp et al. Citation2003). However, some studies in rodents reported that diazepam does not have any effects on REM sleep (Tobler et al. Citation2001). Z-compounds display EEG spectra of BNZs, encompassing spindles activity (frequency range: ∼10–15 Hz). Also, in previous studies, it was shown that administration of BNZs causes an elevation in NREM sleep. In agreement with the former reports, the results showed that diazepam (2 mg/kg) promoted NREM sleep and the same results were revealed by injection of M. communis extract at a dose of 200 mg/kg.
Until now, no electrophysiological studies were conducted to evaluate the M. communis extract as well as its known active compounds. We are considering further work to isolate the active compound(s) in order to clarify the mechanisms of action.
Conclusion
Muscle relaxant effects of the extract demonstrate that this effect may be mediated by the α2 subunit. Also, the lack of the anticonvulsant property indicates (or the lack of anticonvulsant properties indicate) that it has low affinity towards the α1 subunit. Our results show that the M. communis extract also suppresses REM sleep. Although the alteration of sleep architecture keeps the extract far from as an ideal hypnotic, its safety can make it a worthy candidate for the treatment of anxiety disorders.
Funding information
This study was supported by the grant No. 91004268 from the Deputy of Iranian National Science Foundation (INSF).
Disclosure statement
All authors of the present article disclose that they have no other financial or affiliation involvement with any financial interest organization.
References
- Azad N, Byszewski A, Sarazin FF, McLean W, Koziarz P. 2003. Hospitalized patients’ preference in the treatment of insomnia: pharmacological versus non-pharmacological. Can J Clin Pharmacol. 10:89–92.
- Borbely AA, Achermann P. 1991. Ultradian dynamics of sleep after a single dose of benzodiazepine hypnotics. Eur J Pharmacol. 195:11–18.
- Brankack J, Kukushka VI, Vyssotski AL, Draguhn A. 2010. EEG gamma frequency and sleep–wake scoring in mice: comparing two types of supervised classifiers. Brain Res. 1322:59–71.
- Buchbauer G, Jirovetz L, Jáger W, Plank C, Dietrich H. 1993. Fragrance compounds and essential oils with sedative effects upon inhalation. J Pharm Sci. 82:660–664.
- Dijk DJ, Shanahan TL, Duffy JF, Ronda JM, Czeisler CA. 1997. Variation of electroencephalographic activity during non-rapid eye movement and rapid eye movement sleep with phase of circadian melatonin rhythm in humans. J Physiol. 505:851–858.
- Doghramji K. 2006. The epidemiology and diagnosis of insomnia. Am J Manag Care. 12:S214–S220.
- Ebrahimi F, Mikaeili M, Estrada E, Nazeran H. 2008. Automatic sleep stage classification based on EEG signals by using neural networks and wavelet packet coefficients. Conf Proc IEEE Eng Med Biol Soc. 2008:1151–1154.
- Elisabetsky E, Coelho D, Souza G, Dos Santos M, Siquieira I, Amador T, Nunes D. 1995. Sedative properties of linalool. Fitoterapia. 66:407–414.
- Erden BF, Ulak G, Yildiran G, Gacar N. 1997. The effect of 7-nitro- indazole on pentobarbital-induced sleep in mice. Pharmacol Res. 36:265–267.
- Faizi M, Sheikhha M, Ahangar N, Tabatabaei Ghomi H, Shafaghi B, Shafiee A, Tabatabai SA. 2012. Design, synthesis and pharmacological evaluation of novel 2-[2-(2-chlorophenoxy) phenyl]-1,3,4-oxadiazole derivatives as benzodiazepine receptor agonists. Iranian J Pharm Res. 11:83.
- Feißt C, Franke L, Appendino G, Werz O. 2005. Identification of molecular targets of the oligomeric nonprenylated acylphloroglucinols from Myrtus communis and their implication as anti-inflammatory compounds. J Pharmacol Exp Ther. 315:389–396.
- Griebel G, Perrault G, Letang V, Granger P, Avenet P, Schoemaker H, Sanger DJ. 1999. New evidence that the pharmacological effects of benzodiazepine receptor ligands can be associated with activities at different bz (omega) receptor subtypes. Psychopharmacology. 146:205–213.
- Hayder N, Abdelwahed A, Kilani S, Ammar RB, Mahmoud A, Ghedira K, Chekir-Ghedira L. 2004. Anti-genotoxic and free-radical scavenging activities of extracts from (Tunisian) Myrtus communis. Mutat Res. 564:89–95.
- Jung HY, Sohn YH, Mason A, Considine E, Hallett M. 2004. Flumazenil does not affect intracortical motor excitability in humans: a transcranial magnetic stimulation study. Clin Neurophysiol. 115:325–329.
- Kopp C, Rudolph U, Keist R, Tobler I. 2003. Diazepam-induced changes on sleep and the EEG spectrum in mice: role of the alpha3-gaba(a) receptor subtype. Eur J Neurosci. 17:2226–2230.
- Kopp C, Rudolph U, Low K, Tobler I. 2004. Modulation of rhythmic brain activity by diazepam: GABA(a) receptor subtype and state specificity. Proc Natl Acad Sci USA. 101:3674–3679.
- Messaoud C, Zaouali Y, Salah AB, Khoudja M, Boussaid M. 2005. Myrtus communis in Tunisia: variability of the essential oil composition in natural populations. Flavour Frag J. 20:577–582.
- Mulas M, Cani MR. 1999. Germplasm evaluation of spontaneous myrtle (Myrtus communis L.) for cultivar selection and crop development. J Herbs Spices Med Plants. 6:31–49.
- Olson EJ, Boeve BF, Silber MH. 2000. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 123:331–339.
- Pirbalouti A. 2009. Medicinal plants used in Chaharmahal and Bakhtyari districts of Iran. Herba Polonica. 55:69–77.
- Pivik RT, Broughton RJ, Coppola R, Davidson RJ, Fox N, Nuwer MR. 1993. Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology. 30:547–558.
- Rahimmalek M, Mirzakhani M, Pirbalouti AG. 2013. Essential oil variation among 21 wild myrtle (Myrtus communis L.) populations collected from different geographical regions in Iran. Ind Crops Proc. 51:328–333.
- Richey SM, Krystal AD. 2011. Pharmacological advances in the treatment of insomnia. Curr Pharm Des. 17:1471–1475.
- Santos F, Rao V. 2000. Antiinflammatory and antinociceptive effects of 1,8-cineole a terpenoid oxide present in many plant essential oils. Phytother Res. 14:240–244.
- Shannon HE, Guzman F, Cook JM. 1984. Beta-carboline-3-carboxylate-t-butyl ester: a selective bz1 benzodiazepine receptor antagonist. Life Sci. 35:2227–2236.
- Sieghart W, Sperk G. 2002. Subunit composition, distribution and function of GABA(a) receptor subtypes. Curr Top Med Chem. 2:795–816.
- Sukhorukova N, Stranieri A, Ofoghi B, Vamplew P, Saleem M, Ma L, Ugon A, Ugon J, Muecke N, Amiel H. 2010. Automatic sleep stage identification: difficulties and possible solutions. In: Proceedings of the Fourth Australasian Workshop on Health Informatics and Knowledge Management. Vol. 108. Queensland, Australia: Australian Computer Society, Inc. p. 39–44.
- Tallarida RJ. 2000. Dose–response analysis. Drug synergism and dose-effect data analysis. New York (NY): Chapman & Hall/CRC.
- Tobler I, Kopp C, Deboer T, Rudolph U. 2001. Diazepam-induced changes in sleep: role of the alpha 1 GABA(a) receptor subtype. Proc Natl Acad Sci USA. 98:6464–6469.
- White HS. 1997. Clinical significance of animal seizure models and mechanism of action studies of potential antiepileptic drugs. Epilepsia. 38:S9–S17.
