Abstract
Context Nutraceuticals possessing antioxidant potential have been used to alleviate side effects exerted by many chemotherapeutics, including cisplatin. Since Apodytes dimidiata E. Mey. Ex Arn. (Icacinaceae) shows antioxidant potential, it may possess significant chemoprotective effects.
Objectives The study investigated whether A. dimidiata could attenuate cisplatin-induced renal damage.
Materials and methods Nephrotoxicity was induced by cisplatin (single i.p., 16 mg/kg b wt.) in Wistar rats. Methanolic leaf extract of A. dimidiata (AMF) was administered at a dose of 250 mg/kg b. wt. orally for 5 consecutive days before/after cisplatin administration. Blood and renal parameters were analysed. Total phenolic and flavonoid content in AMF and its NO scavenging effect was determined.
Results Significant protective effect of AMF on cisplatin-induced nephrotoxicity was observed in pre-treated animals. The reduction of urea, creatinine and lipid peroxidation was 58.31%, 42.19% and 60%, respectively, and the increase in haemoglobin and leucocyte count was 28.25% and 42.91%, respectively. The increase calculated for GSH, GPx, SOD and catalase was 35.64%, 18.14%, 74.42% and 35.46%, respectively. Tissue architecture of kidney was almost normal in AMF treated animals. The results were comparable to the standard drug, silymarin. AMF contained high level of polyphenols and flavonoids and was found to scavenge NO radicals (IC50 121.8 μg/mL).
Discussion and conclusion AMF can effectively counteract cisplatin mediated renal acute toxicity possibly by scavenging reactive oxygen and nitrogen species. Accordingly, the study suggests that AMF can ameliorate free radical-induced damage associated with chemotherapeutic drugs.
Introduction
Apodytes dimidiata E. Mey. Ex Arn. (Icacinaceae), a small bushy tree is seen in southern and eastern parts of Africa and different Asian countries along South and Central Sahyadris of India. The tree is valued in Zulu traditional medicine for various ailments. An infusion from the root bark is used as purgative to treat intestinal parasites (Gestner Citation1938; Bryant Citation1966; Hutchings et al. Citation1996) and the leaves are used in the treatment of ear inflammation (Watt & Breyer-Brandwijk Citation1962). Recently, a well-known anticancer alkaloid, camptothecin was isolated from the bark of this plant (Ramesha et al. Citation2013) and also from Fusarium solani (Mart) Sacc. (Nectriaceae), an endophytic fungus of A. dimidiata (Shweta et al. Citation2010). Six new saponins were reported from the leaves of A. dimidiata (Kenn et al. Citation2011). A study on the validation of antimycobacterial plants used by traditional healers in three districts of the Limpopo Province (South Africa) shows that A. dimidiata has antibacterial and antioxidant properties. The phytochemical screening of leaf acetone extract showed the presence of saponins, tannins, terpenes, steroids and flavonoids (Masoko & Nxumalo Citation2013). Moreover, in our previous studies, the antitumour (Divya et al. Citation2015) and chemopreventive (Divya et al. Citation2016) effects of A. dimidiata were revealed.
Free radical mediated toxicity is one of the major side effects of chemotherapeutic drugs. Cisplatin (CP-diamminedichloroplatinum II), a heavy metal complex, is a widely used antineoplastic drug for the treatment of several cancers. But, nephrotoxicity is reported as a major side effect conjoined with cisplatin treatment. Generation of oxidative stress and release of NO are considered to be the prime factors for cisplatin-induced nephrotoxicity (Pan et al. Citation2009). Superoxide (Davis et al. Citation2001), hydrogen peroxide (Kadikoylu et al. Citation2004) and hydroxyl (Shino et al. Citation2003) radicals increased by cisplatin, stimulates lipid peroxidation (Sadzuka et al. Citation1992) and results in the inhibition of antioxidant enzyme activities (Mora et al. Citation2003), which is responsible for nephrotoxicity (Amptoulach & Tsavaris Citation2011; Naqshbandi et al. Citation2012; Ognjanovic et al. Citation2012). To ameliorate these side effects, complementary agents of high antioxidant potential have been recently investigated (Monti & Yang Citation2005). Mansour et al. (Citation2002) reported that antioxidants play an important role in attenuating nephrotoxicity mediated by oxidative stress.
Potential nutraceuticals such as natural antioxidants have been used to reduce side effects as well as to enhance the activities of chemotherapeutics (Zhang et al. Citation2010). Phenol and flavonoid class of compounds are natural antioxidants as they can quench reactive oxygen species efficiently (Korkina & Afanasev Citation1997). In the present study, an effort has been made to assess the protective effect of A. dimidiata on cisplatin-induced nephrotoxicity in rats. Moreover, the nitrogen scavenging activity and the total phenolic and flavonoid content in A. dimidiata are also evaluated.
Material and methods
Plant material
The leaves of Apodytes dimidiata were collected from Periya Tiger Reserve Forest, Wayanad District, Kerala, India (Altitude: 810 m, Geographical location: Lat. N 11°51'03.19″, Long. 75°48'05.54″) during the month of January 2014 and identified by Dr. Sujanapal P, Taxonomist, Kerala Forest Research Institute (KFRI) Peechi, Kerala. A voucher specimen (No. KFRI 28024) is kept at the Herbarium of KFRI.
Preparation of extract
Leaves of A. dimidiata were dried under shade and powdered using a mixer grinder. Approximately, 30 g of the powder was extracted separately with 250 mL of petroleum benzene, chloroform, acetone and methanol using Soxhlet for 24 h. Each extract was concentrated to dryness. The extract obtained in methanol was loaded to a column (600 mm × 30 mm) packed with 60–120 mesh silica gel and eluted successively by passing various solvents (150 mL each) of increasing polarities such as petroleum benzene, chloroform, acetone and methanol. The methanolic fraction (AMF) was used for studies.
Chemicals
Nitrobluetetrazolium (NBT), glutathione (GSH), 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) and riboflavin were purchased from Sisco Research Laboratories Pvt. Ltd. (Mumbai, India). Thiobarbituric acid (TBA) was purchased from Hi-Media Laboratories, Mumbai, India. Cisplatin was obtained from Kemoplat, Dabur Pharma Ltd., New Delhi, India. Silymarin was obtained from Krypton Pharmaceuticals, Mumbai. RPMI-1640 culture medium was purchased from Sigma Aldrich (St. Louis, MO) and FBS from PAN Biotech, Aidenbach, Germany. All other chemicals and reagents used were of analytical grade.
Animals
Wistar rats (120–150 g) used in the study were purchased from Small Animal Breeding Station, Kerala Veterinary and Animal Sciences University (KVASU), Thrissur, Kerala and were housed in well ventilated cages under controlled conditions of light and humidity and provided with normal mouse chow (Sai Durga Food and Feeds, Bangalore, India) and water ad libitum. The animal experiment in the study was carried out with the prior approval from Institutional Animal Ethics Committee by strictly following the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) constituted by Animal Welfare Division, Government of India (No. 149/1999/CPCSEA).
Phytochemical screening and quantitative measurement of total phenolic and flavonoid contents
AMF was subjected to phytochemical screening by standard methods (Harbone Citation1973; Sofowora Citation1993; Evans et al. Citation2002). The total flavonoid content was analysed by colorimetric method (Chang et al. Citation2002). Briefly, the plant extract (0.5 mL) was mixed with 1.5 mL of methanol, 0.1 mL of 10% aluminium chloride, 0.1 mL of 1 M potassium acetate and 2.8 mL of distilled water. The reaction mixture was allowed to stand at room temperature for 30 min and the absorbance of the reaction mixture was measured at 415 nm. A calibration curve was prepared by using quercetin at concentrations of 50 to 250 μg/mL in methanol. Flavonoid content was expressed as μg/mg quercetin equivalent (QE) of dry extract. Total phenolic content was determined by using Folin-Ciocalteu reagent (as modified by Ainsworth & Gillespie Citation2007). Gallic acid (20–100 μg/mL) was used as a reference standard for plotting calibration curve. A volume of 0.5 mL of the plant extract (100 μg/mL) was mixed with 2 mL of the Folin-Ciocalteu reagent (diluted 1:10 with de-ionized water) and were neutralized with 4 mL of sodium carbonate solution (7.5%, w/v). The reaction mixture was incubated at room temperature for 30 min with intermittent shaking and the absorbance of the resulting blue colour was measured at 765 nm. Total phenolic content was determined from the linear equation of a standard curve prepared with gallic acid. The content of total phenolic compounds is expressed as μg/mg gallic acid equivalent (GAE) of dry extract.
Experimental design
Male Wistar rats were divided into six groups of six animals each. Group 1 animals were kept as normal without any treatment. All animals in the other groups received cisplatin i.p. (16 mg/kg b. wt). Group 2 animals (untreated control) received no treatment, Group 3 was given propylene glycol (> 99% pure) and kept as vehicle control (200 μL), Group 4 animals were treated with standard drug silymarin at 100 mg/kg b. wt and groups 5 and 6 received AMF 250 mg/kg b. wt., respectively, by oral gavage for 5 days before and after cisplatin administration. All the mice in groups 2–6 were treated with single dose of cisplatin (16 mg/kg b. wt, i.p.) to induce renal damage (Somani et al. Citation2000). At the end of the experiment, the animals were sacrificed using ether anaesthesia and kidneys were removed and kept at −20 °C until homogenization.
Sample collection and biochemical analysis
Blood was collected directly from the heart in heparinized vials to determine the total WBC count (Chaudhari Citation2000) and haemoglobin content (Drabkin & Austin Citation1932). Blood was collected in non-heparinized vials to separate serum for the analysis of urea by Berthelot reaction method (Allain et al. Citation1974) and creatinine by Jaffe’s reaction method (Moss et al. Citation1975) using commercially available kits (Span Diagnostics, Surat, India).
Kidney was excised out, washed in ice-cold saline and a portion of tissue was fixed in 10% formalin for histopathological analysis. Kidney homogenates (10%, w/v) were prepared in Tris–HCl buffer (0.1 M, pH 7.4). The concentration of malondialdehyde (MDA) as an indicator of lipid peroxidation was determined according to a modified method of Ohkawa et al. (Citation1979) based on the reaction with TBA. Superoxide dismutase (SOD) activity was carried out by the method of McCord and Fridovich (Citation1969). The scavenging ability of the enzyme on superoxide anion radicals was determined by light-induced superoxide generation with riboflavin and subsequent reduction of nitrobluetetrazolium. Reduced GSH was measured by the method described by Moron et al. (Citation1979). Glutathione peroxidase (GPx) was determined by the method described by Hafemann et al. (Citation1974) by the degradation of hydrogen peroxide in the presence of GSH, thereby depleting it. The GSH remaining was measured using DTNB, which yields a coloured complex. Catalase (CAT) activity was determined by measuring the decomposition of hydrogen peroxide at 240 nm, according to the method of Aebi (Citation1974). Furthermore, total protein was determined according to Lowry et al. (Citation1951).
Nitric oxide radical inhibition assay
Macrophages were elicited by injecting 5% sodium caseinate intra-peritoneally in BALB/c mice, washed with PBS and resuspended in RPMI-1640 with 10% FCS. The cells were plated in 96-well culture plates and incubated for 2 h at 37 °C in a 5% CO2 atmosphere. After incubation, non-adherent cells were removed and the adherent macrophages were incubated (2 × 106 cells/well) in complete medium (RPMI-1640, 10% FCS, 100 μg/mL streptomycin and penicillin, 2 mM glutamine). Macrophages were cultured in the presence of various concentrations of AMF and the standard drug, ascorbic acid. After 24 h, plates were centrifuged and the supernatant was used for the estimation of NO by the Griess method (Stuehr & Nathan Citation1989). In brief, 50 μL of supernatant was mixed with 1% sulphanilamide/0.1% naphthylethylenediamine/2.5% H3PO4 and was incubated at room temperature for 10 min to form a chromophore. A typical blank/control solution containing the same solution mixture without plant extract was measured at 550 nm. The percentage inhibition was calculated according to the following equation: % inhibition = (1−A1/A0 × 100), where Al is the absorbance of the extract and A0 is the absorbance of the control. The concentration of sample required to inhibit 50% of nitric oxide radical (IC50 value) was calculated by plotting the graph.
Statistical analysis
Data obtained in the experiment were expressed in terms of mean ± SEM. Statistical significance of data was assessed by the analysis of variance (one-way ANOVA) followed by a comparison between vehicle control and treated groups using ‘Tukey–Kramer’ multiple comparison test with Instat 3 software package (San Diego, CA). The significance level was set at p < 0.05.
Results
Phytochemical analysis of AMF
An estimated amount of total phenolic compounds in methanolic extract of A. dimidiata leaf was found to be 61.22 μg/mg GAE and the total flavonoid compounds in the same was 84.75 μg/mg QE. The other phytoconstituents evidenced by preliminary phytochemical screening were alkaloids, saponins, terpenes, carbohydrates, steroids, flavonoids, glycosides and tannins.
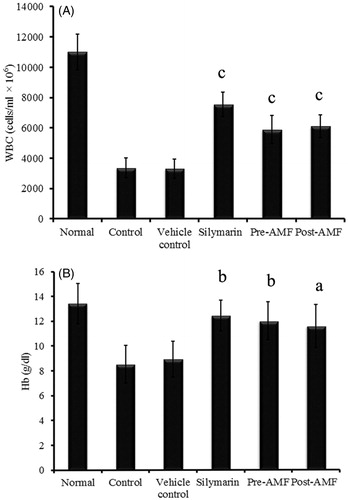
Effect of AMF on haematological parameters
Total WBC count was found to be significantly reduced in cisplatin alone treated animals. There was a proportionate increase in WBC (cells/mL × 106) count with both pre- and post-treatment of AMF and the significance (p < 0.001) was same compared to that of vehicle control (). Similarly, cisplatin-induced decrease in haemoglobin level (Hb in g/dL) was improved to 11.98 ± 1.53 (p < 0.01) and 11.56 ± 1.73 (p < 0.05) with AMF pre- and post-treatment, respectively ().
Figure 1. Effect of AMF on total WBC count (A) and haemoglobin level (B) in cisplatin treated mice. The data obtained were represented as mean ± SEM (n = 6) and analysed using one-way ANOVA and group means were compared using the Tukey–Kramer multiple comparison test. The values are statistically different from vehicle control at p < 0.05a, 0.01b and 0.001c and the values > 0.05 are considered to be non-significant.
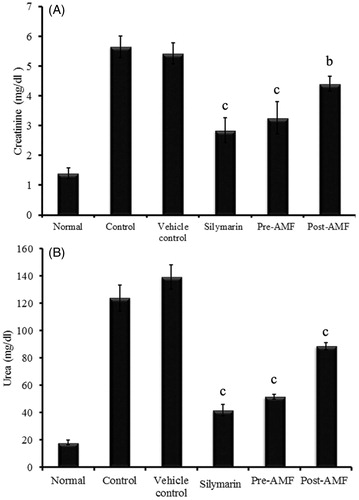
Effect of AMF on biochemical parameters
Serum levels of creatinine (mg/dL) and urea (mg/dL) were increased in cisplatin alone treated group compared to normal group. The level of creatinine in cisplatin treated animals (5.42 ± 0.35) was significantly reduced to 3.26 ± 0.54 (p < 0.001) and 4.41 ± 0.25 (p < 0.001) in AMF pre- and post-treated groups, respectively (). Similarly, the elevated level of urea (139.21 ± 8.9) was significantly decreased to 51.56 ± 1.82 (p < 0.001) and 88.54 ± 2.56 (p < 0.001) in AMF pre-treated and post-treated groups, respectively (). The effect of AMF on the normalization of these kidney markers are comparable to that of silymarin (standard drug) treated animals.
Figure 2. Effect of AMF on serum creatinine (A) and urea (B) levels in cisplatin treated mice. The data were represented as mean ± SEM (n = 6) and analysed using one-way ANOVA and group means were compared using the Tukey–Kramer multiple comparison test. The values are statistically different from the control at p < 0.05a, 0.01b and 0.001c and the values > 0.05 are considered to be non-significant.
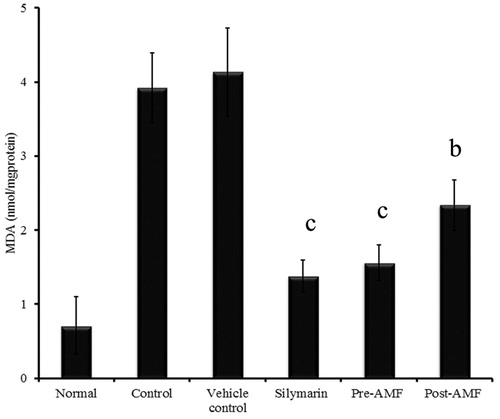
Cisplatin-induced decline in the activity of SOD (0.58 ± 0.16 U/mg protein), GPx (21.98 ± 3.21 U/mg protein) and CAT (3.74 ± 0.29 U/mg protein) was found to be reversed to 1.92 ± 0.23 (p < 0.001), 27.89 ± 2.45 (p < 0.01) and 5.48 ± 0.47 (p < 0.001), respectively, by the pre-treatment of AMF and 1.1 ± 0.29 (p < 0.05), 22.41 ± 1.78 (non-significant) and 4.75 ± 0.43 (p < 0.01) by the post-treatment (). Similar observation was seen with GSH (nmol/mg protein) in which the depleted level (30.34 ± 1.04) was elevated to 45.76 ± 2.08 (p < 0.001) and 35.01 ± 2.32 (p < 0.01) by the pre- and post-treatment of AMF (). Also, a significant reduction in lipid peroxidation status was observed as inferred by the value of MDA (nmol/mg protein), 1.56 ± 0.24 (p < 0.001) and 2.34 ± 0.33 (p < 0.001) for the pre- and post-treated groups. In cisplatin treated animals, the MDA level was raised to 4.13 ± 0.59 ().
Figure 3. Effect of AMF on SOD (A), GPx (B), CAT (C) activities and GSH (D) level in kidney tissue of cisplatin treated mice. The data obtained were represented as mean ± SEM (n = 6) and analysed using one-way ANOVA and group means were compared using the Tukey–Kramer multiple comparison test. The values are statistically different from the control at p < 0.05a, 0.01b and 0.001c and the values >0.05 are considered to be non-significant.
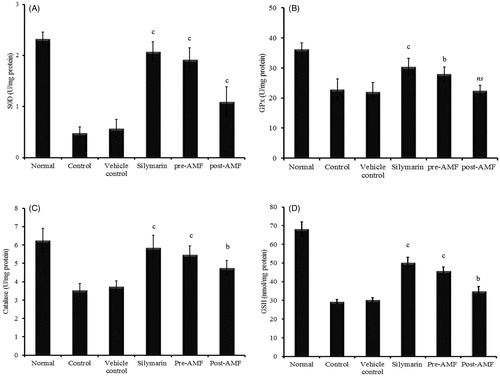
Figure 4. Effect of AMF on lipid peroxidation in the kidney tissue of cisplatin treated mice. The data obtained were represented as mean ± SEM (n = 6) and analysed using one-way ANOVA and group means were compared using the Tukey–Kramer multiple comparison test. The values are statistically different from the control at p < 0.05a, 0.01b and 0.001c and the values >0.05 are considered to be non-significant.
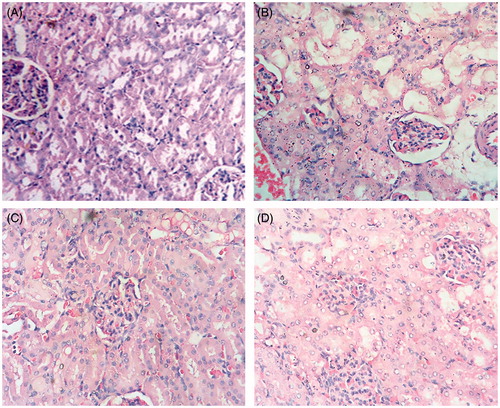
Effect of AMF on cisplatin-induced kidney damage
Histological details of kidney tissue from cisplatin and propylene glycol (vehicle control) treated animals showed a moderate to severe degree of damage. There was glomerular and tubular congestion with abnormal Bowman’s capsule, blood vessel constriction, vacuolation, necrosis and hemorrhagic areas. Inflammatory cells were also observed. Very few collections of lymphocytes were seen in the interstitial tissue. The histological features of both pre- and post-treatment groups showed minimal cellular damage in contrast to the control group. The AMF pre-treated group showed more recovery with almost normal glomerular and tubular arrangements, minimal blood vessel congestion and very few inflammatory cells ().
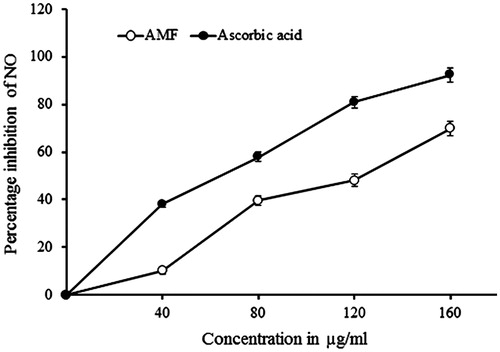
Scavenging effect of AMF on nitric oxide radicals
AMF exhibited potent nitric oxide scavenging activity with an IC50 value of 121.8 μg/mL and the scavenging was found to be in a dose-dependent manner. The standard drug, ascorbic acid, exhibited IC50 value at 68.9 μg/mL ().
Discussion
Cisplatin is a chemotherapeutic drug currently used in the treatment of a wide range of cancers. However, the clinical usefulness of this drug is limited due to its side effects including nephrotoxicity. When cisplatin accumulates in renal epithelial cells, the GSH concentration and antioxidant enzymes activities become depleted which eventually leads to the hike in intracellular concentrations of ROS (Badary et al. Citation2005). Cisplatin also enhances Ca++ independent nitric oxide synthase activity leading to the increased production of nitric oxide (Srivastava et al. Citation1996). Among the approaches used to ameliorate or protect the cisplatin-induced nephrotoxicities, the most consistent effects have been observed with the use of antioxidant agents (Majid et al. Citation2012).
In the present study, the rats treated with single dose of cisplatin showed marked increase in serum creatinine and urea levels. The activity of various antioxidant enzymes like SOD, peroxidase and CAT in the kidney was found reduced. A concomitant increase in lipid peroxidation and depletion of GSH level along with disrupted tissue architecture clearly indicated significant nephrotoxicity by cisplatin administration.
Cisplatin-induced increase in urea and creatinine levels was found to be reduced by the oral administration of AMF. GSH depletion and associated lipid peroxidation was attenuated in these animals. Furthermore, the reduced activities of antioxidant enzymes by cisplatin were found restored by AMF to a considerable extent, indicating its ability to counteract the oxidative stress. Therefore, the nephroprotective effect of AMF observed in this study is likely to be mediated through its antioxidant property. These biochemical results were well corroborated by hisptopathological observations. This protective efficacy of AMF was comparable to that of standard drug, silymarin. Moreover, pre-treatment rather than the post-treatment with AMF exhibited better ameliorative effect. In pre-treatment, AMF might have improved the antioxidant status of the tissue environment and ultimately decreased the peroxidative damages.
The phytochemical screening of AMF had revealed the presence of various antioxidant constituents like saponins, tannins, steroids and appreciable quantities of polyphenols and flavonoids. Polyphenols and flavonoids are proved effective as ROS scavengers and metal chelators due to the presence of multiple hydroxyl groups (Bors et al. Citation1996). Renal content of peroxynitrite and nitric oxide is increased in cisplatin treated rats (Chirino et al. Citation2004), indicating the role of NO in nephrotoxicity. In this study, AMF inhibited NO generation in an in vitro system. Flavonoids which are considered effective scavenger of NO (Shutenko et al. Citation1999) were detected in the AMF. It is thus suggested that nephroprotective effect of A. dimidiata may be due to its polyphenol and flavonoid contents. Moreover, in our previous studies, the presence of an iridioid glycoside, genipin in the AMF fraction was detected while ascertaining the cytotoxic and antitumour properties of AMF (Divya et al. Citation2015). Drewes et al. (Citation1996) had isolated genipin from the bark of A. dimidiata. Genipin has been reported to possess anti-inflammatory activity in air pouch oedema model in rat, by the inhibition of NO production (Koo et al. Citation2006). It is likely that NO scavenging ability of AMF may have a contributory role in nephroprotectivity. In an earlier study, NaF-induced imbalance in redox status was found to be regained by AMF administration in mice (Divya et al. Citation2016). Thus, the nitric oxide and other oxygen free radical scavenging phytoconstituents in AMF might be responsible for the nephroprotective effect. Further investigations are needed to identify and characterize the actual biomolecules in AMF responsible for its nephroprotective action.
Conclusion
AMF offers significant protection against cisplatin mediated nephrotoxicity primarily through its ROS and NO scavenging ability, suggesting its potential as an adjuvant in chemotherapy.
Funding information
The authors are thankful to Kerala State Council for Science Technology and Environment for providing the research fellowship (No 009-26/fshp/09/cste) to undertake this work.
Disclosure statement
The authors report no conflicts of interest.
References
- Aebi H. 1974. Catalase. In: Bergmeyer HV, editor. Methods in enzymatic analysis. Vol. 2. New York (NY): Academic Press. p. 674–684.
- Ainsworth EA, Gillespie KM. 2007. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat Protoc. 2:875–877.
- Allain CC, Poon LS, Chan CSG, Richmond W, Fu PC. 1974. Enzymatic determination of total serum cholesterol. Clin Chem. 20:470–475.
- Amptoulach S, Tsavaris N. 2011. Neurotoxicity caused by the treatment with platinum analogues. Chemother Res Pract. 2011:843019.
- Badary OA, Abdel-Maksoud S, Ahmed WA, Owieda GH. 2005. Naringenin attenuates cisplatin nephrotoxicity in rats. Life Sci. 76:2125–2135.
- Bors W, Heller W, Michel C. 1996. Flavonoids and polyphenols: Chemistry and biology. In: Cadenas E, Packer l, editors. Handbook of antioxidants. New York (NY): Marcel Dekker. p. 409–465.
- Bryant AT. 1966. Zulu medicine and medicine – men. Cape Town: Struik.
- Chang CC, Yang MH, Wen HM, Chern JC. 2002. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 10:178–182.
- Chaudhari AR. 2000. Total leukocyte count. In: Chaudhari AR, editor. A text book of practical physiology. Hyderabad: PARAS Medical Publisher. p. 104–108.
- Chirino YI, Hernandez-Pando R, Pedraza-Chaverri J. 2004. Peroxynitrite decomposition catalyst ameliorates renal damage and protein nitration in cisplatin-induced nephrotoxicity in rats. BMC Pharmacol. 4:20–29.
- Davis CA, Nicks HS, Agarwal A. 2001. Manganese superoxide dismutase attenuates cisplatin induced renal injury: importance of superoxide. J Am Soc Nephrol. 12:2683–2690.
- Divya MK, Salini S, Meera N, Lincy L, Seema M, Raghavamenon AC, Babu TD. 2016. Attenuation of DMBA/croton oil induced mouse skin papilloma by Apodytes dimidiata mediated by its antioxidant and antimutagenic potential. Pharm Biol. [Epub ahead of print]. doi:10.3109/13880209.2015.1107747.
- Divya MK, Salini S, Chubicka T, Raghavamenon AC, Babu TD. 2015. Evaluation of cytotoxic and anti-tumor properties of Apodytes dimidiata and characterisation of the bioactive component. Planta Med. 81:1705–1711.
- Drabkin DL, Austin JM. 1932. Spectrometric studies; spectrometric constants for common hemoglobin derivatives in human, dog and rabbit blood. J Biol Chem. 98:719–733.
- Drewes SE, Kayonga L, Clark TE, Brackenbury TD, Appleton CC. 1996. Iridoid molluscicidal compounds from Apodytes dimidiata. J Nat Prod. 59:1169–1170.
- Evans WC, Trease GE, Evans D. 2002. Trease and Evans pharmacognosy. 15th ed. Edinburgh: Saunders Publishers. p. 249, 454.
- Gestner J. 1938. A preliminary checklist of Zulu names of plants with short notes. Bantu Stud. 12:321–342.
- Hafemann DG, Sunde RA, Houestra WG. 1974. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr. 104:580–584.
- Harbone JB. 1973. Phytochemical methods: a guide to modern techniques for plant analysis. London: Chapman and Hall. p. 3–8.
- Hutchings A, Scott AH, Lewis G, Cunningham AB. 1996. Zulu medicinal plants: an inventory. Pietermaritzburg: University of Natal Press.
- Kadikoylu G, Bolaman Z, Demir S. 2004. The effects of desferrioxamine on cisplatin-induced lipid peroxidation and the activities of antioxidant enzymes in rat kidneys. Hum Exp Toxicol. 23:29–34.
- Kenn F, Filip C, Ann M. 2011. TAntiprotozoal and antiangiogenic saponins from Apodytes dimidiata. Phytochem. 72:1414–1423.
- Koo H, Lim K, Jung H, Park E. 2006. Anti-inflammatory evaluation of Gardenia extract, geniposide and genipin. J Ethnopharmacol. 103:496–500.
- Korkina LG, Afanasev IB. 1997. Antioxidant and chelating properties of flavonoids. Adv Pharmacol. 38:151–163.
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem. 193:265–275.
- Majid T, Hasan A, Pooran T. 2012. Inhibitory effect of olive leaf extract on gentamicin-induced nephrotoxicity in rats. Iran J Kidney Dis. 6:25–32.
- Mansour MA, Mostafa AM, Nagi MN, Khattab MM, Al-Shabanah OA. 2002. Protective effect of aminoguanidine against nephrotoxicity induced by cisplatin in normal rats. Comp Biochem Physiol C Toxicol Pharmacol. 132:123–128.
- Masoko P, Nxumalo KM. 2013. Validation of antimycobacterial plants used by traditional healers in three districts of the Limpopo Province (South Africa). Evid Based Complement Alternat Med. 2013:586247.
- McCord JM, Fridovich I. 1969. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 244:6049–6055.
- Monti DA, Yang J. 2005. Complementary medicine in chronic cancer care. Semin Oncol. 32:225–231.
- Mora LO, Antunes LM, Francescato HD, Bianchi M. 2003. The effects of oral glutamine on cisplatin induced nephrotoxicity in rats. Pharmacol Res. 47:517–522.
- Moron MA, De Pierre JW, Manner VB. 1979. Levels of glutathione, glutathione reductase and glutathione-S-transferase activities in rat liver. Biochim Biophys Acta. 582:67–68.
- Moss GA, Bondar RL, Buzzelli DM. 1975. Kinetic enzymatic method for determining serum creatinine. Clin Chem. 21:1422–1426.
- Naqshbandi A, Khan W, Rizwan S, Khan F. 2012. Studies on the protective effect of flaxseed oil on cisplatin-induced hepatotoxicity. Hum Exp Toxicol. 31:364–375.
- Ognjanovic BI, Djordjevic NZ, Matic MM, Obradovic JM, Mladenovic JM, Stajn AS. 2012. Lipid peroxidastive damage on cisplatin exposure and alterations in antioxidant defence system in rat kidneys: a possible protective effect of selenium. Int J Mol Sci. 13:1790–1803.
- Ohkawa H, Oshishi N, Yagi K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 95:351–358.
- Pan H, Mukhopadhyay P, Rajesh M, Patel V, Mukhopadhyay B. 2009. Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J Pharmacol Exp Ther. 328:708–714.
- Ramesha BT, Suma HK, Senthilkumar U, Priti V, Ravikanth G, Vasudeva R, Kumar TR, Ganeshaiah KN, Shaanker RU. 2013. New plant sources of the anti-cancer alkaloid, camptothecine from the Icacinaceae taxa, India. Phytomedicine. 20:521–527.
- Sadzuka Y, Shoji T, Takino Y. 1992. Effect of cisplatin on the activities of enzymes which protect against lipid peroxidation. Biochem Pharmacol. 43:1873–1875.
- Shino Y, Itoh Y, Kubota T. 2003. Role of poly(ADP-ribose)polymerase in cisplatin-induced injury in LLC-PK1 cells. Free Radic Biol Med. 35:966–977.
- Shutenko Z, Henry Y, Pinard E. 1999. Influence of the antioxidant quercetin in vivo on the level of nitric oxide determined by electron paramagnetic resonance in rat brain during global ischemia and reperfusion. Biochem Pharmacol. 57:199–208.
- Shweta S, Zuehlke S, Ramesha BT, Priti V, Mohana KP, Ravikanth G, Spiteller M, Vasudeva R, Uma SR. 2010. Endophytic fungal strains of Fusarium solani, from Apodytes dimidiata E. Mey. ex Arn (Icacinaceae) produce camptothecin, 10-hydroxycamptothecin and 9-methoxycamptothecin. Phytochemistry. 71:117–122.
- Sofowora A. 1993. Screening plants for bioactive agents. In: Medicinal plants and traditional medicine in Africa. 2nd ed. Ibadan: Sunshine House, Spectrum Books Ltd. p. 134–156.
- Somani SM, Husain K, Whitworth C. 2000. Dose-dependent protection by lipoic acid against cisplatin-induced nephrotoxicity in rats: antioxidant defense system. Pharmacol Toxicol. 86:234–241.
- Srivastava RC, Farook A, Ahmad N, Misra M, Hasan SK, Hussain MM. 1996. Evidence for the involvement of nitric oxide in cisplatin-induced toxicity in rats. Biometals. 9:139–142.
- Stuehr DJ, Nathan CF. 1989. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 169:1543–1555.
- Watt JM, Breyer-Brandwijk MG. (1962). The medicinal and poisonous plants of southern and eastern Africa. 2nd ed. Livingstone: Edinburgh.
- Zhang X, Yeung ED, Wang J, Panzhinskiy EE, Tong C, Li W, Li J. 2010. Isoliquiritigenin, a natural anti-oxidant, selectively inhibits the proliferation of prostate cancer cells. Clin Exp Pharmacol Physiol. 37:841–847.