Abstract
Context Scutellarin (1) has been widely used in China to treat acute cerebral infarction and paralysis induced by cerebrovascular diseases. However, scutellarin (1) has unstable metabolic characteristics.
Objective The metabolic profile of 6-O-scutellarein was studied to determine its metabolic stability in vivo.
Materials and methods In this study, a method of UFLC/Q-TOF MS was used to study the 6-O-methyl-scutellarein metabolites in rat plasma, urine, bile and faeces after oral administration of 6-O-methyl-scutellarein (3). One hour after oral administration of 6-O-methyl-scutellarein (3) (34 mg/kg), approximately 1 mL blood samples were collected in EP tubes from all groups. Bile, urine and faeces samples were collected from eight SD rats during 0–24 h after oral administration. The mass defect filtering, dynamic background subtraction and information dependent acquisition techniques were also used to identify the 6-O-methyl-scutellarein metabolites.
Results The parent compound 6-O-methyl-scutellarein (3) was found in rat urine, plasma, bile and faeces. The glucuronide conjugate of 6-O-methyl-scutellarein (M1, M2), diglucuronide conjugate of 6-O-methyl-scutellarein (M3), sulphate conjugate of 6-O-methyl-scutellarein (M4), glucuronide and sulphate conjugate of 6-O-methyl-scutellarein (M5), methylated conjugate of 6-O-methyl-scutellarein (M6) were detected in rat urine. M1, M2 and M3 were detected in rat bile. M1 was found in rat plasma and M7 was detected in faeces.
Discussion and conclusion Because the parent compound 6-O-methyl-scutellarein (3) was found in rat urine, plasma, bile and faeces, we speculate that 6-O-methyl-scutellarein (3) had good metabolic stability in vivo. This warrants further study to develop it as a promising candidate for the treatment of ischemic cerebrovascular disease.
Introduction
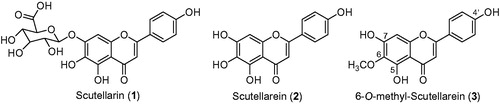
Breviscapine, which is extracted from the Chinese herb of Erigeron breviscapus (Vant) Hand-Mazz (Asteraceae), has been widely used since 1984 in China to treat acute cerebral infarction and paralysis induced by cerebrovascular diseases such as hypertension, cerebral thrombosis and cerebral haemorrhaging (Liu et al. Citation2003). Modern pharmacology has shown that scutellarin (1) (), which is the major active constituent in breviscapine, can dilate blood vessels, improve microcirculation, decrease the viscosity of blood, reduce the blood platelet count and inhibit platelet aggregation and so on (Hong & Liu Citation2004; Liu et al. Citation2005). Unfortunately, scutellarin (1) is mainly hydrolysed into scutellarein (2) () in the intestine (Zhang et al. Citation2003), and scutellarein (2) was absorbed more easily than scutellarin (1) after oral administration (Che et al. Citation2006). Our previous studies have found that scutellarein (2) had a better protective effect than scutellarin (1) in rat cerebral ischemia (Qian et al. Citation2012; Tang, et al. Citation2014b). Furthermore, scutellarein (2) could be metabolized by glucuronide conjugation, dehydroxylation, sulphation, methylation and acetylation in vivo after oral administration in rats (Tang, et al. Citation2014a). 6-O-Methyl-scutellarein (3), which is one of the major metabolites of scutellarein (2) in rats (Tang, et al. Citation2014a), might have better neuroprotective effects and stronger metabolic stability than scutellarin (1) against brain ischemia. However, there is little information on the metabolism of 6-O-methyl-scutellarein (3). In order to fully understand the action mechanism of 6-O-methyl-scutellarein (3), it is important to study the metabolic profile of 6-O-methyl-scutellarein (3) in vivo.
Figure 1. The chemical structures of scutellarin (1), scutellarein (2) and 6-O-methyl-scutellarein (3).
Currently, many analytical methods such as high performance liquid chromatography coupled with ultraviolet or mass spectrometry (HPLC/UV or HPLC/MS) have been used to study the metabolic profile of drugs in vivo. However, HPLC/UV can only determine the parent drug or the known metabolites, thus limiting its usage (Zhong et al. Citation2003; Hao et al. Citation2005; Xiong et al. Citation2006). Although HPLC/MS could provide some information on molecular formulae and fragments for identification and quantification, the ultra-flow liquid chromatography (UFLC) has higher peak capacity, greater resolution, increased sensitivity and higher speed of analysis compared to HPLC (Atila et al. Citation2014). In this paper, UFLC/quadrupole-time-of-flight mass spectrometry (UFLC/QTOF/MS) with automated data analysis (MetaboLynx™) was used for the fast determination of the metabolic profile of 6-O-methyl-scutellarein (3) after oral administration to rats. With minimal operator intervention, this method could reduce the time for data interpretation and produce efficiently the high-quality information for structure.
Materials and methods
Reagents and chemicals
6-O-Methyl-scutellarein (3) was prepared according to our previous method (Shen et al. Citation2013). Ultra-pure water, LC–MS grade acetonitrile and formic acid were purchased from Merck KGaA (Darmstadt, Germany).
Preparation of 6-O-methyl-scutellarein (3)
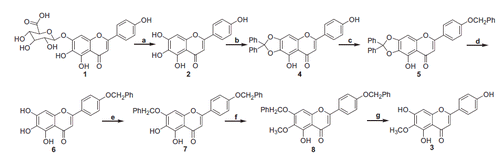
The chemical synthetic route for the construction of 6-O-methyl-scutellarein (3) is shown in Scheme 1. First, scutellarein (2) was obtained from the hydrolysis of scutellarin (1) by refluxing it with 6 N HCl in ethanol under N2 protection. Then, the treatment of 2 with 1.5 equiv. of dichlorodiphenylmethane at 175 °C using diphenyl ether as solvent, afforded the desired product 4 in good yield, where the hydroxyl groups in C6 and C7 were both protected simultaneously. Taken into account that the protecting groups of diphenylmethylene ketal and benzyl group could be removed by the different reaction condition, 1.5 equiv. benzyl bromide was used to selectively protect the hydroxyl group at C-4′ in compound 4 in the presence of K2CO3 afforded 5. Subsequently, we focused our attention on the selective deprotection conditions of the diphenylmethylene ketal in 5, the two available different methods which are hydrogenolysis or hydrolysis could work for the cleavage of this protecting group. Fortunately, the diphenylmethylene ketal was deprotected under hydrolysis conditions using HAc in H2O to afford 6 in 95% yield. Selective benzylation of 6 with 1.3 equiv. of benzyl bromide afforded the desired di-benzyl ether substituted product 7, treatment of 7 with 1.2 equiv. of iodomethane produced the desired 6-OH nethylated product 8 in 94% yield. Finally, the di-benzyl groups in 8 were deprotected under hydrogenation conditions with 10% palladium on carbon as the catalyst produced 3 in 96% yield.
Scheme 1. Reagents and conditions: (a) HCl, EtOH, N2, reflux, 36 h, 17%; (b) Ph2CCl2 (1.5 equiv.), Ph2O, 175 °C, 30 min, 85%; (c) PhCH2Br (1.5 equiv), K2CO3 (1.75 equiv), DMF, 25 °C, 12 h, 93%; (d) HAc–H2O (4:1), reflux, 1.5 h, 95%; (e) PhCH2Br (1.3 equiv), K2CO3 (1.5 equiv), DMF, 25 °C, 12 h, 93%; (f) CH3I (1.3 equiv), K2CO3 (1.5 equiv), DMF, 25 °C, 4 h, 94%; (g) Pd/C (10 wt%), H2 (atm), THF/EtOH, 8 h, 96%.
Animals
All the male SD rats (200 ± 20 g, clean grade, Certification SCXK 2009-0001) were purchased from Shanghai SLAC Laboratory Animal Co. Ltd. (Shanghai, China). With a temperature of 23 ± 2 °C, humidity of 60 ± 5%, all rats were bred in a 12-h dark/light cycle, and they had free access to food and water. The protocol was approved by the Shanghai SLAC Laboratory Animal Co. Ltd. All efforts were made to ameliorate any suffering of the animals.
Biological sample collection
Blank plasma, urine, bile and faeces samples were collected before oral administration of 6-O-methyl-scutellarein (3). One hour after oral administration of 6-O-methyl-scutellarein (3) (34 mg/kg), approximately 1 mL blood samples were collected in EP tubes from all groups, then the plasma was immediately prepared at 4 °C by centrifugation at 3000 rpm for 10 min. Urine and faeces samples were collected from four rats during 0–24 h after the administration. Bile samples were collected from another four rats during 0–24 h after the administration. All samples were kept at −78 °C until analysis.
After the plasma (200 μL) mixed with acetic acid (20 μL, 1 mol/L) on a vortex-mixer for 30 s, 2 mL ethyl acetate was added. Then, the mixture was vortex mixed for 1 min and centrifuged at 3000 rpm for 5 min at 4 °C. Supernatant solution (1.8 mL) was transferred into a 2-mL centrifuge tube and evaporated to dryness under vacuum at 37 °C. After the residue was dissolved in 100 μL mobile phase, the mixture was vortex mixed for 1 min and then centrifuged at 13 000 rpm for 10 min. For the analysis of urine and bile, the samples (1 mL) were mixed with acetonitrile (0.42 mL) and centrifuged at 13 000 rpm for 10 min. The upper layer (0.3 mL) was transferred into another tube. For the analysis of faeces, the samples (1 g) were mixed with 420 μL mobile phase on ultrasounds for 10 min and centrifuged at 13 000 rpm for 10 min, the upper layer (0.5 mL) was diluted with 670 μL distilled water, and vortex mixed for 1 min, then centrifuged at 13 000 rpm for 10 min at 4 °C and 5 μL sample was used for analysis.
LC condition
LC separation was performed on a UFLC 20ADXR LC system in-line (Shimadzu Corporation, Kyoto, Japan) coupled with hybrid quadrupole time-of-flight tandem mass spectrometer Q-TOF MS (Triple TOF™5600 MS system, AB Sciex Corporation, Foster City, CA). The auto-sampler temperature was set at 10 °C, and the injection volume was set at 5 μL. LC was performed at 40 °C using ACQUITY UPLC® HSS T3 column (2.1 mm × 50 mm, 1.8 μm) and a gradient system with a gradient mobile phase of solvent A (0.1% formic acid in ultra-pure water) and solvent B (acetonitrile) at a flow rate of 400 μL/min. The mobile phase was composed of solvent A (acetonitrile) and B (0.1% formic acid) with gradient elution: 0–6 min, increase from 5% to 35% A, 7–8 min, increase to 80% A; 8-9 min, maintain at 80% A; 9–10 min, decrease to 5% A.
Mass spectrometric condition
MS and MS/MS data were acquired by analyst®software using Triple TOF™5600 mass spectrometer with MS scan and information dependent acquisition (IDA) MS/MS scans. The mass spectrometer was operated in negative ion mode with a source temperature of 550 °C by electrospray ionization (ESI). Nitrogen gas was used as ion source gases and collision gas. The instrument was optimized by using 6-O-methyl-scutellarein (3) to obtain the best parameters in MS and MS/MS experiments. The MS mass spectrometric analysis was conducted in the range of m/z 50–1000, with the following parameters: ion source gases 1 and 2 (GS1 and GS2), 55 psi; declustering potential, 100 V; entrance potential, 13 V and collision energy (CE) 10. MS/MS data were acquired using IDA methods. Most intense 10 peaks for ions with m/z 50–1000 trigger IDA criteria using real time mass defect filtering and dynamic background subtraction. The MS/MS CE was set at 38 with spread ± 15. External mass calibration was performed automatically using calibrate delivery system.
Results and discussion
Metabolism profiles by UFLC–MS analysis
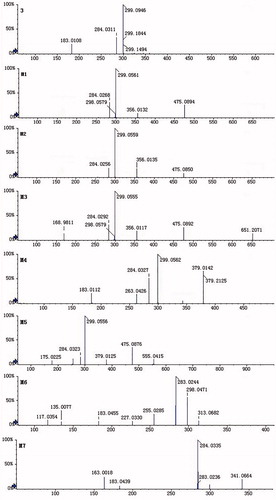
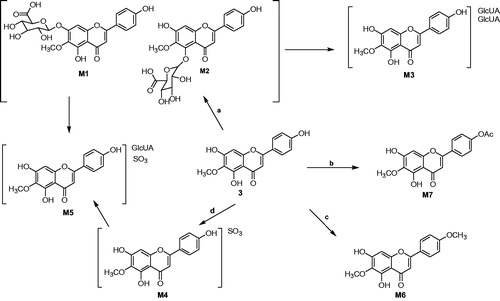
Chromatographic profiles of 6-O-methyl-scutellarein (3) in biological samples were analysed by UFLC/Q-TOF MS (shown in ). By careful setting of the key parameters of Metabolitepilot™, this method could determine a wide range of metabolites with only a limited requirement for manual intervention. Using negative ion electrospray tandem mass spectrometry, the parent compound 6-O-methyl-scutellarein (3) and seven 6-O-methyl-scutellarein metabolites (M1–M7) were identified by comparison with blank samples, the results are shown in . The results indicated that 6-O-methyl-scutellarein (3) had four major metabolic pathways in vivo including glucuronidation, sulphation, methylation and acetylation.
Figure 2. UFLC/MS chromatograms of rat urine, plasma, faeces and bile samples before and after oral administration of 6-O-methyl-scutellarein (3): (a) urine sample in ESI-mode, (b) plasma sample in ESI− mode, (c) faeces sample in ESI− mode, (d) bile sample in ESI− mode.
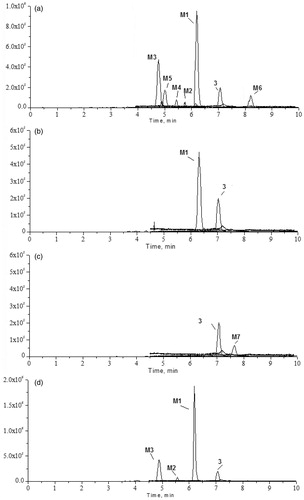
Table 1. UFLC/ESI-MS, retention time and fragment ions of 6-O-methyl-scuetellarin (3) and its metabolites in rats.
LC–MS/MS analysis of 6-O-methyl-scutelellarien and metabolites
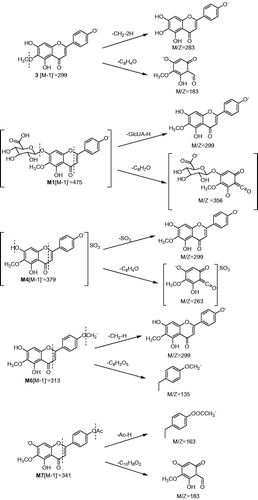
Parent compound (3): In rat plasma, urine, bile and faeces, the parent compound 6-O-methyl-scutellarein (3) was detected on the same retention time and MS spectra of the standard solution of 6-O-methyl-scutellarein (3) which indicated that a lot of 6-O-methyl-scutellarein (3) was absorbed in the gastrointestinal tract and a fraction of 6-O-methyl-scutellarein (3) which was not absorbed was discharged in vitro by faeces. The deprotonated ion of 6-O-methyl-scutellarein (3) was at m/z 299 and the mass spectrum yielded fragment ions at m/z 284 and 183 in ).. The fragment ion at m/z 183 was generated after the Retro-Diels Alder (RDA) cleavage of A ring (
Metabolites (M1, M2)
The retention time of two compounds (M1, M2) with m/z 475 [M − 1]− was found at 6.21 and 5.72 min () in rat urine and bile, respectively. M1 and M2 were 176 Da higher than the deprotonated ion of 6-O-methyl-scutellarein (3), the predominant fragmentation ions at m/z 299 [M − H-GlcUA]− and m/z 284 [M − 2H-GlcUA-CH2]− were detected in the mass spectra of M1 and M2 (). The fragment ion at m/z 299 was generated by deglycosylation from the loss of m/z 176, the other fragment ion at m/z 284 was generated by further loss of methyl group from the fragment ion at m/z 299, the m/z 356 was generated after the RDA cleavage (). Therefore, we speculated that M1 and M2 were identified as glucuronide conjugates of 6-O-methyl-scutellarein and the glucuronide was conjugated in A ring. Recently, Xia et al. (Citation2007) found scutellarein-6-O-glucuronide and scutellarein-5-O-glucuronide in rat urine after oral administration of breviscapine, these two metabolites were isolated by open-column chromatography and preparative HPLC, and their structures were confirmed by MS, 1H NMR, 13C NMR and nuclear Overhauser enhancement spectroscopy. They also found that the chemical polarity of scutellarein-5-O-glucuronide was relatively larger than scutellarein-6-O-glucuronide. Based on this research, we speculated that the chemical polarity of 6-O-methyl-scutellarein-5-O-glucuronide was relatively larger than 6-O-methyl-scutellarein-7-O-glucuronide. So, one 6-O-methyl-scutellarein glucuronide with the retention time at 5.72 min in this study was identified as 6-O-methyl-scutellarein-5-O-glucuronide (M2).
Metabolite (M3)
The diglucuronide metabolite M3 was observed in the UFLC chromatogram in rat urine and bile with a retention time at 4.73 min () and was not found in rat plasma and faeces. MS determinations indicated that there was a molecular ion at m/z 651, and this molecular ion was 352 Da (GlcUA) higher than the protonated ion of 6-O-methyl-scutellarein (3). The MS/MS spectrum of M3 in provided some fragment ions which were at m/z 475 [M − GlcUA-H]−, m/z 356, m/z 299 [M − H-2GlcUA]− and m/z 284 [M − 2H-2GlcUA-CH2]. The fragment ion at m/z 475 was generated by the loss of glucuronide group from the precursor ion at m/z 651, another fragment ion at m/z 299 was attributed to further loss of glucuronide group from the fragment ion at m/z 475 and the fragment ion at m/z 284 was produced by the loss of methyl group from the fragment ion at m/z 299. The fragment ion at m/z 356 was also the characteristic product ion () and was generated after the RDA cleavage. Based on this analysis, we speculated that compound M3 should be diglucuronide conjugates of 6-O-methyl-scutellarein. However, the positions of diglucuronide could not be identified.
Metabolites (M4)
Diagnostic losses of 80 Da corresponding to a sulphate group were observed in the mass spectra of M4 (m/z 379) with a retention time at 5.43 min () in rat urine. The molecular weight of M4 was 80 Da higher than the deprotonated ion of 6-O-methy-scutellarein (3). From the mass spectra of M4, the peak at 299 [M-SO3-H]- and 284 [M-SO3-2H-CH2]- is observed in . So, compound M4 was identified as one metabolite which contains a sulphate moiety. In the chemical structure of 6-O-methyl-scutellarein (3), every hydroxyl group at C-5, C-7 and C-4′ position was a possible site for conjugation with sulphate group. Interestingly, the predominant fragmentation ions at m/z 263 which was generated after the RDA cleavage indicated that sulphate group should not occupy the C-4′ position (). However, we could not be sure that the sulphate group was conjugated at C-5 or C-7 position.
Metabolites (M5)
In the urine, the retention time at 4.92 () min was metabolite M5 with fragment ion at m/z 555, and the fragment ions at m/z 475 [M − H-SO3]−, m/z 379 [M − H-GlcUA]−, m/z 299 [M − H-GlcUA-SO3]− and m/z 284 [M − 2H-GlcUA-SO3-CH2]− were observed in the mass spectra of M5 in . The fragment ion at m/z 475 was produced by the loss of sulphate group from the precursor ion at m/z 555, the m/z 379 was generated by loss of glucuronide group from the precursor ion at m/z 555, the m/z 299 was obtained by further loss of glucuronide group from the fragment ion at m/z 475 or sulphate group from the fragment ion at m/z 379, and the fragment ion at m/z 284 appeared after the loss of methyl group from the fragment ion at m/z 299. Based on this analysis, compound M5 should be 6-O-methyl-scutellarein conjugated with both glucuronide group and sulphate group, unfortunately, the positions of glucuronide and sulphate were not identified.
Metabolites (M6)
In rat urine, the retention time of one metabolite M6 was at 8.22 min with its ion at m/z 313 (). The fragment ions at m/z 298 [M − 2H-CH2]− and m/z 283 [M − 3H-CH2-CH2]− were found in the mass spectra of M6 in , this compound was 15 Da (CH3) higher than the deprotonated ion of 6-O-methyl-scutellarein (3) and was 28 Da (2CH2) higher than the deprotonated ion of scutellarein (2), this phenomenon indicated that there were two methyl groups conjugated with 6-O-methyl-scutellarein. From the MS/MS spectra of M6, the fragment ion at m/z 183 and 135 was generated after the RDA cleavage (), this result indicated that the metabolite M6 was identified as 4′,6-di-O-methyl-scutellarein.
Metabolites (M7)
Diagnostic losses corresponding to acetyl group (Ac, 42 Da) were observed in the mass spectra of M7 (m/z 341) with a retention time at 7.67 min in rat faeces (). As shown in , the fragment ion at m/z 299 [M − H-Ac]− was observed in the mass spectra of M7, and it was 42 Da higher than the deprotonated ion 6-O-methyl-scutellarein (3), which was generated by the loss of acetyl group from the precursor ion at m/z 341. From the MS/MS spectra of M7, the fragment ion at m/z 183 and 163 was generated after the RDA cleavage (), this phenomenon indicated that the metabolite M7 was identified as 4′-O-acetyl-6-O-methyl-scutellarein.
Conclusion
In this study, the 6-O-methyl-scutellarein (3) metabolites in rats after oral administration have been studied by UFLC/Q-TOF MS combined with automated data analysis (Metabololitepilot™). The parent compound 6-O-methyl-scutellarein (3) and its seven metabolites (M1–M7) including the glucuronide conjugate (M1, M2), diglucuronide conjugate of 6-O-methyl-scutellarein (M3), sulphate conjugate of 6-O-methyl-scutellarein (M4), glucuronide and sulphate conjugate of 6-O-methyl-scutellarein (M5), 4′,6-di-O-methyl-scutellarein (M6), 4′-O-acetyl-6-O-methyl-acetyl-scutellarein (M7) were detected and identified simultaneously according to their retention times, accurate molecular masses and fingerprint product ions after comparison with blank samples, most of these metabolites were discovered for the first time.
Interestingly, the parent compound of 6-O-methyl-scutellarein (3) has been found in rats urine, plasma, bile and faeces, this result indicated that 6-O-methyl-scutellarein (3) had better metabolic stability in vivo than the scutellarin. Furthermore, the glucuronide conjugate of 6-O-methyl-scutellarein (M1, M2), diglucuronide conjugate of 6-O-methyl-scutellarein (M3), the sulphate conjugate of 6-O-methyl-scutellarein (M4), the glucuronide and sulphate conjugate of 6-O-methyl-scutellarein (M5), the methylated conjugate of 6-O-methyl-scutellarein (M6) were detected in rat urine. M1, M2 and M3 were detected in rat bile. M1 was detected in rat plasma and M7 was found in faeces. These results showed that 6-O-methyl-scutellarein (3) had some main metabolic pathways in rats including methylation, sulphation, acetylation and glucuronidation in vivo after oral administration (). Because 6-O-methyl-scutellarein (3) had better metabolic stability than scutellarin in vivo, it could be a promising candidate for treating ischemic cerebrovascular disease, the neuroprotective effects of 6-O-methyl-scutellarein (3) against brain ischemia will be studied in the near future.
Funding information
This work was supported by National Natural Science Foundation of China (No. 81274058), the Program for New Century Excellent Talents by the Ministry of Education (NCET-12-0741), 333 High-level Talents Training Project Funded by Jiangsu Province, Six Talents Project Funded by Jiangsu Province (2013-YY-010), Technology Innovation Venture Fund by Nanjing University of Chinese Medicine (CX201301), Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (ysxk-2010), Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions and Project Funded by the Flagship Major Development of Jiangsu Higher Education Institutions (PPZY2015A070) and Jiangsu Collaborative Innovation Center of Chinese Medicinal Resources Industrialization (ZDXMHT-1-13).
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Atila A, Ozturk M, Kadioglu Y, Halici Z, Turkan D, Yayla M. 2014. Development and validation of UFLC–MS/MS method for determination of bosentan in rat plasma. J Pharm Biomed Anal Biomed. 97:33–38.
- Che QM, Chen Y, Pan LY, He H. 2006. Scutellarein’s pharmacokinetics in rats. Chin J New Drugs. 15:1557–1561.
- Hao X, Cheng G, Sun J, Zou M, Yu J, Zhang S, Cui F. 2005. Validation of an HPLC method for the determination of scutellarin in rat plasma and its pharmacokinetics. J Pharm Biomed Anal Biomed. 38:360–363.
- Hong H, Liu GQ. 2004. Protection against hydrogen peroxide-induced cytotoxicity in PC12 cells by scutellarin. Life Sci. 74:2959–2973.
- Liu H, Yang X, Tang R, Liu J, Xu H. 2005. Effect of scutellarin on nitric oxide production in early stages of neuron damage induced by hydrogen peroxide. Pharmacol Res. 51:205–210.
- Liu YM, Lin AH, Chen H, Zeng FD. 2003. Study on pharmacokinetics of scutellarin in rabbits. Yao Xue Xue Bao. 38:775–778.
- Qian LH, Shen MZ, Tang H, Tang YP, Zhang L, Fu YF, Shi QP, Li NG. 2012. Synthesis and protective effect of scutellarein on focal cerebral ischemia/reperfusion in rats. Molecules. 17:10667–10674.
- Shen MZ, Li NG, Shi ZH, Tang H, Shi QP, Tang YP, Yang JP, Duan JA. 2013. Efficient synthesis of 6-O-methyl-scutellarein from scutellarin via selective methylation. Lett Org Chem. 10:733–737.
- Tang H, Li NG, Tang YP, Shi QP, Guo JM, Zhang W, Shen MZ, Duan JA. 2014a. Identification of scutellarein metabolites in rat using ultra performance liquid chromatography quadrupole-time-of-flight mass spectrometry. Anal Methods. 6:4667–4673.
- Tang H, Tang YP, Li NG, Shi QP, Guo JM, Shang EX, Duan JA. 2014b. Neuroprotective effects of scuetellarin and scutellarin repeatedly cerebral ischemia–reperfusion in rats. Pharmacol Biochem Behav. 118:51–59.
- Xia HJ, Qiu F, Zhu S, Zhang TY, Qu GX, Yao XS. 2007. Isolation and identification of ten metabolites of breviscapine in rat urine. Biol Pharm Bull. 30:1308–1316.
- Xiong F, Wang H, Cheng J, Zhu J. 2006. Determination of scutellarin in mouse plasma and different tissues by high-performance liquid chromatography. J Chromatogr B Anal Technol Biomed Life Sci. 835:114–118.
- Zhang JM, Che QM, Li SZ, Zhou TH. 2003. Study on metabolism of scutellarin in rats by HPLC–MS and HPLC-NMR. J Asian Nat Prod Res. 5:249–256.
- Zhong DF, Yang BH, Chen XY, Li K, Xu JH. 2003. Determination of scutellarin in rat plasma by high-performance liquid chromatography with ultraviolet detection. J Chromatogr B Analyt Technol Biomed. Life Sci 796:439–444.