Abstract
Context Metformin induced AMP-activated protein kinase (AMPK) and protected neurons in cerebral ischaemia.
Objective This study examined pretreatment with metformin and activation of AMPK in molecular and behavioral levels associated with memory.
Materials and methods Rats were pretreated with metformin (200 mg/kg) for 2 weeks and 4-vessels occlusion global cerebral ischaemia was induced. Three days after ischaemia, memory improvement was done by passive avoidance task and neurological scores were evaluated. The amount of Brain-Derived Neurotropic Factor (BDNF) and phosphorylated and total P70S6 kinase (P70S6K) were measured.
Results Pretreatment with metformin (met) in the met + ischaemia/reperfusion (I/R) group reduced latency time for enter to dark chamber compared with the sham group (p < 0.001) and increased latency time compared with the I/R group (p < 0.001). Injection of Compound C (CC) (as an AMPK inhibitor) concomitant with metformin reduced latency time in I/R rats compared with the I/R + met group (p < 0.05). Neurological scores were reduced in met treated rats compared with the sham group. Pretreatment with metformin in I/R animals reduced levels of pro-BDNF compared with the I/R group (p < 0.001) but increased that compared with the sham group (p < 0.001). The level of pro-BDNF decreased in the met + CC + I/R group compared with the met + I/R group (p < 0.01). Pretreatment with metformin in I/R animals significantly increased P70S6K compared with the I/R group (p < 0.001).
Conclusion Short-term memory in ischaemic rats treated with metformin increased step-through latency; sensory-motor evaluation was applied and a group of ischaemia rats that were pretreated with metformin showed high levels of BDNF, P70S6K that seemed to be due to increasing AMPK.
Introduction
Cerebral ischaemia is the second cause of death and one of the reasons for the prolonged neurological disorders and dementia in the world (Li et al. Citation2014). Many lines of evidence indicated that nearly 87% of brain strokes were ischaemia (Truong et al. Citation2012). Acute cerbral ischaemia due to the occlusion of intracranial vessels leads to a high rate of mortality and is generally considered to be heterogeneous and multi-factorial disorder (Dichgans Citation2007; Ajami et al. Citation2011; Walcott et al. Citation2013). There are two types of cerebral ischaemia: focal and global cerebral ischaemia. In focal cerebral ischaemia/reperfusion (I/R), blood flow interrupted in some areas of the brain and, in global cerebral I/R, interruption of cerebral blood flow occurs when patients undergo cardiac arrest, shock or respiratory arrest, and this is usually associated with wide ranging neuronal death in the hippocampus, cortex and striatum (Petito et al. Citation1987; Globus et al. Citation1991; Zare Mehrjerdi et al. Citation2013; Ashabi et al. Citation2014).
The vulnerable areas of the brain which are damaged during global cerebral I/R are involved in memory and learning processes (Vorhees & Williams Citation2014). Clinical and experimental data have revealed that vascular dementia during global cerebral ischaemia (Cummins et al. Citation1988; Wu et al. Citation2009).
Metformin is one of the major anti-diabetic drugs used for hyperglycaemia. Metformin activates AMP-activated protein kinase (AMPK) by phosphorylation of the Thr172 residue (Hardie Citation2008). Recently, a wide range of therapeutic actions of metformin against brain vascular disruption and cerebral cancer cortexes have been determined (Jun et al. Citation2015). Metformin improved learning and memory deficits in diabetic models, Alzheimer’s and Parkinson’s diseases, which many researchers agree relates to the activation of AMPK by metformin (Wilson & Cook Citation1994; DiTacchio et al. Citation2015; Chen et al. Citation2016).
Collected data from our laboratory have reported that metformin induced its protective role by the phosphorylation of AMPK. Metformin reduced apoptosis and enhanced the levels of antioxidants in the hippocampus (Ashabi et al. Citation2014, Citation2015). According to our recent paper (Farbood et al. Citation2015), metformin modulated alterations of cerebral blood flow during and after ischaemia and then facilitated the activity of neuronal discharge.
Near relations among synaptic plasticity and memory regulatory proteins [such as Brain-Derived Neurotrophic Factor (BDNF), P70S6 kinase (P70S6K)] and AMPK were detected (Ishizuka et al. Citation2013). However, on one hand, a distinct mechanism of AMPK related to expression of BDNF is under consideration (Ishizuka et al. Citation2013). On the other hand, some data have shown that AMPK directly activated BDNF and increased long-term potentiation (LTP) in hippocampal neurons (Potter et al. Citation2010). Indeed, many events underlying plasticity are related to protein translation. Phosphorylation of P70S6K leads to mRNA transcription and protein synthesis which finally activates other kinases such as mitogen activated protein kinase and phosphoinositide 3-kinase and so enhances viability of the cells (Lehman et al. Citation2003; Lu Citation2003). In this respect, the signalling pathway of AMPK/P70S6K through modulation of mammalian target of rapamycin was interestingly documented in carcinoma cells (Zhang et al. Citation2014; Plews et al. Citation2015); while unfortunately, there was no evidence in the brain study.
The main idea of the present study was to investigate the role of metformin against global cerebral ischaemia focused on learning and memory. We have shown the role of activation and/or inhibition of AMPK on memory improvement by behavioural assessment (passive avoidance performance). Then, BDNF and activation of P70S6K were detected in the hippocampal neurons in the presence of metformin or in the inhibition of AMPK.
Materials and methods
Animals
Adult male Wister rats were kept in cages under standard conditions (22 ± 2 °C), humidity and a 12 h light/dark cycle (light on 07:00–19:00), with food and water provided ad libitum. Experimentation was approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences in accordance with international guidelines for animal experiments.
Experimental design
The rats were randomly divided into eight groups: (1) “Sham”, (2) metformin (200 mg/kg); “Met”, (3) compound C (CC); “CC”, (4) metformin and CC; “Met + CC”, (5) ischaemia/reperfusion; “I/R”, (6) met pretreatment plus I/R; “Met + I/R”, (7) CC and I/R; “CC + I/R”, (8) met pretreatment plus CC and I/R “Met + CC + I/R”.
Preparation and administration of drugs
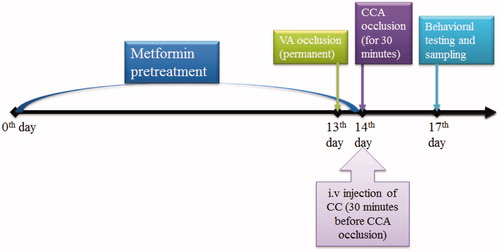
Metformin (200 mg/kg) was dissolved in the phosphate buffer saline (PBS, 0.1 M) and gavage was performing once daily for 14 d (Ashabi et al. Citation2014). On the 13th day, the vertebra arteries were coagulated and, on the 14th day, common carotid artery (CCA) was clipped for 30 min. CC was dissolved in DMSO 10% and aliquots of CC at a concentration of 5 μg/μL prepared in PBS (0.1 M) for an intravenous (5 μL) injection at 30 min before induction of cerebral ischaemia (14th day) ().
Figure 1. Outline scheme of experimental designs. Rats were pretreated with metformin over 14 d and on days 13 and 14, the 4VO surgery was done. On the day 17, the behavioural tests for passive avoidance task and neurological scores were evaluated and rats were scarified for molecular measurements (CCA, common carotid arteries; VA, vertebral arteries; CC, compound C).
Surgical procedure
Rats underwent transient forebrain global ischaemia as described by Pulsinelli and Brierley (Citation1979). Briefly, on the first day, rats were anesthetized by chloral hydrate (400 mg/kg). A sterile string was loosely placed around each CCA without interrupting carotid blood flow and the incision was sutured. Both vertebral arteries were permanently electro coagulated. All changes in cerebral blood flow were monitored by laser Doppler Flowmetry whereas cerebral blood flow was less than 25%, the rats were used as global cerebral ischaemia ones. Seventy-two hour reperfusion was initiated by opening the carotid clamps after 30 min of ischaemia. Sham surgery involved exposure of common carotid and vertebral arteries. Rectal temperature was monitored (Citizen-513w) and kept at 37 °C by surface heating and cooling during surgery.
Neurological scores and experimental groups
Neurological outcomes were assessed using standard neurological score parameters (a maximum score of 25) (Shi et al. Citation2011). In brief, the symptoms of roughed hair raising, movement decreasing, improving the response to ear-palpating and eyelid ptosis, were 1 point, separately for each other and splayed-out hind limb, eye patency, circulating around body, hooked position, and clonus were 3 points, respectively; and myasthenia of limbs was 6 points. The first total scores were obtained after being conscious immediately after reperfusion. Twenty-four hours later, the second neurological scores were examined. The higher score showed the more neurological damage (Shi et al. Citation2011).
Passive avoidance task
The two-way shuttle box instrument (ST-5500, Borj Sanat Co., Tehran, Iran) has been used for this test, which was formed with two adjacent Plexiglas boxes of identical sizes (27 cm × 14.5 cm × 14 cm) with plexus floors. The floors from two compartments have been covered with stainless steel bars (2 mm diameter) are separated by 1 cm distance. The bright compartment was lightened by a 5 W lamp attached on the wall, just under a mobile transparent Plexiglas roof. For passive avoidance test, Tamburella method was used with little modification (Lipton & Rosenberg Citation1994; Mansouri et al. Citation2013). The procedure was performed in 3 days, on the first day; the rats were adapted for recognition of compartments. Each rat was allowed over a period of 10 min adaptation to free access to either bright or dark compartment to avoid training box after being placed in a shuttle box (to make familiar with tools). On the second day, the rats were placed in the bright compartment and, 10 s later, the sliding door was elevated, and latency of step-down was recorded as a learning phase (initial latency). After entering the dark compartment, the door was closed and when the rats touched the grid by all four paws, a low-level electric shock (0.3 mA, 3 s) was delivered. After 3 min, the rats were removed from the dark compartment and were placed in their home cage. On the third day, in order to assess short-term memory, they were placed in the bright chamber again, and, 10 s later, the sliding door went up, the latency to enter the dark compartment (step-through latency) and the time duration in a dark chamber were recorded. The cut-off time was 300 s and no shock was delivered on this day (Lipton & Rosenberg Citation1994; Mansouri et al. Citation2013).
Sacrifice and tissue preparation
After 72 h reperfusion, each group (n = 6) was killed by asphyxiation with CO2. The rats were decapitated, their brains were removed and the hippocampi were isolated on ice, frozen in liquid nitrogen and stored at −80 °C for Western blotting assay.
Western blotting
The hippocampi of the brains were dissected in lysis-buffer containing protease inhibitor cocktails. Total protein extract was collected after centrifuging at 13 000 g for 5 min. Protein concentrations in collected supernatant were assayed using the Bradford method (Bradford Citation1976). Standard plots were generated using bovine serum albumin. Then, the total proteins (60 μg) were electrophoresed on 12% SDS-PAGE gels and then transferred to poly vinylidine fluoride (PVDF) membranes and probed with specific antibodies. Immune-reactive polypeptides were detected by chemo-luminescence using enhanced electro-chemo-luminescence (ECL) reagents and a subsequent autoradiography. A densitometry scan of films was performed to quantify the results. Finally data analysis was done by Image J software (NIH, Bethesda, MD), measuring integrated densities of obtained bands after background subtraction.
Statistical analysis
All data were expressed as mean ± SEM. The difference between data was assessed using one-way ANOVA followed by Tukey HSD as a post-test in SPSS 16.0 software (SPSS Inc., Chicago, IL). p Values < 0.05 were considered statistically significant.
Results
Metformin increased neurological scores in the ischaemic rats in two measured time-points
The rats were scored immediately after reperfusion and 24 h after reperfusion. As shown in , neurological scores were 1.50 ± 0.4 in the sham-operated group immediately after reperfusion that was significantly different with the I/R group (p < 0.001). Neurological scores were reduced in the met + I/R group compared with the I/R group immediately after reperfusion (p < 0.001). The neurological scores increased in the met + CC + I/R group compared with the met + I/R group immediately after reperfusion (p < 0.001). Neurological scores were zero in the sham, met, CC and met + CC groups, 24 h after reperfusion. The neurological outcomes increased in the met + I/R group compared with the I/R group 24 h after reperfusion (p < 0.001). Neurological outcomes increased in the met + CC + I/R group compared with the met + I/R group 24 h after reperfusion (p < 0.001) ().
Table. 1. Metformin improves neurological scores in the global cerebral ischaemia/reperfusion models.
Evaluation of metformin role in the passive avoidance task against cerebral I/R animals
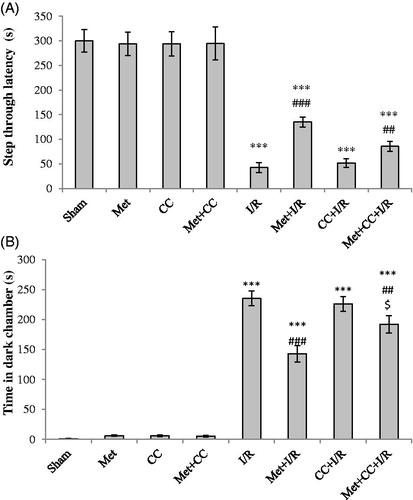
In , step-through latency time reduced in the I/R group compared with the sham group (p < 0.001). Also, spent time increased in a dark chamber in the I/R group compared with the sham group (p < 0.001). Pretreatment of rats with metformin in the metformin + I/R group increased the time of latency in dark chamber compared with the sham group (p < 0.001) and compared with the I/R group (p < 0.001, ). It is expected that in ischemic rats, which pretreated with metformin, spent time had reduced in a dark chamber compared with the I/R group (p < 0.001). Administration of the metformin group compared with the sham group has no significant role on memory and learning in the passive avoidance test (p > 0.05).
Figure 2. Evaluated effects of administration of metformin and CC on step through latency in ischemic rats. (A) Latency time to the dark chamber was detected for 5 min and expressed as seconds. (B) Time spent in dark chamber was recorded over 5 min. Bars indicate the mean ± SEM. ***p< 0.001 versus the sham group, ##p< 0.01, ###p< 0.001 versus the I/R group. $p< 0.05 versus the met + I/R group. Met, metformin (200 mg/kg); CC, compound C; I/R, ischaemia/reperfusion.
Inhibition of AMPK by CC reversed the beneficial effects of metformin in the passive avoidance task
The latency time reduced in ischemic animals which were treated with CC + metformin and spent time increased in the dark chamber compared with the sham group (p < 0.001 for both, ). In addition, the treatment of ischemic animals by CC + metformin significantly increased latency time and the time reduced in a dark chamber compared with the metformin + I/R group (p < 0.05 for both, ). Besides, injection of CC alone has no effect on latency time compared with the sham group (p > 0.05).
The role of metformin on pro-BDNF levels in hippocampus of I/R rats
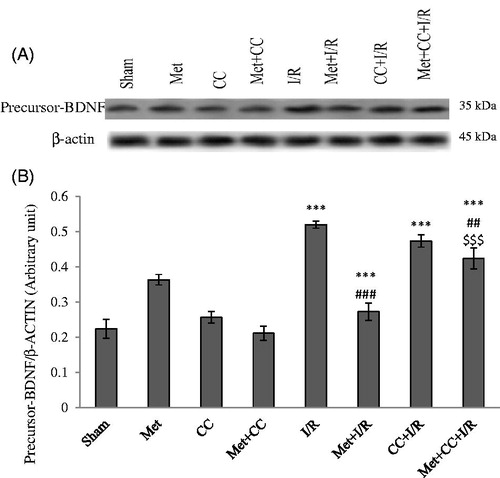
As shown in , the results showed that the level of pro-BDNF increased in the I/R group compared with the sham group (p < 0.001). Pretreatment of I/R animals by metformin reduced the level of pro-BDNF compared with the I/R group (p < 0.001) but it significantly increased compared with the sham group (p < 0.001).
Figure 3. Western blot analysis to measure the effect of metformin pretreatment on the mature BDNF levels in the hippocampus. (A) Western blots for pro-BDNF are shown. (B) The density of pro-BDNF bands was measured and their ratio was calculated. Bars indicate the mean ± SEM. ***p< 0.001 versus the sham group, ###p< 0.01, ###p< 0.001 versus the I/R group, $$$p < 0.001 versus the met + I/R group. Met, metformin (200 mg/kg); CC, compound C; I/R, ischaemia/reperfusion.
Inhibition of AMPK reduced the action of metformin on pro-BDNF levels in I/R rats
Pretreatment of I/R animals by CC or CC + metformin did not have significant changes in the level of pro-BDNF compared with the I/R group (p > 0.05) but these groups had a statistical difference compared with the sham group (p < 0.001). The level of pro-BDNF increased in the CC + metformin + I/R group compared with the met + I/R group (p < 0.001, ).
Activation of AMPK by metformin effects on phosphorylation of P70S6K after I/R
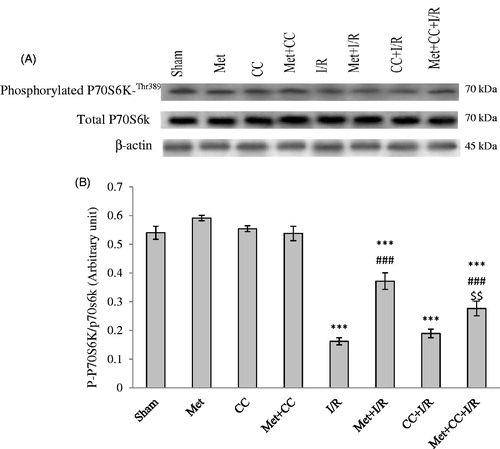
As shown in , data revealed that the level of phospho-P70S6K/total P70S6K reduced in the I/R group compared with the sham group (p < 0.001). Pretreatment of I/R animals by metformin enhanced the levels of phospho-P70S6K/total P70S6K compared with the I/R group (p < 0.001).
Figure 4. The western blotting analysis was used to measure the effect of pretreatment with metformin on the phosphorylated P70S6K, total P70S6K levels in the hippocampus. (A) Western blots for phosphorylated P70S6K, total P70S6K are shown. (B) The density of phosphorylated P70S6K to total P70S6K bands was measured and their ratio was calculated. Bars indicate the mean ± SEM. ***p< 0.001 versus the sham group, ###p< 0.001 versus the I/R group, $$p < 0.01 versus the met + I/R group. Met, metformin (200 mg/kg); CC, compound C; I/R, ischaemia/reperfusion.
Inhibition of AMPK effects on phosphorylation of P70S6K followed by the model of cerebral ischaemia
Pretreatment of I/R animals by CC or CC + metformin compared with the I/R group did not have significant changes on P70S6K (p > 0.05) but these groups have statistical differences compared with the sham group (p < 0.001). The level of phospho-P70S6K decreased in the CC + metformin + I/R group compared with the met + I/R group (p < 0.01) ().
Discussion
This study explained the role of metformin in improving memory function in the global cerebral I/R. Besides, recent investigations revealed that metformin induced phosphorylation of AMPK and consequently increased neurological scores as well as memory and some of molecules involved in cognition. Metformin increased BDNF and P70S6K in hippocampal neurons and enhanced the formation of memory in passive avoidance task. As expected, in line with our previous studies, AMPK might be responsible for the protective function of metformin.
One of the main disruptive roles of cerebral ischaemia was neurovascular damage in which blood–brain-barrier (BBB) and reactive hyperaemia impaired after reperfusion. The vascular damage considered in the pathophysiology of cerebral ischaemia and impaired neuronal homeostasis in the brain. Our previous findings declared that neuronal homeostasis has impaired through BBB disruption and increased reactive hypermedia (Farbood et al. Citation2015).
The protective role of metformin in the neuronal homeostasis and associated electrophysiological activity was obtained in our past findings (Ashabi et al. Citation2014; Farbood et al. Citation2015). Indeed, we suggested that activation of AMPK through treatment by metformin increased locomotor performance in behavioural data (Sarkaki et al. Citation2015). So, we were planning to investigate the role of metformin in the memory function in behavioural and molecular process. Brain damage followed by global cerebral ischaemia which raised the behavioural and pathophysiological deficits. Neurological outcomes were one of the major indicators of cerebral I/R (Lenzser et al. Citation2005). Metformin increased neurological scores and injection of CC plus metformin reduced neurological scores which determined the protective role of AMPK in global cerebral ischaemia.
According to our previous reports, the sensory motor functions were improved by metformin (Sarkaki et al. Citation2015) and the protocol introduced by Shi et al. (Citation2011) while revealed additional methods in neurological scores assessments. Subsequently, we declared the role of metformin treatment on learning and memory; passive avoidance test presented as the behavioral method in many studies of learning and memory, probably because it required little specialized training for subjects, and results are available quickly (Wilson & Cook Citation1994).
Here, behavioural assessments using passive avoidance test showed that metformin increased short-term memory which impaired over ischaemia and also determined the importance of activation of AMPK by CC injection (reversed role of CC versus metformin). The therapeutic potential of metformin against oxidative damages to brain tissue such as neurodegenerative diseases was accepted and our results confirmed previous works (Ishizuka et al. Citation2013; Lennox et al. Citation2014; Mousavi et al. Citation2015).
In the molecular step, we focused on neurotrophins in the brain, neurotrophic factors could play a functional role in the adult brain, given that trophic signals protected target innervations, improved cell viability, plasticity and neuronal regeneration, controlled synthesis of the neurotransmitter and the neuronal excitability under oxidative condition (Fahnestock et al. Citation2002).
BDNF is one of the most important neurotrophin factors in the brain and clearly demonstrated both in vitro and in vivo whereas reduction of BDNF leading to neuronal atrophy and finally death. Like other neurotrophins, a pro-BDNF was cleaved to a mature-BDNF (m-BDNF) in the endoplasmic reticulum or in other way, pro-BDNF is released into the extracellular environment and cleaved by plasmin (Barker Citation2009). Some studies have demonstrated that the pro-BDNF and the m-BDNF had opposite biological potentials (Barker Citation2009; Yeh et al. Citation2015), while other studies indicated that pro-BDNF and m-BDNF collaborated with each other in the neurodegenerative diseases (Fahnestock et al. Citation2002).
Our data indicated that the levels of pro-BDNF were increased during cerebral I/R and metformin decreased hippocampal level of BDNF. Current results confirmed previous studies that the pro-BDNF and the m-BDNF in the neuronal contexts presented opposite functions (Numakawa et al. Citation2010). Tsai et al. reported that the neuronal apoptosis improved in high levels of pro-BDNF (Tsai Citation2007). However, the pro-BNDF cleaved to m-BDNF by several enzymes such as Furin (Lu Citation2003); this process may be disrupted during cerebral ischaemia and metformin administration has recovered cellular enzymes in Golgi apparatus and consequently decreased the level of pro-BDNF, which means that m-BDNF might be increased after pretreatment by metformin.
Also, injection of CC reversed the role of metformin and activation of AMPK which was consistent with our previous findings about the role of AMPK in the I/R (Ashabi et al. Citation2014, Citation2015). Unfortunately, our selected antibody could not detect m-BDNF in the hippocampus but according to mentioned reports and determined phosphorylation of P70S6K, the level of AMPK/BDNF reduced during forebrain ischaemia.
P70S6K is one of the last downstream proteins that was phosphorylated through activation of BDNF and enhanced the plasticity in degenerated regions of the brain (Fang et al. Citation2013; Koskimaki et al. Citation2015). The ratio of phospho-P70S6K/total-P70S6K reduced in the I/R group and probably metformin induction increased phospho-P70S6K/total-P70S6K levels through AMPK activation. Zhou et al. (Citation2010) demonstrated the role of BDNF on phosphorylation of P70S6K in the neurons; the current study presented activation of AMPK as activation of one of the up-stream proteins in this pathway against global cerebral I/R. However, Ishizuka et al. (Citation2013) established the role of phosphorylation of AMPK on BDNF/P70S6K pathways in vitro and similarly our data agreed with Ishizuka findings in vivo.
Conclusion
In conclusion, accordance with our electrophysiological assessments which identified the advantages of metformin therapy effects on neuronal discharge activity (Farbood et al. Citation2015) and LTP (unpublished data) in I/R rats, we continued to investigate the neurological outcome, learning and memory improvements in behavioural aspect and moreover, detected the memory related molecules in the brain. Both behavioural and molecular performances showed the protective effect of metformin in memory formation and neuronal plasticity in the hippocampus. Short-term memory which measured by step-through test was improved in ischaemic rats were treated by metformin and the level of BDNF and phosphorylation of P70S6K were increased through activation of AMPK which suggested the AMPK/BDNF/P70S6K pathway in an experimental model of the global cerebral ischaemia. However, post treatment by metformin should be examined to accept our working hypothesis in metformin therapy.
Funding information
The authors thank the research council of Alborz Medical University and Ahvaz Jundishapur University of medical sciences for the funding of this project.
Acknowledgements
The authors thank the research council of Alborz Medical University and Ahvaz Jundishapur University of medical sciences for the funding of this project.
Disclosure statement
The authors report that they have no conflicts of interest.
References
- Ajami M, Eghtesadi S, Razaz JM, Kalantari N, Habibey R, Nilforoushzadeh MA, Zarrindast M, Pazoki-Toroudi H. 2011. Expression of Bcl-2 and Bax after hippocampal ischemia in DHA + EPA treated rats. Neurol Sci. 32:811–818.
- Ashabi G, Khalaj L, Khodagholi F, Goudarzvand M, Sarkaki A. 2015. Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab Brain Dis. 30:747–754.
- Ashabi G, Khodagholi F, Khalaj L, Goudarzvand M, Nasiri M. 2014. Activation of AMP-activated protein kinase by metformin protects against global cerebral ischemia in male rats: interference of AMPK/PGC-1α pathway. Metab Brain Dis. 29:47–58.
- Barker PA. 2009. Whither pro BDNF? Nat Neurosci. 12:105–106.
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254.
- Chen F, Dong RR, Zhong KL, Ghosh A, Tang SS, Long Y, Hu M, Miao MX, Liao JM, Sun HB, et al. 2016. Antidiabetic drugs restore abnormal transport of amyloid-beta across the blood-brain barrier and memory impairment in db/db mice. Neuropharmacology. 101:123–136.
- Cummins RO, Austin D Jr, Graves JR, Hambly C. 1988. An innovative approach to medical control: semiautomatic defibrillators with solid-state memory modules for recording cardiac arrest events. Ann Emerg Med. 17:818–824.
- Dichgans M. 2007. Genetics of ischaemic stroke. Lancet Neurol. 6:149–161.
- Ditacchio KA, Heinemann SF, Dziewczapolski G. 2015. Metformin treatment alters memory function in a mouse model of Alzheimer's disease. J Alzheimers Dis. 44:43–48.
- Fahnestock M, Garzon D, Holsinger RM, Michalski B. 2002. Neurotrophic factors and Alzheimer's disease: are we focusing on the wrong molecule? J Neural Transm Suppl. 1:241–252.
- Fang ZH, Lee CH, Seo MK, Cho H, Lee JG, Lee BJ, Park SW, Kim YH. 2013. Effect of treadmill exercise on the BDNF-mediated pathway in the hippocampus of stressed rats. Neurosci Res. 76:187–194.
- Farbood Y, Sarkaki A, Khalaj L, Khodagholi F, Badavi M, Ashabi G. 2015. Targeting adenosine monophosphate-activated protein kinase by metformin adjusts post-ischemic hyperemia and extracellular neuronal discharge in transient global cerebral ischemia. Microcirculation. 22:534–541.
- Globus MY, Busto R, Martinez E, Valdés I, Dietrich WD, Ginsberg MD. 1991. Comparative effect of transient global ischemia on extracellular levels of glutamate, glycine, and gamma-aminobutyric acid in vulnerable and nonvulnerable brain regions in the rat. J Neurochem. 57:470–478.
- Hardie DG. 2008. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes (Lond). 32:S7–S12.
- Ishizuka Y, Kakiya N, Witters LA, Oshiro N, Shirao T, Nawa H, Takei N. 2013. AMP-activated protein kinase counteracts brain-derived neurotrophic factor-induced mammalian target of rapamycin complex 1 signaling in neurons. J Neurochem. 127:66–77.
- Jun KH, Lee JE, Kim SH, Jung JH, Choi HJ, Kim YI, Chin HM, Yang SH. 2015. Clinicopathological significance of N-cadherin and VEGF in advanced gastric cancer brain metastasis and the effects of metformin in preclinical models. Oncol Rep. 34:2047–2053.
- Koskimaki J, Matsui N, Umemori J, Rantamäki T, Castrén E. 2015. Nimodipine activates TrkB neurotrophin receptors and induces neuroplastic and neuroprotective signaling events in the mouse hippocampus and prefrontal cortex. Cell Mol Neurobiol. 35:189–196.
- Lehman JA, Calvo V, Gomez-Cambronero J. 2003. Mechanism of ribosomal p70S6 kinase activation by granulocyte macrophage colony-stimulating factor in neutrophils: cooperation of a MEK-related, THR421/SER424 kinase and a rapamycin-sensitive, m-TOR-related THR389 kinase. J Biol Chem. 278:28130–28138.
- Lennox R, Porter DW, Flatt PR, Holscher C, Irwin N, Gault VA. 2014. Comparison of the independent and combined effects of sub-chronic therapy with metformin and a stable GLP-1 receptor agonist on cognitive function, hippocampal synaptic plasticity and metabolic control in high-fat fed mice. Neuropharmacology. 86:22–30.
- Lenzser G, Kis B, Bari F, Busija DW. 2005. Diazoxide preconditioning attenuates global cerebral ischemia-induced blood–brain barrier permeability. Brain Res. 1051:72–80.
- Li L, Zhang H, Meng SQ, Qian HZ. 2014. An updated meta-analysis of the efficacy and safety of acupuncture treatment for cerebral infarction. PLoS One. 9:e114057.
- Lipton SA, Rosenberg PA. 1994. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 330:613–622.
- Lu B. 2003. Pro-region of neurotrophins: role in synaptic modulation. Neuron. 39:735–738.
- Mansouri MT, Farbood Y, Sameri MJ, Sarkaki A, Naghizadeh B, Rafeirad M. 2013. Neuroprotective effects of oral gallic acid against oxidative stress induced by 6-hydroxydopamine in rats. Food Chem. 138:1028–1033.
- Mousavi SM, Niazmand S, Hosseini M, Hassanzadeh Z, Sadeghnia HR, Vafaee F, Keshavarzi Z. 2015. Beneficial effects of teucrium polium and metformin on diabetes-induced memory impairments and brain tissue oxidative damage in rats. Int J Alzheimers Dis. 2015:493729.
- Numakawa T, Suzuki S, Kumamaru E, Adachi N, Richards M, Kunugi H. 2010. BDNF function and intracellular signaling in neurons. Histol Histopathol. 25:237–258.
- Petito CK, Feldmann E, Pulsinelli WA, Plum F. 1987. Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology. 37:1281–1286.
- Plews RL, Mohd Yusof A, Wang C, Saji M, Zhang X, Chen CS, Ringel MD, Phay JE. 2015. A novel dual AMPK activator/mTOR inhibitor inhibits thyroid cancer cell growth. J Clin Endocrinol Metab. 100:E748–E756.
- Potter WB, O'riordan KJ, Barnett D, Osting SM, Wagoner M, Burger C, Roopra A. 2010. Metabolic regulation of neuronal plasticity by the energy sensor AMPK. PLoS One. 5:e8996.
- Pulsinelli WA, Brierley JB. 1979. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 10:267–272.
- Sarkaki A, Farbood Y, Badavi M, Khalaj L, Khodagholi F, Ashabi G. 2015. Metformin improves anxiety-like behaviors through AMPK-dependent regulation of autophagy following transient forebrain ischemia. Metab Brain Dis. 30:1139–1150.
- Shi LL, Chen BN, Gao M, Zhang HA, Li YJ, Wang L, Du GH. 2011. The characteristics of therapeutic effect of pinocembrin in transient global brain ischemia/reperfusion rats. Life Sci. 88:521–528.
- Truong DT, Venna VR, McCullough LD, Fitch RH. 2012. Deficits in auditory, cognitive, and motor processing following reversible middle cerebral artery occlusion in mice. Exp Neurol. 238:114–121.
- Tsai SJ. 2007. The P11, tPA/plasminogen system and brain-derived neurotrophic factor: implications for the pathogenesis of major depression and the therapeutic mechanism of antidepressants. Med Hypotheses. 68:180–183.
- Vorhees CV, Williams MT. 2014. Assessing spatial learning and memory in rodents. Ilar J. 55:310–332.
- Walcott BP, Boehm KM, Stapleton CJ, Mehta BP, Nahed BV, Ogilvy CS. 2013. Retrievable stent thrombectomy in the treatment of acute ischemic stroke: analysis of a revolutionizing treatment technique. J Clin Neurosci. 20:1346–1349.
- Wilson WJ, Cook JA. 1994. Cholinergic manipulations and passive avoidance in the rat: effects on acquisition and recall. Acta Neurobiol Exp (Wars). 54:377–391.
- Wu HQ, Guo HN, Wang HQ, Chang MZ, Zhang GL, Zhao YX. 2009. Protective effects and mechanism of puerarin on learning-memory disorder after global cerebral ischemia–reperfusion injury in rats. Chin J Integr Med. 15:54–59.
- Yeh FC, Kao CF, Kuo PH. 2015. Explore the features of brain-derived neurotrophic factor in mood disorders. PLoS One. 10:e0128605.
- Zare Mehrjerdi F, Aboutaleb N, Habibey R, Ajami M, Soleimani M, Arabian M, Niknazar S, Hossein Davoodi S, Pazoki-Toroudi H. 2013. Increased phosphorylation of mTOR is involved in remote ischemic preconditioning of hippocampus in mice. Brain Res. 1526:94–101.
- Zhang T, Wang X, He D, Jin X, Guo P. 2014. Metformin sensitizes human bladder cancer cells to TRAIL-induced apoptosis through mTOR/S6K1-mediated downregulation of c-FLIP. Anticancer Drugs. 25:887–897.
- Zhou X, Lin DS, Zheng F, Sutton MA, Wang H. 2010. Intracellular calcium and calmodulin link brain-derived neurotrophic factor to p70S6 kinase phosphorylation and dendritic protein synthesis. J Neurosci Res. 88:1420–1432.
