Abstract
Context Dunaliella salina Teodoresco (Dunaliellaceae) is one of the promising microalgae consumed as food and medicine for many years.
Objective Dunaliella salina was grown under different stress conditions for enhancing carotene production. The carotene enriched extract was evaluated for antioxidant and cytotoxic activity.
Materials and methods Carotene content was calculated under salinity, nitrogen and temperature stress conditions. Antioxidant activity was determined through DPPH assay by incubating the samples for 45 min with 250 μg/mL of extract and reducing power assay was performed with 50, 100, 150 and 200 μg/mL of extract. Cytotoxicity was determined by incubating ∼2 × 104 MCF-7 (breast cancer) cells with 250 μg of extract in each well for 72 h by MTT assay.
Result Carotene content was significantly increased to 9.8 (3.5 M NaCl), 13.9 (37 °C), 8.2 (250 mM KNO3) and 10.6 μg/mL (nitrogen-depleted medium) as compared with 3.2 μg/mL in normal conditions (1.7 M NaCl, 0.75 mM KNO3 and 28 °C). Free radical scavenging activity increased at 3.0 and 3.5 M NaCl (27.8 and 57.5%, respectively), 37 °C (31.4%) and in nitrogen-depleted medium (41.9%) compared with normal (15%) conditions. Carotene content and scavenging activity were positively correlated under salinity (r = 0.97), temperature (r = 0.85) and nitrogen (r = 0.7) stress conditions. Cytotoxicity against MCF-7 cell lines increased due to increase in carotene content suggesting that cytotoxicity may be associated with carotene accumulation.
Discussion and conclusions Carotene content enhanced by D. salina under stress conditions increased the antioxidant and cytotoxic activity.
Keywords:
Introduction
Microalgal biotechnology has gained noteworthy attention in recent decades, as they are used in production of biofuels, pharmaceutical, food additives and cosmetics (Cardozo et al. Citation2007). Some of the microalgae such as Botryococcus Kützing (Botryococcaceae), Chlorella Beyerinck (Chlorellaceae), Dunaliella Teodoresco (Dunaliellaceae), Haematococcus Flotow (Haematococcaceae) and Spirulina Turpin Ex Gomont (Spirulinaceae) are extensively used for biotechnological and biopharmaceutical products. Among different species of Dunaliella, Dunaliella salina Teodoresco (Dunaliellaceae) and Dunaliella viridis Teodoresco (Dunaliellaceae) are most often reported from natural salt lakes and saltern ponds (Oren Citation2014). Dunaliella salina is a halotolerant, unicellular, uninucleate, red-green microalgae which lacks a rigid polysaccharide cell wall. It is becoming commercially and economically important because it can grow and acclimatize in salinities ranging from 0.05 to 5.0 M NaCl with constant and low intracellular sodium concentration by regulating the metabolism of osmolyte, glycerol to balance external diverse stress conditions (Raja et al. Citation2007). It can accumulate three valuable products (carotenes, glycerol and proteins) under abiotic stress such as high salinity or high intensity of light.
Carotenes are of particular interest at the commercial level as they are extremely important for human nutrition. Dunaliella salina is known to produce carotenes and extensively exploited for its production. It contains valuable carotene pigments such as α- and β-carotene, violaxanthin, neoxanthin, zeaxanthin and lutein (Hosseini Tafreshi & Shariati Citation2009). Lutein and zeaxanthin play role in reducing the risk of age related macular degeneration (Carpentier et al. Citation2009). β-Carotene is precursor of vitamin A which is important for the function of retina. In addition, β-carotene protects the skin by means of its antioxidant activity. Antioxidants neutralize reactive oxygen species (ROS) which are detrimental to human health. ROS is associated with ageing process and the pathogenesis of several diseases such as cardiovascular and diabetes. ROS is also found to promote the development and progression of different cancers (Tong et al. Citation2015). Epidemiological evidence suggests that β-carotene prevents various cancers by means of antioxidant activity (Van Poppel & Goldbohm Citation1995). It has been shown that carotenoids of D. salina are capable of augmenting or preserving the activity of hepatic enzymes such as catalase, peroxidase and super oxide dismutase, which are involved in combating ROS (Murthy et al. Citation2005). Madkour and Abdel-Daim (Citation2013) reported that D. salina serves as a potential source of natural antioxidant with hepatoprotective activity. Carotenoid extract of D. salina was found to have significantly higher antioxidant activity than commercial α-carotene, β-carotene, lutein and zeaxanthin (Hu et al. Citation2008). Cakmak et al. (Citation2014) demonstrated that the extracts from D. salina can be used as natural antimicrobials and antioxidants in the food/feed and pharmaceutical industry. Anticancer activity of D. salina has been studied in different cancer cell lines (Emtyazjoo et al. Citation2012; Atasever-Arslan et al. Citation2015). Dunaliella salina was also reported to increase NK cell activity and phagocytosis of macrophages in WEHI-3 leukaemic mice (Chuang et al. Citation2014). Further, Abdel-Daim et al. (Citation2015) demonstrated the anti-inflammatory and immunomodulatory effects of D. salina in acetic-acid-induced ulcerative colitis.
In the present study, attempts were made to enhance the production of carotenes by growing D. salina under different stress conditions such as nitrogen, salinity and temperature. Further, carotenes enriched extracts of D. salina grown under stress were tested for their antioxidant potential and cytotoxic effects in human breast cancer cell line (MCF-7).
Materials and methods
Dunaliella salina culture used in this study was obtained from Birla Institute of Scientific Research, Jaipur, India, and maintained in AS-100 medium (Vonshak Citation1986) at 28 °C under 12/12 (light and dark cycle) illuminated with fluorescent white lamps with average light intensity. Three stress conditions, salinity, temperature and nitrogen concentration were taken for enhancement of carotene production and studying antioxidant and cytotoxic activities. In normal condition, cells were cultured in AS-100 medium comprising 1.7 M NaCl (salinity), 0.75 mM KNO3 (nitrogen source) at 28 °C temperature. AS-100 medium comprising of 1.0, 3.0 and 3.5 M concentrations of NaCl as salinity stress while medium with nitrogen depleted (0 mM KNO3) and nitrogen excess conditions (250 mM KNO3) as nitrogen stress were chosen. For temperature stress, cultures were kept at 37 °C and oscillating between 15 °C and 25 °C were selected in this study. Growth of D. salina was measured in terms of cell number by using haemocytometer. Since the algae are motile, cells were fixed by adding a drop of Lugol’s iodine solution. The cell count was expressed as cells per mL.
Pigment estimation
Log phase culture of D. salina cells was taken and centrifuged at 8000 rpm for 15 min. Supernatant was discarded and cell pellet was diluted with 80% acetone and water (Ben‐Amotz & Avron 1983). Further, it was subjected to vortexing for 2 min and then kept in dark conditions for 24 h at 4 °C. Extract was again centrifuged at 3000 rpm for 10 min. The absorbance of the supernatant was measured spectrophotometrically at 645 and 661.5 nm. Chlorophyll was estimated according to Lichtentaler equations (Kichtenthaler & Wellburn Citation1983). Carotene content was determined according to method described by Davies (Citation1976) using extinction co-efficient 2500:
Preparation of extracts
Dunaliella salina cells extraction was done when cells reached a maximum specific growth rate (18–20 d). A homogeneous suspension of D. salina from each triplicate flask was taken in graduated falcons and centrifuged at 5000 rpm for 15 min. Pellet obtained after removing supernatant was washed with distilled water and then centrifuged at 5000 rpm for 10 min to remove extra salts. The pellet was dried for 48 h at 37 °C and then resuspended in 100% ethanol with strong vortexing for 15 s. Cell wall was disrupted using ultrasonication for 10 min. Cells were homogenized with solvents for 4 d and then filtered using 0.2 μm filter units to get crude ethanol extract. Finally, the solvents were removed under reduced pressure by rotary evaporator (Yamoto Scientific Co. Ltd, Tokyo, Japan) and extracts were reconstituted in dimethyl sulphoxide (DMSO).
Antioxidant assay
In order to know the antioxidant potential of crude extract of both normal and stressed D. salina cells, two well-known antioxidant assays namely DPPH (2,2-diphenyl-1-picrylhydrazyl) assay and total reducing assay were performed.
DPPH assay: This assay is based on the classic method which was developed by Blois (Citation1958). Crude extract was diluted appropriately in methanol to make a final concentration of 250 μg/mL and was mixed with 170 μL of DPPH (100 μM) in methanol, added in wells of a 96-well microtitre plate. A standard of 100 μg/mL ascorbic acid was run simultaneously as a positive control. The plate was incubated in dark for 45 min, after which the absorbance of the solution was measured at 517 nm in ELISA microtitre plate (Tecan Infinite, Groedig, Austria). Free radical scavenging activity was expressed as the inhibition percentage calculated using following formula:
Total reducing power assay: For this assay, different concentrations of crude extract (50, 100, 150 and 200 μg/mL) were mixed with 2.5 mL of phosphate buffer (2.0 M, pH 6.6) and 2.5 mL of potassium ferric cyanide (1%) (Duh & Yen Citation1997). Reaction mixture was incubated in a water bath at 50 °C for 30 min. After that, 2.5 mL of trichloroacetic acid (10% of TCA) was added and centrifuged for 10 min. From the upper layer, 2.5 mL solution was mixed with 2.5 mL distilled water and 0.5 mL of FeCl3 (0.1%). The absorbance of all the solutions was measured at 700 nm. Ascorbic acid is used as a positive control. An increase in absorbance indicates the reducing potential of the samples.
Cell culture maintenance
Human breast cancer cell line (MCF-7) was procured from National Center for Cell Science (NCCS), Pune, India. The cells were maintained in DMEM (Sigma, St. Louis, MO), containing 10% (v/v) foetal bovine serum (Gibco, Waltham, MA), 100 IU/mL penicillin, 100 μg/mL streptomycin and 2.5 μg/mL amphotericin (Sigma, St. Louis, MO). Cells were maintained in a humidified incubator with 5% CO2 at 37 °C.
Cytotoxic assay
The response of extracts of D. salina on the growth of breast cancer cell lines was evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT assay) (Denizot & Lang Citation1986). The cells were trypsinised and seeded at a density of ∼2 × 104 cells per well of 96-well cell culture plate and incubated overnight. After 16 h, sample extracts (250 μg/well) were added to the wells. The assay was carried out in triplicates. After 72 h of incubation, 20 μL of MTT reagent (Sigma, St. Louis, MO, 5 mg/mL) was added to each well and again incubated for 4 h. The formazan crystals formed were solubilized in 100 μL DMSO (Merck, Darmstadt, Germany). Finally, the absorbance of each well was recorded at 570 nm, taking 630 nm as the reference wavelength, using the microplate reader (Tecan Infinite, Groedig, Austria). Paclitaxel was used as a positive control at the concentration of 20 μg/mL. Percentage of inhibition was calculated by the following formula:
Statistical analysis
The data were expressed as the mean ± standard error of mean (SEM) of three independent experiments. Data were analyzed using one-way analysis of variance (ANOVA), followed by Tukey's multiple comparison test using GraphPad Prism (GraphPad Software, Inc., San Diego, CA).
Results
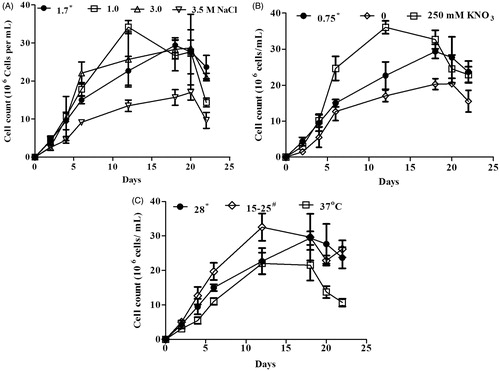
The cell growth of D. salina was represented by cell count which was recorded at different time intervals. Under normal condition, the maximum cell count was observed on the 18th day and the cell count decreased thereafter (). Three different concentrations (1.0, 3.0 and 3.5 M) of NaCl were considered as salinity stress and 1.7 M NaCl as normal condition. Two different temperatures 37 °C and 15–25 °C (oscillating) were considered as stress and 28 °C as normal. The cell growth was decreased at 3.5 M NaCl and 37 °C as compared with normal conditions ( and ). Nitrogen-depleted medium (0 mM) has less cell growth and nitrogen excess medium (250 mM) has more growth compared to normal levels (KNO3: 0.75 mM) ().
Estimation of carotenoid and chlorophyll
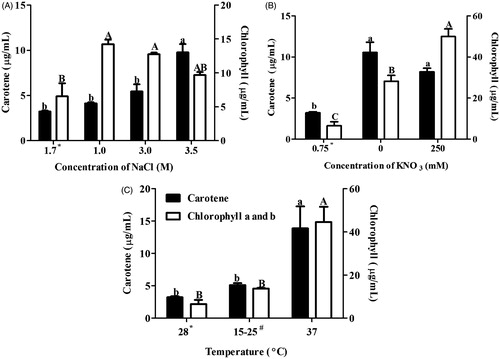
Dunaliella salina extracts were evaluated for carotene and chlorophyll content in order to know their accumulation under stress conditions. Carotene along with chlorophyll content was found to be elevated in salinity, nitrogen and temperature stress as compared with normal condition. The carotene content significantly increased at 3.5 M NaCl while chlorophyll content was higher at 1.0 and 3.0 M NaCl as compared with normal conditions (). Carotene and chlorophyll contents were significantly higher in both nitrogen stress and 37 °C ( and ).
Figure 2. Effect of salinity (A), nitrogen (B) and temperature (C) stress on the carotene and chlorophyll content of Dunaliella salina. Means followed by the same letter are not significant at p < 0.05. *Under normal condition, Dunaliella salina was cultivated in 1.7 M NaCl, 0.75 mM KNO3 and 28 °C. #Dunaliella cultures were oscillating between 15 °C and 25 °C.
Antioxidant activity
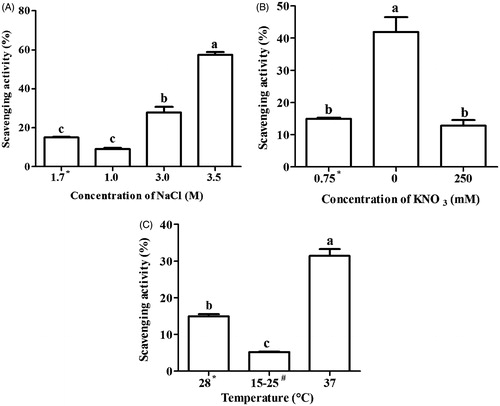
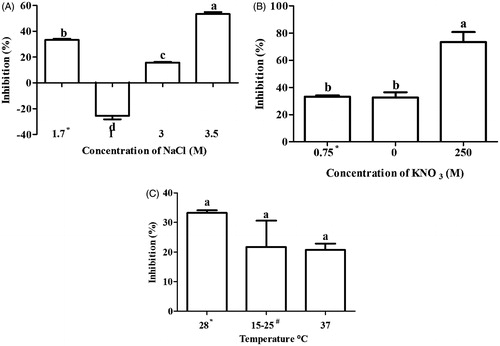
Free radical scavenging activity and total reducing power assay were preformed to assess the antioxidant activity in extract of D. salina. The result of free radical scavenging activity showed a variable pattern in stressed condition. Free radical scavenging activity was significantly increased at 3.0 (27.8%) and 3.5 M (57.5%) NaCl, 37 °C (31.4%) and in nitrogen-depleted medium (0 M) (41.9%) as compared with normal condition (15%) (). Ascorbic acid was used as a positive control where free radical scavenging activity was found to be 95.4 ± 0.8%. There was positive correlation between carotene content and free radical scavenging activity in salinity (r = 0.97), nitrogen (r = 0.70) and temperature (r = 0.85) condition.
Figure 3. Free radical scavenging (DPPH) activity of Dunaliella extracts under different stress conditions (A) Salinity, (B) nitrogen and (C) temperature stress. Means followed by the same letter are not significant at p < 0.05. *Under normal condition, D. Salina was cultivated in 1.7 M NaCl, 0.75 mM KNO3 and 28 °C. #Dunaliella cultures were oscillating between 15 °C–25 °C.
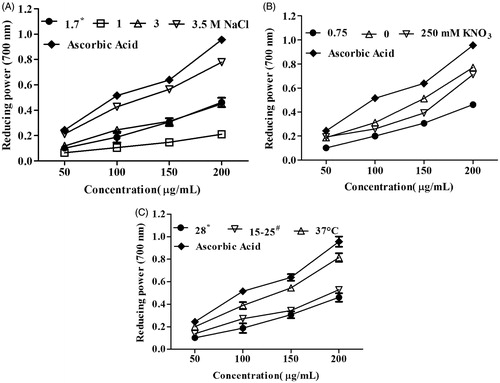
Reducing power is reflection of antioxidant activity where an increase in absorbance after the addition of extracts indicates their reducing potential. Hence, different concentrations of extracts were used to assess their reducing potential. It was found that reducing power increased with the concentration of both stressed and normal conditions. Reducing power was found to be more at 3.5 M NaCl and 37 °C in three concentrations (100, 150 and 200 μg/mL) as compared with normal condition ( and ). It was found to be higher reducing power of both nitrogen stresses as compared with normal condition at 200 μg/mL of extract ().
Figure 4. Total reducing power of Dunaliella extracts under different stress conditions: (A) salinity, (B) nitrogen and (C) temperature stress. *Under normal condition, Dunaliella salina was cultivated in 1.7 M NaCl, 0.75 mM KNO3 and 28 °C. #Dunaliella cultures were oscillating between 15 °C and 25 °C.
Effect of D. salina extract on breast cancer cells
It was interesting to look for the effect of these extracts on the cancer cell growth. Cell growth inhibition (cytotoxic effect) was observed in most of the stressed and normal condition. Cytotoxic effect was found to be significantly higher at 3.5 M NaCl and in nitrogen-depleted medium (0 M) as compared with normal condition ( and ). In temperature stress, cytotoxic effect was observed but not significantly higher than normal condition.
Figure 5. Effect of Dunaliella salina grown under salinity (A) nitrogen (B) and temperature (C) stress condition on the cell proliferation of MCF-7 (breast cancer) cell line. Means followed by the same letter are not significant at p < 0.05. *Under normal condition, Dunaliella salina was cultivated in 1.7 M NaCl, 0.75 mM KNO3 and 28 °C. #Dunaliella cultures were oscillating between 15 °C and 25 °C.
Discussion
Photosynthetic algae are known for biofuel production but they are also enriched with several bioactive properties. Dunaliella salina is one of the photosynthetic algae which are known to have medicinal value. In the present study, D. salina which is grown under different stress conditions has shown to increase carotene production. Further, antioxidant and cytotoxic activities are increased under different stress conditions and found to be associated with accumulation of carotenes.
Dunaliella salina grows in a wide range of salinities (Ben-Amotz et al. Citation1991; García et al. Citation2007), hence different salinity stresses were explored for the carotenogenesis as well as antioxidant and cytotoxic activities. Carotenogenesis in stressed cells are found to be associated with suboptimal growth conditions and the carotenoid content in algal cells is inversely correlated with their specific growth rate (Ben‐Amotz et al. Citation1982; Raja et al. Citation2007). In agreement with these reports, cell growth was found to be decreased while the carotene content was increased at high salinity (3.5 M NaCl). Narváez-Zapata et al. (Citation2011) reported the variations in carotene contents of D. salina isolates when grown in hypersaline conditions. The variation in carotene production may be explained based on the source. D. salina used in the present study was isolated from salt lake (Shambar lake, Rajasthan, India) (Sharma et al. Citation2012). Carotenoids biosynthesis is associated with biogenesis of chlorophyll and proteins (Bohne & Linden Citation2002). Thus, chlorophyll content was also evaluated and results have shown an increase in chlorophyll content with salinity stress.
Nitrogen stress is found to be associated with carotene production in D. salina (Lamers et al. Citation2012). It was found that cell growth is increased with an increase in concentration of KNO3 and lowest cell growth was recorded in nitrogen-deprived medium. Similar growth patterns were reported where three different concentrations of NaNO3 (0.05, 0.5 and 5 mM) were used in this microalgae by Yilancioglu et al. (Citation2014). However, they reported no difference in carotene content in these concentrations. In contrast, the current study showed significantly higher carotene content in nitrogen-depleted and excess medium as compared with normal condition. Saha et al. (Citation2013) also reported enhanced carotenoid production in a medium depleted with manganese, zinc, iron and nitrogen. It indicates that macro- and micro-nutrient limitation enhances the accumulation of metabolites in algae.
Studies were also done on the effect of temperature stress on carotene, chlorophyll production and cell growth but in most of the studies, low temperature was taken as stress factor (Raja et al. Citation2007; Haghjou et al. Citation2009). The present study considered low as well as high temperature for the study against D. salina. Low temperature did not show effect on cell growth as well as carotene and chlorophyll production while high temperature resulted into significant increase in carotene and chlorophyll content with decrease in cell growth which put forward that high temperature may be better stress factor than low temperature.
Generation of free radical is toxic to human health as their role in wide variety of pathological manifestations is well established (Tong et al. Citation2015). There is immense interest in natural antioxidants because they provide protection by scavenging the free radicals. Studies have shown that D. salina exhibits antioxidant activity by means of carotenes production (Murthy et al. Citation2005; Tran et al. Citation2014). Madkour and Abdel-Daim (Citation2013) reported the hepatoprotective effect of D. salina due to the presence of carotenoids in the extract which are known for their antioxidant activity. Abdel-Daim et al. (Citation2015) demonstrated that D. salina exhibits a modulatory effect on acetic-acid induced colitis in rats due to a significant increase of antioxidant enzymes activity and inhibition of lipid peroxidation. An interesting part of this study was to assess the antioxidant activities of D. salina which are grown in different abiotic stresses. DPPH and reducing power assay results have shown the antioxidant activities in all stressed and normal conditions of D. salina. DPPH results have shown that the free radical scavenging is significantly higher at 3 and 3.5 M NaCl, 37 °C and nitrogen-depleted medium than normal condition. Total reducing power assay has shown antioxidant effect at 3.5 M NaCl, 37 °C and in both nitrogen stress conditions. Both assays have shown the higher antioxidant effect in common stress conditions except few variations which could be explained due to different experimental conditions. The antioxidant effect was found to be positively correlated with carotene production in all stressed conditions. Hence, these results suggest that antioxidant effect may be due to an increase in carotene production in D. salina under different stress conditions.
Microalgae have been explored for bioactive compounds having anticancer properties with intention to overcome the side effects associated with existing cancer therapy (Moussavou et al. Citation2014). There have been a few studies which have shown anticancer effect of D. salina. Ethanol extract of D. salina was reported to exhibit anticancer effect in A549 (human lung cancer) cell lines by inducing apoptosis and cell-cycle arrest (Sheu et al. Citation2008). Atasever-Arslan et al. (Citation2015) demonstrated the cytotoxic effect of D. salina on SH-SY5Y human neuroblastoma cells. In the present study, different cancer cell lines, i.e., breast cancer (MCF-7) cell lines were taken to study the effect on cancer cell growth. Most of the stressed and normal D. salina extracts have shown cytotoxic effect in MCF-7 cell lines. Cytotoxic effect was found to be significantly higher in 3.5 M NaCl (salinity stress) and 250 mM KNO3 (nitrogen stress). Dunaliella salina grown in nitrogen-devoid medium and temperature stress has not shown a significant difference in cytotoxic effect as compared with normal condition. Beside the antioxidant effect of carotenes, these compounds are reported to play role in cancer prevention (Raja et al. Citation2007). As compared with normal conditions, carotene content and cytotoxic effect were found to be higher in stress condition, i.e., 3.5 M NaCl and 250 mM KNO3 which indicates that cytotoxic effect may be associated with carotene accumulation.
Conclusion
The current study has shown different stress conditions which may lead to increase in carotene production. Further, antioxidant and cytotoxic effects in breast cancer cell lines were found to be significantly higher in some stress conditions. This could be due to an increase in carotene production or may be involvement of some other bioactive compounds.
Acknowledgements
The authors would like to acknowledge Dr, M. Krishna Mohan, Birla Institute of Scientific Research, Jaipur, India, for providing the culture of Dunaliella salina.
Disclosure statement
No competing financial interests exist.
References
- Abdel-Daim MM, Farouk SM, Madkour FF, Azab SS. 2015. Anti-inflammatory and immunomodulatory effects of Spirulina platensis in comparison to Dunaliella salina in acetic acid-induced rat experimental colitis. Immunopharmacol Immunotoxicol. 37:126–139.
- Atasever-Arslan B, Yilancioglu K, Bekaroglu MG, Taskin E, Altinoz E, Cetiner S. 2015. Cytotoxic effect of extract from Dunaliella salina against SH-SY5Y neuroblastoma cells. Gen Physiol Biophys. 34:201–217.
- Ben-Amotz A, Avron M. 1983. On the factors which determine massive beta-carotene accumulation in the halotolerant alga Dunaliella bardawil. Plant Physiol. 72:593–597.
- Ben‐Amotz A, Katz A, Avron M. 1982. Accumulation of β‐carotene in halotolerant algae: purification and characterization of β‐carotene‐rich globules from Dunaliella bardawil (Chlorophyceae). J Phycol. 18:529–537.
- Ben-Amotz A, Shaish A, Avron M. 1991. The biotechnology of cultivating Dunaliella for production of β-carotene rich algae. Bioresour Technol. 38:233–235.
- Blois MS. 1958. Antioxidant determinations by the use of a stable free radical. Nature. 181:1199–1200.
- Bohne F, Linden H. 2002. Regulation of carotenoid biosynthesis genes in response to light in Chlamydomonas reinhardtii. Biochim Biophys Acta. 1579:26–34.
- Cakmak YS, Kaya M, Asan-Ozusaglam M. 2014. Biochemical composition and bioactivity screening of various extracts from Dunaliella salina, a green microalga. Excli J. 13:679–690.
- Cardozo KH, Guaratini T, Barros MP, Falcao VR, Tonon AP, Lopes NP, Campos S, Torres MA, Souza AO, Colepicolo P, et al. 2007. Metabolites from algae with economical impact. Comp Biochem Physiol C Toxicol Pharmacol. 146:60–78.
- Carpentier S, Knaus M, Suh MY. 2009. Associations between lutein, zeaxanthin, and age-related macular degeneration: an overview. Crit Rev Food Sci Nutr. 49:313–326.
- Chuang WC, Ho YC, Liao JW, Lu FJ. 2014. Dunaliella salina exhibits an antileukemic immunity in a mouse model of WEHI-3 leukemia cells. J Agric Food Chem. 62:11479–11487.
- Davies B. 1976. Carotenoids. In: Goodwin TW, editor. Chemistry and biochemistry of plant pigments. New York: Academic Press. p. 38–165.
- Denizot F, Lang R. 1986. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 89:271–277.
- Duh PD, Yen GC. 1997. Antioxidative activity of three herbal water extracts. Food Chem. 60:639–645.
- Emtyazjoo M, Moghadasi Z, Rabbani M, Samadi S, Mossaffa N. 2012. Anticancer effect of Dunaliella salina under stress and normal conditions against skin carcinoma cell line A431 in vitro. Iran J Fish Sci. 11:283–293.
- García F, Freile-Pelegrín Y, Robledo D. 2007. Physiological characterization of Dunaliella sp. (Chlorophyta, Volvocales) from Yucatan, Mexico. Bioresour Technol. 98:1359–1365.
- Haghjou MM, Shariati M, Smirnoff N. 2009. The effect of acute high light and low temperature stresses on the ascorbate-glutathione cycle and superoxide dismutase activity in two Dunaliella salina strains.. Physiol Plant. 135:272–280.
- Hosseini Tafreshi A, Shariati M. 2009. Dunaliella biotechnology: methods and applications. J Appl Microbiol. 107:14–35.
- Hu CC, Lin JT, Lu FJ, Chou FP, Yang DJ. 2008. Determination of carotenoids in Dunaliella salina cultivated in Taiwan and antioxidant capacity of the algal carotenoid extract. Food Chem. 109:439–446.
- Kichtenthaler H, Wellburn A. 1983. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvent. Biochem Soc Trans. 603:591–593.
- Lamers PP, Janssen M, De Vos RC, Bino RJ, Wijffels RH. 2012. Carotenoid and fatty acid metabolism in nitrogen-starved Dunaliella salina, a unicellular green microalga. J Biotechnol. 162:21–27.
- Madkour FF, Abdel-Daim MM. 2013. Hepatoprotective and antioxidant activity of Dunaliella salina in paracetamol-induced acute toxicity in rats. Indian J Pharm Sci. 75:642–648.
- Moussavou G, Kwak DH, Obiang-Obonou BW, Maranguy CAO, Dinzouna-Boutamba SD, Lee DH, Pissibanganga OGM, Ko K, Seo JI, Choo YK. 2014. Anticancer effects of different seaweeds on human colon and breast cancers. Mar Drugs. 12:4898–4911.
- Murthy KC, Vanitha A, Rajesha J, Mahadeva Swamy M, Sowmya PR, Ravishankar GA. 2005. In vivo antioxidant activity of carotenoids from Dunaliella salina – a green microalga. Life Sci. 76:1381–1390.
- Narváez-Zapata JA, Rojas-Herrera R, López-Uc Y, Sánchez-Estudillo L. 2011. Different physiological responses influenced by salinity in genetically related Dunaliella salina isolates. Biotechnol Lett. 33:1021–1026.
- Oren A. 2014. The ecology of Dunaliella in high-salt environments. J Biol Res (Thessalon). 21:23. DOI: 10.1186/s40709-014-0023-y.
- Raja R, Hemaiswarya S, Rengasamy R. 2007. Exploitation of Dunaliella for beta-carotene production. Appl Microbiol Biotechnol. 74:517–523.
- Saha SK, Moane S, Murray P. 2013. Effect of macro-and micro-nutrient limitation on superoxide dismutase activities and carotenoid levels in microalga Dunaliella salina CCAP 19/18. Bioresour Technol. 147:23–28.
- Sharma P, Agarwal V, Mohan MK, Kachhwaha S, Kothari S. 2012. Isolation and characterization of Dunaliella species from Sambhar lake (India) and its phylogenetic position in the genus Dunaliella using 18S rDNA. Natl Acad Sci Lett. 35:207–213.
- Sheu MJ, Huang GJ, Wu CH, Chen JS, Chang HY, Chang SJ, Chung JG. 2008. Ethanol extract of Dunaliella salina induces cell cycle arrest and apoptosis in A549 human non-small cell lung cancer cells. In Vivo. 22:369–378.
- Tong L, Chuang CC, Wu S, Zuo L. 2015. Reactive oxygen species in redox cancer therapy. Cancer Lett. 367:18–25.
- Tran D, Doan N, Louime C, Giordano M, Portilla S. 2014. Growth, antioxidant capacity and total carotene of Dunaliella salina DCCBC15 in a low cost enriched natural seawater medium. World J Microbiol Biotechnol. 30:317–322.
- van Poppel G, Goldbohm RA. 1995. Epidemiologic evidence for beta-carotene and cancer prevention. Am J Clin Nutr. 62:1393S–1402S.
- Vonshak A. 1986. Laboratory techniques for the cultivation of microalgae. In: Richmond A, editor. Handbook of microalgal mass culture. California: CRC Press. p. 117–145.
- Yilancioglu K, Cokol M, Pastirmaci I, Erman B, Cetiner S. 2014. Oxidative stress is a mediator for increased lipid accumulation in a newly isolated Dunaliella salina strain. PLoS One. 9:e91957.