Abstract
Context The emergence of resistant pathogens and toxicity of antifungals have encouraged an active search for novel candidates to manage Candida biofilms.
Objective In this study, the little known species Sideroxylon obtusifolium T.D. Penn (Sapotacea) and Syzygium cumini (L.) Skeels (Myrtaceae), from the Caatinga biome in Brazil were chemically characterized and explored for their antifungal potential against C. albicans.
Materials and methods We determined the effects of hydroalcoholic extracts/fractions upon fungal growth (minimum inhibitory and fungicidal concentrations, MIC/MFC), biofilm morphology (scanning electron microscopy) and viability (confocal laser scanning microscopy), proposed their mode of action (sorbitol and ergosterol assays), and finally investigated their effects against macrophage and keratinocyte cells in a cell-based assay. Data were analysed using one-way analysis of variance with Tukey-Kramer post-test (α = 0.05).
Results The n-butanol (Nb) fraction from S. obtusifolium and S. cumini extract (Sc) showed flavonoids (39.11 ± 6.62 mg/g) and saponins (820.35 ± 225.38 mg/g), respectively, in their chemical composition and demonstrated antifungal activity, with MICs of 62.5 and 125 μg/mL, respectively. Nb and Sc may complex with ergosterol as there was a 4–16-fold increase in MICs in the presence of exogenous ergosterol, leading to disrupted permeability of cell membrane. Deleterious effects were observed on morphology and viability of treated biofilms from concentrations as low as their MICs and higher. Sc was not toxic to macrophages and keratinocytes at these concentrations (p > 0.05), unlike Nb.
Conclusions Nb and Sc demonstrated considerable antifungal activity and should be further investigated as potential alternative candidates to treat Candida biofilms.
Introduction
Oral candidiasis is a common superficial and opportunistic infection caused by Candida spp., mainly C. albicans, when the host immunity becomes compromised (Dangi et al. Citation2010). It is considered a global health issue affecting a wide portion of individuals in the population, such as newborns (Ali et al. Citation2012), oral cancer patients (Alnuaimi et al. Citation2015), denture wearers (Dar-Odeh & Shehabi Citation2003), among others, presenting different clinical features. Recently, oral candidiasis has also been reported to be a risk factor for disseminated bacterial infections, which constitute a critical and life-threatening trait, particularly in immunocompromised individuals (Kong et al. Citation2015).
There are still a limited number of antifungal classes commercially available, many of which have the same mechanism of action, as a result of the few targets so far known in the fungal cell (Pierce et al. Citation2013). Not surprisingly, this non-diverse arsenal is sometimes ineffective upon microbial resistance (Ramage et al. Citation2012) leading to either persistent or recurrent fungal infections in the clinical setting. In addition, some of synthetic antifungals are potentially toxic for the host cells due to the similarity in both eukaryotic fungal and human cell components, such as sterols (Berman & Sudbery Citation2002). Biochemical engineering has been used to modify standard antifungals or even develop novel low-toxicity drugs (Boros-Majewska et al. Citation2014; Peng et al. Citation2015), but most drugs have not been tested in humans. Taken together, all these aspects have encouraged an active search for novel candidates to manage oral candidiasis in susceptible patients.
With this purpose, naturally-occurring agents are considered a promising source of bioactive molecules with antifungal properties (Arif et al. Citation2009; Negri et al. Citation2014). Over the last century, research on medicinal plant species has assessed their viability, sustainability and affordability to be used as natural drugs (Iwu Citation2000; Negri et al. Citation2014) or to prototype the development of synthetic compounds. Previous studies have suggested that the little known species Sideroxylon obtusifolium T.D. Penn (Sapotacea) (Albuquerque et al. Citation2011), native to northeastern Brazil, and Syzygium cumini (L.) Skeels (Myrtaceae) (Oliveira et al. Citation2007), common in tropical Asia, could be promising antifungal agents.
S. obtusifolium, known as ‘quixabeira’, ‘quixaba’ or ‘rompe-gibão’, is a medicinal plant species native to the Brazilian Caatinga, which is a unique biome that has aroused attention for its exuberant and unexplored biodiversity. It is threatened with extinction mainly by the extraction of its bark in popular medicine to treat pain and inflammation (Araújo-Neto et al. Citation2010). However, other parts of this plant seem to have an unexplored potential against oral microorganisms of clinical relevance, such as streptococci and yeasts.
S. cumini, commonly known as jambolan, is a large evergreen and foliaceous tree whose seeds, bark, fruit and leaves have been used in traditional medicine. It is reported to have antioxidant, antinociceptive, hypoglycemic, anti-Leishmania and antimicrobial properties (Eshwarappa et al. Citation2014; Quintans et al. Citation2014; Rodrigues et al. Citation2015).
Interestingly, the antifungal properties of S. obtusifolium and S. cumini have never been comprehensively studied despite their use in folk medicine as antimicrobial (Albuquerque et al. Citation2011), which could open avenues for discovery of novel bioactive molecules and drug development and also aggregate value to the ethnopharmacological knowledge of the communities living in the Caatinga biome (28 million people as of 2013, according to the Brazilian Institute of Geography and Statistics - IBGE).
In this pioneering study, the antifungal activity of extracts and/or fractions from the leaves of S. obtusifolium and S. cumini from the Caatinga biome was determined on planktonic cells of C. albicans. Their effects on biofilm morphology and viability and mode of action were further established. With the perspective of future clinical use, the cytotoxic effects of these extracts were investigated upon murine and human cells. Further scientific exploration of these species could provide new opportunities for the development of needed novel antifungals to manage oral candidiasis.
Materials and methods
Plant material
Sideroxylon obtusifolium and Syzygium cumini leaves were collected in April and August 2013, respectively, in the semi-arid region in the countryside area of Campina Grande, Paraíba State, Brazil (7° 22′ 25″ S, 35° 59′ 32″ W), under permission of the Brazilian Ministry of Environment (Council for the Administration and Management of Genetic Patrimony - CGEN) via the National Council for Scientific and Technological Development (CNPq) [010637/2014-1]. Botanical specimens were identified by Prof. Maria Regina de Vasconcellos Barbosa, curator of Lauro Pires Xavier Herbarium (http://inct.florabrasil.net/participantes/herbarios-curadores/jpb/) at the Department of Molecular Biology, Federal University of Paraíba, João Pessoa, Paraíba, under voucher numbers JPB 57.985 and JPB 58.543, respectively.
After cleaning processing, the leaves were dried in an air-circulating oven at 40 °C until stabilization of final weight, and then ground in a Wiley mill (SL 30 Solab, Piracicaba, SP, Brazil). Hydroalcoholic extracts of the leaves were prepared by maceration following the proportion 200 g of ground plant to 1 L of 70% hydroalcoholic solution. The extracts were subjected to evaporation under reduced pressure, lyophilized at −20 to −40 °C and stored in glass containers, protected from light and under refrigeration.
Extract fractionation
S. obtusifolium extract was subjected to liquid–liquid fractionation (Cunha et al. Citation2013) using organic solvents in order of increasing polarity: hexane, dichloromethane, ethyl acetate and n-butanol (Nb). The final portion corresponded to the aqueous phase. Our preliminary experiments (not shown) indicated that the dichloromethane and Nb fractions had the best antimicrobial activity and therefore were selected for further testing.
Phytochemical analysis
The extract(s)/fraction(s) with the best activity were quantified for their content of total polyphenols, total flavonoids, condensed tannins and saponins. The method described by Chandra and Mejía (Citation2004) was used to determine the content of total polyphenols in equivalent milligrams of gallic acid, with absorbance reading at 757 nm. The content of total flavonoids, expressed in equivalent milligrams of quercetin, was determined according to Meda et al. (Citation2005) with absorbance reading at 420 nm. The content of condensed tannins, expressed as milligrams of catechin equivalents, was quantified using the method proposed by Makkar and Becker (Citation1993) with absorbance reading at 500 nm. Finally, the content of saponins in milligrams of diosgenin was determined according to Makkar et al. (Citation2007) with absorbance reading at 544 nm.
Microorganism and growth conditions
In all microbiological assays a reference strain of Candida albicans ATCC 10231, obtained from the American Type Culture Collection, was used. Cultures were kept in a glycerol stock solution at −80 °C and grown in Sabouraud dextrose broth and agar (HIMEDIA Laboratories Pvt. Ltd., Mumbai, India) at 35 °C for 24 h in aerobic atmosphere.
Inhibitory and lethal effects of the plant extracts on C. albicans cells
This study investigated the antifungal susceptibility of C. albicans to the action of S. obtusifolium extract and fractions and S. cumini extract (Sc). Their minimum inhibitory concentration (MIC) was established using 96-well U-bottom tissue culture microplates. Fresh solutions of the extracts and fractions were prepared using 10% (v/v) ethanol as vehicle. Then, aliquots of 100 μL of the samples were used to perform serial dilutions in RPMI-1640 medium with 0.165 M MOPS (Sigma-Aldrich, St. Louis, MO). Nystatin, caspofungin diacetate (Sigma-Aldrich, St. Louis, MO) and amphotericin B (União Química Farmacêutica Nacional, SP, Brazil) were used as positive controls, and the vehicle as the negative control. Sterility of the culture medium and samples and culture viability were also monitored. Fungal inoculum was adjusted at 530 nm to 2.5 × 103 CFU/mL in the wells totaling a volume of 200 μL. Plates were incubated at 35 °C for 24 h. The MIC was defined as the lowest concentration of the sample that inhibited visible microbial growth (CLSI Citation2002).
The lethal effects of the samples were determined through their minimum fungicidal concentrations (MFC) by subculturing on Sabouraud dextrose agar plates an aliquot from each well having a concentration equal to or higher than the MIC. The plates were incubated at 35 °C for 48 h. The MFC was defined as the lowest concentration of the extract or bioactive fraction that showed no visible growth on the agar plate (Manohar et al. Citation2001).
Finally, the MFC/MIC ratio was calculated to determine if the extracts and their bioactive fractions have fungistatic (MFC/MIC ≥ 4) or fungicidal (MFC/MIC < 4) properties (Siddiqui et al. Citation2013).
Antifungal mode of action
These assays aimed to elucidate the antifungal mechanisms through which the extracts and bioactive fractions interact with the fungal cells. Two major mechanisms were investigated: direct interaction with the cell wall biosynthesis (sorbitol assay) or membrane ionic permeability (ergosterol assay).
Effects on cell wall biosynthesis
The MIC of the extracts or bioactive fractions was determined in the presence of an osmotic protector through an adaptation of the microdilution technique proposed by the CLSI (Citation2002). Culture medium (RPMI-1640) was added to each well of the microplate and serial dilutions of the samples and controls were carried out. Finally, 100 μL of inoculum (2.5 × 105 CFU/mL) prepared with RPMI-1640 supplemented with 0.8 M sorbitol (final concentration) (Sigma-Aldrich, St. Louis, MO) was transferred to the wells. Caspofungin diacetate (Sigma-Aldrich, St. Louis, MO) was used as positive control. Yeast growth, media sterility and inertness of vehicle (negative control) were also monitored. Plates were incubated at 35 °C and read after 2 and 7 d (Lima et al. Citation2013; Freires et al. Citation2014).
Effects on membrane permeability
The MIC of the extracts or bioactive fractions was determined in absence and presence of exogenous ergosterol (Sigma-Aldrich, St. Louis, MO) at concentrations of 100, 200 and 400 μg/mL through an adaptation of the microdilution technique (CLSI Citation2002). Amphotericin B, which highly binds to ergosterol, was used as positive control. Yeast viability in presence of different concentrations of ergosterol and of vehicle was checked as a negative control. Plates were read after 24 h of incubation at 35 °C (Lima et al. Citation2013; Freires et al. Citation2014).
Scanning electron microscopy (SEM)
Biofilm cultures (2.5 × 105 CFU/mL) were grown in chambered glass slides (BD Falcon™, Bedford, MA) containing RPMI-1640 and were treated with the extract/bioactive fraction at different concentrations (MIC, MFC, 5 x MIC and 10 x MIC) or with nystatin (MIC). The chambered slides were kept shaking at 100 rpm, 35 °C for 72 h to form consistent biofilms. After that, the samples were washed twice to remove planktonic cells and kept in a 3% glutaraldehyde solution (pH 7.4) for 12 h at room temperature, followed by serial dehydration with ethanol (50, 70 and 90%) for 10 min and drying for 45 min under laminar flow. At last, the chambers were separated from the slides and the latter were coated with a gold pellicle in a metallizer device to be observed under scanning electron microscopy (Jeon JSM 5600LV microscope, Tokyo, Japan) (Freires et al. Citation2014).
Confocal laser scanning microscopy (CLSM)
C. albicans cultures were started in yeast nitrogen base (YNB) medium (Himedia®, Mumbai, India) supplemented with 50 mM glucose for 24 h at 35 °C. Cells were harvested (1200 rpm, 10 min, 10 °C), washed twice, resuspended in PBS (0.15 M, pH 7.2, Ca2+- and Mg2-free) and adjusted to 5 × 106 CFU/mL (530 nm, λ = 0.08–0.1). Final inocula concentration in the wells was 2.5 × 105 CFU/mL. For preparation of C. albicans biofilms, glass slides (324 mm2 surface area) were immersed in 12-well flat-bottom plates (TPP, Trasadingen, Switzerland) containing fetal bovine serum (FBS) (Vitrocell Embriolife, Campinas, SP, Brazil), followed by incubation for 24 h at 35 °C. Then the slides were washed with PBS to remove residual FBS and transferred to new plates containing the C. albicans suspension, followed by incubation for 90 min at 35 °C. Subsequently, the slides were gently washed to ensure the removal of non-adhered cells before immersion in YNB medium containing the extract/bioactive fraction, vehicle and nystatin. The plates were incubated at 35 °C for 48 h for biofilm formation (adapted from Kuhn et al. Citation2002; Freires et al. Citation2015). 72 h biofilm formation was not possible using this method as the biofilm pellicle on the slide turns to be thick and detaches from the surface after 48 h.
For the CLSM analysis, the biofilms were placed in wells containing 10 μM FUN-1 (F-7030, Life Technologies, Carlsbad, CA) and 25 μg/mL Concanavalin A, Alexa Fluor® 488 Conjugate (C11252, Life Technologies, Carlsbad, CA) in 3 mL of PBS. The plates were then incubated for 45 min at 35 °C and the slides were subsequently visualized on a Leica TCS-SP5 confocal microscope (Mannheim, Germany) equipped with an HCX-PL-APO 63x oil immersion objective lens. Each biofilm sample was scanned at five randomly selected points and representative images were selected. 2D images were generated and processed using LAS X Suite for Windows®.
Cytotoxic activity on murine and human cell cultures
Murine macrophage RAW264.7 were grown in RPMI medium and human keratinocyte HaCat were cultured in DMEM medium, both supplemented with 10% fetal bovine serum (Gibco BR, Gaithersburg, MD). A viability assay was carried out in which RAW264.7 and HaCat cells were seeded onto 96-well plates at a density of 1 × 105 and 1 × 104 cells/well, respectively, and allowed to adhere for 30 h. After that, the adhered cells were exposed to the extracts/bioactive fraction at the concentrations of 200, 100, 20 and 10 μg/mL in dimethyl sulfoxide (DMSO)/medium and vehicle control. Final DMSO concentration (1%) did not affect cell viability. After 24 h of incubation, viability was determined by the addition of 200 μL of MTT solution (0.3 mg/mL). The precipitated formazan crystals were solubilized in ethanol, and the absorbance was determined at 570 nm using a microplate reader (Mosmann Citation1983).
Statistical analysis
All the tests in this study were carried out in triplicate in three independent experiments. Descriptive and inferential statistics were used to analyse the data on GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA). One-way analysis of variance followed by Tukey-Kramer post-test was used to analyse the intra- and inter-group differences in the cytotoxicity assays, with type I error set as 0.05.
Results
Extracts and fractions yields
The extracts and fractions yields, expressed in relation to dry weight of plant material (%, w/w) were as follows: S. obtusifolium extract (3.64%) – dichloromethane fraction (13.1% in relation to crude extract)/ Nb fraction (11.8%, in relation to crude extract) – and Sc (6.25%).
Phytochemical analysis
The extract and/or fraction with the best antifungal activity were chemically characterized. shows the phytochemical makers of the Nb fraction from S. obtusifolium extract and Sc. The results showed a predominance of total flavonoids in the Nb fraction and saponins in the Sc.
Table 1. Phytochemical markers of the Nb fraction from Sideroxylon obtusifolium hydroalcoholic extract and Syzygium cumini hydroalcoholic extract.
Antifungal activity and mode of action
The inhibitory and lethal concentrations of the extracts, bioactive fractions and standard drugs against C. albicans can be found in . The Nb fraction from S. obtusifolium hydroalcoholic extract was shown to concentrate the most active constituents of S. obtusifolium with MIC/MFC values lower than those of the crude extract and dichloromethane fraction. Therefore, the Nb fraction was selected to be further tested as to mode of action, effects on biofilm morphology and viability and cytotoxicity. The crude extract of S. cumini was also tested in this study, which was also proven to have considerable antifungal activity against planktonic C. albicans cells.
Table 2. Antifungal activity of Sideroxylon obtusifolium extract and its fractions, Syzygium cumini extract, and standard drugs on Candida albicans ATCC 10231 (values are expressed as μg/mL).
Considering the promising antifungal effects of the Nb fraction and Sc, we next studied their modes of action upon yeast cells. The findings showed that Nb fraction and Sc do not interfere with cell wall biosynthesis pathways as does Caspofungin (used as a control), because the antifungal susceptibility of yeasts to them was unaltered either in the presence or absence of an osmotic protector (). Instead, their MIC values increased between 4 and 16 times with the addition of exogenous ergosterol, which indicates that they seem to complex with membrane ergosterol and this could potentially lead to increased membrane ionic permeability, cell leakage and death ().
Table 3. Effects of the Nb fraction from Sideroxylon obtusifolium extract, and Sc on Candida albicans (ATCC 10231) cell wall biosynthesis (sorbitol assay). Values are expressed as μg/mL.
Table 4. Effect of different concentrations of exogenous ergosterol (100–400 μg/mL) on the antifungal activity of the Nb fraction from Sideroxylon obtusifolium extract; Syzygium cumini extract; and Amphotericin B on Candida albicans ATCC10231.
Effects on biofilm morphology and viability
SEM analysis was initially carried out to evaluate the effects of the extract and bioactive fraction on the morphology and integrity of C. albicans biofilms.
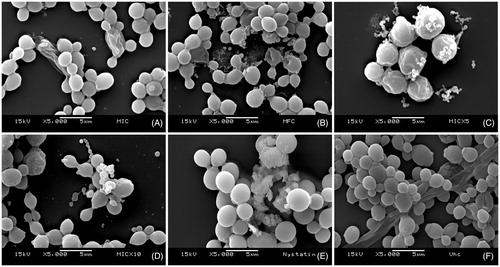
We observed that the Nb fraction from S. obtusifolium severely affected the biofilm cell structures even at low inhibitory concentrations (62.5 μg/mL), and that at higher concentrations the damages were lethal to the cells. Marked structural changes with membrane rupture and possible leakage of cellular contents were observed. Therefore, the Nb fraction was shown to exert antifungal effects on C. albicans biofilms ().
Figure 1. Effects of the Nb fraction from Sideroxylon obtusifolium extract on biofilm morphology/integrity. SEM photomicrographs (5000x) showing Candida albicans biofilm cells treated with different concentrations of the Nb fraction (A) 62.5 μg/mL (MIC), (B) 250 μg/mL (MFC), (C) 312.5 μg/mL (5xMIC), (D) 625 μg/mL (10xMIC); as well as (E) 0.97 μg/mL nystatin and (F) vehicle. The Nb fraction affected cell structures even at low concentrations (MIC). Nystatin was used as positive control, and the vehicle did not affect the biofilm cells.
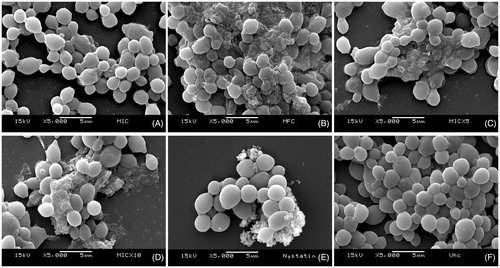
Similarly, Sc also affected C. albicans biofilm cells, with deleterious effects at concentrations as low as the MIC (125 μg/mL) and evident destruction from 500 μg/mL (MFC). Nystatin, used as positive control, was effective in disrupting biofilm integrity; and the vehicle did not affect the cells ().
Figure 2. Effects of the Syzygium cumini extract on biofilm morphology/integrity. SEM photomicrographs (5000x) showing Candida albicans biofilm cells treated with different concentrations of Sc: (A) 125 μg/mL (MIC), (B) 500 μg/mL (MFC), (C) 625 μg/mL (5xMIC), (D) 1250 μg/mL (10xMIC); as well as (E) 0.97 μg/mL nystatin and (F) vehicle. It can be noted that Sc affected cell structures at concentrations as low as 125 μg/mL. Nystatin was used as positive control, and the vehicle did not affect the biofilm cells.
The inhibitory effects of the Nb fraction and Sc on the viability of C. albicans biofilms were visualized by CLSM (). Viability of fungal cells upon the action of the tested substances could be observed through Concanavalin A (Alexa Fluor® 488 conjugate) and FUN® 1 cell staining. Both the fraction and the extract affected the viability of the biofilm cells when compared to the vehicle.
Figure 3. Effects on biofilm viability. This is a representative 2-D confocal imaging of Candida albicans biofilms treated with (A) Nb fraction from S. obtusifolium extract (5xMIC: 312.5 μg/mL); (B) S. cumini hydroalcoholic extract (5xMIC: 625 μg/mL); (C) Nystatin (MIC: 0.97 μg/mL) [positive control]; and (D) Vehicle (negative control). The structures depicted in green (Concanavalin A, Alexa Fluor® 488 Conjugate) represent the yeast cell wall, and those depicted in yellow (FUN® 1 Cell Stain) are nonviable cells, metabolically inactive. The viable cells, in turn, convert the dye FUN-1 to red fluorescent aggregates (63x optical magnitude, 2.85 zoomed-in). Concanavalin A selectively binds to polysaccharides, including alpha-mannopyranosyl and alpha-gluco-pyranosyl residues, and gives a green fluorescence. FUN-1 is a fluorescent dye taken up by yeast cells; in the presence of metabolic viability it is converted from a diffuse yellow cytoplasmic stain to red. It can be noted that both Sc and Nb fraction affected the viability of C. albicans cells when compared to the vehicle.
![Figure 3. Effects on biofilm viability. This is a representative 2-D confocal imaging of Candida albicans biofilms treated with (A) Nb fraction from S. obtusifolium extract (5xMIC: 312.5 μg/mL); (B) S. cumini hydroalcoholic extract (5xMIC: 625 μg/mL); (C) Nystatin (MIC: 0.97 μg/mL) [positive control]; and (D) Vehicle (negative control). The structures depicted in green (Concanavalin A, Alexa Fluor® 488 Conjugate) represent the yeast cell wall, and those depicted in yellow (FUN® 1 Cell Stain) are nonviable cells, metabolically inactive. The viable cells, in turn, convert the dye FUN-1 to red fluorescent aggregates (63x optical magnitude, 2.85 zoomed-in). Concanavalin A selectively binds to polysaccharides, including alpha-mannopyranosyl and alpha-gluco-pyranosyl residues, and gives a green fluorescence. FUN-1 is a fluorescent dye taken up by yeast cells; in the presence of metabolic viability it is converted from a diffuse yellow cytoplasmic stain to red. It can be noted that both Sc and Nb fraction affected the viability of C. albicans cells when compared to the vehicle.](/cms/asset/2a78829e-33c8-4c4f-a9a0-911d0cce9ffe/iphb_a_1155629_f0003_c.jpg)
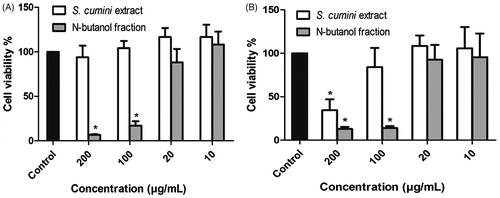
Cytotoxic activity
Cytotoxicity assays were carried out to provide preliminary evidence on whether these antifungal agents would affect or not host cells in a future clinical setting. shows that Sc did not show toxicity on murine macrophage cells, with no significant difference at any tested concentration (10–200 μg/mL) when compared to the vehicle. However, the Nb fraction from S. obtusifolium extract showed toxicity on macrophages at concentrations close to its MIC/MFC. On human keratinocytes, the toxicity threshold (limitrophe concentration that does not affect cell viability) for Sc and Nb fraction from S. obtusifolium extract was found to be 100 and 20 μg/mL, respectively, again lower than their MIC/MFC values.
Discussion
This study describes the antifungal potential and mode of action of S. obtusifolium and S. cumini against C. albicans. The plant material was collected in a semi-arid biome in northeastern Brazil termed ‘Caatinga’. It is a unique biogeographic region and considered one of the largest intermittently dry tropical forests in America (Penninghton et al. Citation2009). This biodiverse ecological setting is a shelter for 4 478 native plants species (Siqueira-Filho et al. Citation2012), including S. obtusifolium and exotic species, such as S. cumini, which are mostly explored by the local communities in folk medicine and for commercial purposes.
Due to the presence of major compounds in the leaves of S. obtusifolium and S. cumini that are known to have antifungal activity, such as total flavonoids (Onsare & Arora Citation2014; Yixi et al. Citation2015) and saponins (Sparg et al. Citation2004; Coleman et al. Citation2010), respectively, and in view of the potential sustainability of their use for therapeutic purposes, this study was carried out to elucidate the antifungal mode of action of these species and their effects upon yeast cell morphology and viability in biofilm cultures.
In agreement with other studies, the extract of S. obtusifolium showed weak antifungal activity (Cruz et al. Citation2007; Almeida et al. Citation2012), as well as its dichloromethane fraction. However, its Nb fraction showed better activity (lower MIC/MFC values) than the crude extract, indicating that the fractionation scheme adopted in this study (Jeon et al. Citation2011) was successful. Likely, the predominance of flavonoids in the Nb fraction may have favored its anti-Candida properties. Flavonoids extracted from different plant species were found to have strong inhibitory effects on the formation and metabolic activity of C. albicans biofilms or planktonic cells (Onsare & Arora Citation2014), which means that this phytochemical class could be a marker for antifungal activity.
The crude extract of S. cumini was also tested in this study, which showed fungistatic activity. Other studies in the literature have reported on the antifungal effects of S. cumini on C. albicans (Oliveira et al. Citation2007; Höfling et al. Citation2010; Santos et al. Citation2012), but have not further described its mode of action and effects on biofilm morphology and viability (see further in the discussion). As to the chemical profile, the major compounds found in the extract of the leaves in this study were saponins. Coleman et al. (Citation2010) found that two saponin family members disrupted C. albicans hyphae and biofilm formation; thus, it is assumed that saponins are possibly the active compounds in the Sc.
The next step in this bioprospection study was to elucidate the mode of action of the fraction or extract with the best antimicrobial activity determined by MIC/MFC. Herein, two possible chief mechanisms were investigated for Nb and Sc antifungal effects on yeasts: (i) disrupting cell wall biosynthesis or (ii) increasing membrane ionic permeability (Freires et al. Citation2014). The findings showed that both the Nb fraction from S. obtusifolium extract and Sc consistently bind to membrane ergosterol, leading to increased cell membrane permeability due to the formation of pores in the membrane. When at higher concentrations (250–500 μg/mL), their effects on the yeast cells are lethal, because ergosterol is crucial to maintain cell integrity, viability and normal growth (Ghannoum & Rice Citation1999). It is worth noting that other cell targets might also be involved in the antifungal activity of Nb fraction and Sc, warranting further investigation. In this study, the plant extract/fraction were found to be fungistatic (MFC/MIC = 4) and amphotericin B, fungicidal (MFC/MIC = 2), although both showed similar modes of action. This could be explained by other aspects that play a role in the major properties of antifungal drugs, such as drug affinity to ergosterol and chemical stability of the molecules determining the biological effects (Milhaud et al. Citation2002).
The study of the antimicrobial properties of natural products should prioritize biofilm growth models, in order to provide greater reliability of data and closer approach to clinically relevant conditions (Freires et al. Citation2014). In this study, scanning electron and confocal laser microscopy were used to visualize C. albicans biofilms grown on abiotic surfaces and treated with the Nb bioactive fraction and Sc. It was found that both products disrupted the structure and viability of biofilm cells. The association between biofilm formation and antimicrobial resistance remains to be a serious health issue in the current days (Simões Citation2011), which encourages the search for active molecules able to disrupt biofilm integrity. Hence, the harmful effects of the extract/fraction upon biofilm assembly seen in this study could contribute to render the biofilm more susceptible to other antifungal drugs. In addition to oral candidiasis, this result may also be useful for studies addressing yeast biofilm formation on the surfaces of medical devices, such as central venous catheters and endotracheal tubes. Further studies should also perform a quantitative analysis of the effects of the extract and fraction on the biofilm composition (e.g., estimation of extracellular polysaccharides and other components of the biofilm matrix), biovolume (e.g., number of cells vs. exposure time, biofilm inhibitory concentration) and viability (e.g., XTT assay).
Following validation of antifungal activity, the experimental samples were finally checked for their cytotoxic activity on murine and human cell cultures, in order to provide evidence for future clinical use. Overall, cytotoxic activity was detected on both cell lines, particularly the Nb fraction, as the cytotoxicity values found were lower than those of the MIC/MFC of the extract/fraction. As expected, Sc showed a less cytotoxic profile when compared to the Nb fraction, given that the fractionation process may concentrate toxic compounds in the bioactive fractions. Nevertheless, it is a preliminary cell-based assay with 24 h exposure of cells to the test substances, which would be rather different from the clinical use. Ribeiro et al. (Citation2014) reported that S. cumini hexanic extract has low toxicity on murine macrophages, with IC50 of 31.64 μg/mL. There have been no reports on the toxicity of S. obtusifolium in vitro.
An in vivo study by Ruela et al. (Citation2011) evaluated the acute toxicity in mice of the ethyl acetate fraction from the stem bark of S. obtusifolium. The authors found that such fraction, rich in polyphenols, has very low cytotoxicity with LD50 of 777 mg/kg by intraperitoneal route. With regard to S. cumini, an in vivo study by Silva et al. (Citation2012) showed that the hydroalcoholic extract of S. cumini leaves did not exert acute or chronic toxic effects on rodents by oral route, with LD50 of 489 mg/kg. More sophisticated toxicological assays are now encouraged to further determine whether the Nb fraction and Sc would pose a risk or not against the host cells.
The clinical management of oral candidiasis in most patients includes the administration of topical or systemic antifungal drugs as well as disinfection of dentures in patients presenting denture-related atrophic stomatitis (Gendreau & Loewy Citation2011). Hence, the Nb fraction and Sc may be of interest in both approaches, with particular focus on the denture disinfection protocol due to their potential toxicity toward host cells. In addition, several studies have also suggested the combination of synthetic drugs with natural products in order to improve the efficacy of both drugs and manage toxicity upon clinical use (Hemaiswaryaa et al. Citation2008; Castro et al. Citation2015).
Taken altogether, the findings of this bioprospection study support the view that these plant materials warrant further exploration of their biological properties and toxicity in future non-clinical studies and clinical trials. In summary, the Nb fraction from S. obtusifolium extract, and Sc, which are species from the unique Caatinga biome, showed antifungal activity against C. albicans biofilm, probably due to the predominance of flavonoids and saponins, respectively, in their chemical composition. Their ability to bind to ergosterol and thus increase cell membrane permeability may explain the deleterious effects observed on biofilm morphology and viability. The Nb fraction and Sc showed relative cytotoxic effects on murine and human cells. Other toxicological assays and in vivo studies of efficacy are now encouraged to further investigate the antifungal activity of the Nb fraction from S. obtusifolium and Sc as alternative candidates for the treatment of oral candidiasis.
Funding information
This work was supported by São Paulo Research Foundation (SP, Brazil) with grants no. 2013/25080-7, no. 2014/01723-9 and no. 2011/23635-6; National Council for Scientific and Technological Development (CNPq, Brazil) with grants no. 308644/2011-5 and no. 150682/2014-0; and a partnership between the CNPq and the Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil) with grant no. 552561/2011-8.
Acknowledgements
The authors thank the Department of Morphology (Microscopy Core), Piracicaba Dental School, University of Campinas, for the technical support in the scanning electron and confocal analyses.
Disclosure statement
The authors have no conflict of interest.
References
- Albuquerque UL, Soldati GT, Sieber SS, Sá EMF, Souza LC, Lins-Neto EMF. 2011. Use and extraction of medicinal plants by the Fulni-ô indians in northeastern Brazil – implications for local conservation. Sitientibus Sér Ci Biol11:309–320. Available at: https://doi.org/http://pkp.uefs.br/ojs/index.php/sitientibusBiologia/article/viewFile/78/60. Accessed on 12 March 2015.
- Ali GY, Algohary EHSS, Rashed KA, Almoghanum M, Khalifa AA. 2012. Prevalence of Candida colonization in preterm newborns and VLBW in neonatal intensive care unit: role of maternal colonization as a risk factor in transmission of disease. J Matern Fetal Neonatal Med. 25:789–795.
- Almeida CFCBR, Cabral DLV, Almeida CCBR, Amorim ELC, Araújo JM, Albuquerque UP. 2012. Comparative study of the antimicrobial activity of native and exotic plants from the Caatinga and Atlantic Forest selected through an ethnobotanical survey. Pharm Biol. 50:201–207.
- Alnuaimi AD, Wiesenfelda D, O’Brien-Simpson NM, Reynolds EC, McCullough MJ. 2015. Oral Candida colonization in oral cancer patients and its relationship with traditional risk factors of oral cancer: A matched case-control study. Oral Oncol. 51:139–145.
- Araújo-Neto V, Bomfim RR, Oliveira VOB, Passos AMPR, Oliveira JPR, Lima CA, Mendes SS, Estevam CS, Thomazzi SM. 2010. Therapeutic benefits of Sideroxylon obtusifolium (Humb. ex Roem. & Schult.) T.D. Penn., Sapotaceae, in experimental models of pain and inflammation. Braz J Pharmacog. 20:933–938.
- Arif T, Bhosale JD, Kumar N, Mandal TK, Bendre RS, Lavekar GS, Dabur R. 2009. Natural products-antifungal agents derived from plants. – J Asian Nat Prod Res. 11:621–638.
- Berman J, Sudbery PE. 2002. Candida albicans: a molecular revolution built on lessons from budding yeast. Nat Rev Genet. 3:918–930.
- Boros-Majewska J, Salewska N, Borowski E, Milewski S, Malic S, Wei XQ, Hayes AJ, Wilson MJ, Williams DW. 2014. Novel nystatin A1 derivatives exhibiting low host cell toxicity and antifungal activity in an in vitro model of oral candidosis. Med Microbiol Immunol. 203:341–355.
- Castro RD, Souza TMPA, Bezerra LMD, Ferreira GLS, Costa EMMB, Cavalcanti AL. 2015. Antifungal activity and mode of action of thymol and its synergism with nystatin against Candida species involved with infections in the oral cavity: an in vitro study. BMC Complement Altern Med. 15:417 DOI: 10.1186/s12906-015-0947-2.
- Chandra S, Mejía EG. 2004. Polyphenolic compounds, antioxidant capacity, and quinone reductase activity of an aqueous extract of Ardisia compressa in comparison to mate (Ilex paraguariensis) and green (Camellia sinensis) teas. J Agric Food Chem. 52:3583–3589.
- Clinical and Laboratory Standards Institute (CLSI). 2002. Protocol M27-A2. reference method for mroth dilution antifungal susceptibility testing of yeasts. 2nd ed. Pennsylvania (PA): NCCLS, 51p.
- Coleman JJ, Okoli I, Tegos GP, Holson EB, Wagner FF, Hamblin MR, Mylonakis E. 2010. Characterization of plant-derived saponin natural products against Candida albicans. ACS Chem Biol. 5:321–332.
- Cruz MC, Santos PO, Barbosa AM. Jr, de Mélo DL, Alviano CS, Antoniolli AR, Alviano DS, Trindade RC. 2007. Antifungal activity of Brazilian medicinal plants involved in popular treatment of mycoses. J Ethnopharmacol. 111:409–412.
- Cunha MG, Franchin M, Galvão LCC, de Ruiz AL, de Carvalho JE, Ikegaki M, de Alencar SM, Koo H, Rosalen PL. 2013. Antimicrobial and antiproliferative activities of stingless bee Melipona scutellaris geopropolis. BMC Complement Altern Med. 13:23. DOI: 10.1186/1472-6882-13-23.
- Dangi YS, Soni ML, Namdeo KP. 2010. Oral candidiasis: a review. Int J Pharm Pharm Sci. 2:36–41.
- Dar-Odeh NS, Shehabi AA. 2003. Oral candidosis in patients with removable dentures. Mycoses. 46:187–191.
- Eshwarappa RS, Iyer RS, Subbaramaiah SR, Richard SA, Dhananjaya BL. 2014. Antioxidant activity of Syzygium cumini leaf gall extracts. Bioimpacts. 4:101–107.
- Freires IA, Furletti VF, Sartoratto A, de Alencar SM, Figueira GM, Rodrigues JAO, Duarte MCT, Rosalen PL. 2014. Coriandrum sativum L. (coriander) Essential oil: antifungal activity and mode of action on Candida spp., and molecular targets affected in human whole-genome expression. Plos One. 9:e99086 doi 10.1371/journal.pone.0099086.
- Freires IA, Bueno-Silva B, Galvão LCC, Duarte MCT, Sartoratto A, Figueira GM, de Alencar SM, Rosalen PL. 2015. The effect of essential oils and bioactive fractions on Streptococcus mutans and Candida albicans biofilms: a confocal analysis. Evid Based Complement Alternat Med. 2015: 871316. doi: 10.1155/2015/871316.
- Gendreau L, Loewy ZG. 2011. Epidemiology and etiology of denture stomatitis. J Prosthodont. 20:251–260.
- Ghannoum MA, Rice LB. 1999. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev. 12:501–517.
- Hemaiswaryaa S, Kruthiventi AK, Doble M. 2008. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine. 15:639–652.
- Höfling JF, Anibal PC, Obando-Pereda GA, Peixoto IA, Furletti VF, Foglio MA, Gonçalves RB. 2010. Antimicrobial potential of some plant extracts against Candida species. Braz J Biol. 70:1065–1068.
- IBGE, Instituto Brasileiro de Geografia e Estatística [Brazilian Institute of Geography and Statistics]. Brazil in synthesis. Available at https://www.brasilemsintese.ibge.gov.br/territorio. Accessed on 15 May 2015.
- Iwu M. 2000. International conference on ethnomedicine and drug discovery. J Altern Complement Med. 6:3–5.
- Jeon JG, Rosalen PL, Falsetta ML, Koo H. 2011. Natural products in caries research: current (limited) knowledge, challenges and future perspective. Caries Res. 45:243–263.
- Kong EF, Kucharíkovác S, Van Dijck P, Peters BM, Shirtliff ME, Jabra-Rizk MA. 2015. Clinical implications of oral candidiasis: host tissue damage and disseminated bacterial disease. Infect Immun. 83:604–613.
- Kuhn DM, Chandra J, Mukherjee JPK, Ghannoum MA. 2002. Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect Immun. 70:878–888.
- Lima IO, Pereira FO, Oliveira WA, Lima EO, Menezes EA, Cunha FA, Diniz MFFM. 2013. Antifungal activity and mode of action of carvacrol against Candida albicans strains. J Essent Oil Res. 25:138–142.
- Manohar V, Ingram C, Gray J, Talpur NA, Echard BW, Bagchi D, Preuss HG. 2001. Antifungal activities of origanum oil against Candida albicans. Mol Cell Biochem. 228:111–117.
- Makkar HPS, Becker K. 1993. Vanillin-HCl method for condensed tannins: effect of organic solvents used for extraction of tannins. J Chem Ecol. 19:613–621.
- Makkar HPS, Siddhuraju P, Becker K. 2007. Plant secondary metabolities. 1st ed. New Jersey (NJ): Human Press.
- Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. 2005. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 91:571–577.
- Milhaud J, Ponsinet V, Takashi M, Michels B. 2002. Interactions of the drug amphotericin B with phospholipid membranes containing or not ergosterol: new insight into the role of ergosterol. Biochim Biophys Acta. 1558:95–108.
- Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 65:55–63.
- Negri M, Salci TP, Shinobu-Mesquita CS, Capoci IRG, Svidzinski TIE, Kioshima ES. 2014. Early state research on antifungal natural products. Molecules. 19:2925–2956.
- Oliveira GF, Furtado NAJC, Silva-Filho AA, Martins CHM, Bastos JN, Cunha WR, Silva MLA. 2007. Antimicrobial activity of Syzygium cumini (Myrtaceae) leaves extract. Braz J Microbiol. 38:381–384.
- Onsare JG, Arora DS. 2014. Antibiofilm potential of flavonoids extracted from Moringa oleifera seed coat against Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans. J Appl Microbiol. 118:313–325.
- Peng LP, Nagarajan S, Rasheeda S, Zhou CH. 2015. Synthesis and biological evaluation of a new class of quinazolinone azoles as potential antimicrobial agents and their interactions with calf thymus DNA and human serum albumin. Med Chem Commun. 6:222–229.
- Penninghton RT, Lavin M, Oliveira-Filho A. 2009. Woody plant diversity, evolution, and ecology in the tropics: perspectives from seasonally dry tropical forests. Annu Rev Ecol Evol Syst. 40:437–457.
- Pierce CG, Srinivasan A, Uppuluri P, Ramasubramanian AK, López-Ribot JL. 2013. Antifungal therapy with an emphasis on biofilms. Curr Opin Pharmacol. 13:726–730.
- Quintans JS, Brito RG, Aquino PG, França PH, Siqueira-Lima PS, Santana AE, Ribeiro EA, Salvador MJ, Araújo-Júnior JX, Quintans-Júnior LJ. 2014. Antinociceptive activity of Syzygium cumini leaves ethanol extract on orofacial nociception protocols in rodents. Pharm Biol. 52:762–766.
- Ramage G, Rajendran R, Sherry L, Williams C. 2012. Fungal biofilm resistance. Int J Microbiol. 2012:528521 doi: 10.1155/2012/528521.
- Ribeiro TG, Chávez-Fumagalli MA, Valadares DG, Valadares DG, Franca JR, Lage PS, Duarte MC, Andrade PH, Martins VT, Costa LE, et al. 2014. Antileishmanial activity and cytotoxicity of Brazilian plants. Exp Parasitol. 143:60–68.
- Rodrigues KAF, Amorim LV, Dias CN, Moraes DF, Carneiro SM, Carvalho FA. 2015. Syzygium cumini (L.) Skeels essential oil and its major constituent α-pinene exhibit anti-Leishmania activity through immunomodulation in vitro. J Ethnopharmacol. 160:32–40.
- Ruela HS, Leal ICR, Almeida MRA, Santos KRN, Wessjohann LA, Kuster RM. 2011. Antibacterial and antioxidant activities and acute toxicity of Brumelia sartorum, a Brazilian medicinal plant. Braz J Pharmacog. 21:86–91.
- Santos KKA, Matias EFF, Tintino SR, Souza CE, Braga MF, Guedes GM, Rolœn M, Vega C, de Arias AR, Costa JG, et al. 2012. Cytotoxic, trypanocidal, and antifungal activities of Eugenia jambolana L. J Med Food. 15:66–70.
- Siddiqui ZN, Farooq F, Musthafa TNM, Ahmad A, Khan AU. 2013. Synthesis, characterization and antimicrobial evaluation of novel halopyrazole derivatives. J Saudi Chem Soc. 17:237–243.
- Silva SN, Abreu IC, Silva GFC, Ribeiro RM, Lopes AS, Cartágenes MSS, Freire SMS, Borges ACR, Borges MOR. 2012. The toxicity evaluation of Syzygium cumini leaves in rodents. Braz J Pharmacogn. 22:102–108.
- Simões M. 2011. Antimicrobial strategies effective against infectious bacterial biofilms. Curr Med Chem. 18:2129–2145.
- Siqueira-Filho JA, Souza DP, Siqueira AA, Meiado MV, Correa LC, Ramos RRD. 2012. A queda do mito: composição, riqueza e conservação das plantas vasculares das Caatingas do rio São Francisco [The fall of the myth: composition, richness and conservation of vascular plants of Caatingas by the São Francisco River]. In: Siqueira-Filho JA. A flora das Caatingas do rio São Francisco: história natural e conservação, Andrea Jakobsson, Rio de Janeiro: 162–191. Portuguese.
- Sparg SG, Light ME, Van Staden J. 2004. Biological activities and distribution of plant saponins. J Ethnopharmacol. 94:219–243.
- Xie Y, Yang W, Tang F, Chen X, Ren L. 2015. Antibacterial activities of flavonoids: structure-activity relationship and mechanism. Curr Med Chem. 22:132–149.