Abstract
Context Some mushrooms of the order Polyporales are known for their immunomodulatory actions.
Objective The objective of this study is to evaluate the in vitro phagocytic and cytotoxic effects of extracts from polyporales native to Central Europe.
Materials and methods The effects of ethanol extracts from 27 polypore species on opsonized zymosan-induced phagocytosis of isolated human neutrophils were tested by a chemiluminescence method. Colon epithelial cell lines, Caco-2 and HT-29, were used for cytotoxicity assays, and extracts were chemically characterized in terms of total phenolic and β-glucan content.
Results We observed phagocytosis or respiratory burst enhancing activity in 17 extracts, of which five species, namely Aurantiporus fissilis (Berk. & M.A. Curtis) H. Jahn ex Ryvarden, Trametes gibbosa (Pers.) Fr., Piptoporus betulinus (Bull.) P. Karst, Neolentinus lepideus (Fr.) Redhead & Ginns, Polyporus squamosus (Huds.) Fr., significantly increased phagocytosis in granulocytes by 205, 181, 158, 155 and 141%, respectively. The β-glucan content of the three most potent extracts was 58, 42 and 74 mg/g, respectively, and the polyphenol content was 155.6, 133.5 and 155.2 μmol of gallic acid equivalent/g, respectively. Some extracts showed cytotoxic activity, with higher cytotoxicity in Caco-2 than in HT-29 cells. Pycnoporus cinnabarinus (Jacq.) P. Karst. extract was cytotoxic to both cell lines, with IC50 values of 81 and 31 μg/mL, respectively.
Discussion and conclusion The most promising extracts were from N. lepideus and Polyporus squamosus, which are edible species and may be considered safe. Our findings support their use as culinary preparations or food supplements for various immunological gut disorders.
Introduction
For millennia, mushrooms have been valued for their culinary use and as a source of medicinal substances. Polypore fungi are among major categories in the pharmacopeias and medicines used by indigenous people worldwide (Grienke et al. Citation2014). Ganoderma lucidum (Curtis) P. Karst. (Polyporales) is a species widely used in traditional Chinese medicine for its tonifying, immunomodulatory properties, and for overall promotion of health and longevity. It is a common belief that its use lowers risk of cancer and heart disease and boosts the immune system (Ko & Leung Citation2007). Mushroom extracts have potential clinical applications as preventive agents against intestinal injury, which is a common adverse effect of chemotherapy (Kashimoto et al. Citation2010) while some recent studies suggest their positive role in inflammatory bowel disease (Hanaoka et al. Citation2011).
Immunomodulatory properties of mushrooms are mostly associated with their polysaccharide fraction. Mushroom β-glucans are a diverse group of medium to high molecular weight compounds. They vary in structure, with a β-(1 → 3) backbone, and additional β-(1 → 6) side chains of varying lengths, with attached protein residues. These structural variations result in specific activities (Wasser Citation2002). After ingestion, recognition of these β-glucans by immune phagocytic cells can trigger immune responses, primarily to control fungal pathogens (Brown & Gordon Citation2003). In vitro and in vivo studies suggest that high molecular weight β-glucans can directly activate leukocytes, stimulating their phagocytic, cytotoxic and antimicrobial activities, including release of reactive oxygen and nitrogen intermediates in addition to various proinflammatory mediators (Tzianabos Citation2000; Vetvicka & Vetvickova Citation2010).
Besides the polysaccharide fraction of mushrooms, a vast spectrum of biological activities are associated with other small molecule components such as steroids, triterpenes or phenolics (Tomasi Citation2008; Grienke et al. Citation2014). Ethanol or methanol extracts rich in these metabolites or derived pure compounds have shown antimicrobial, anti-inflammatory, radical-scavenging or cytotoxic activities (Barros et al. Citation2007; Macakova et al. Citation2009, Citation2010; Grienke et al. Citation2014).
Polypores are distributed worldwide, but compared with the Asian or tropical species, there is much less data regarding the biological activity of European species. These mushrooms are often found on decaying trees, but few are considered suitable for consumption. However, some species are edible especially when their fruiting bodies are young and soft (). The importance of Central European polypores in ancient folk medicine has been recently emphasized in a review by Grienke et al. (Citation2014).
Table 1. Properties and taxonomical relations of the sample set.
Our aims were to assess the immunomodulatory potential of ethanol extracts from 27 species of the order Polyporales, belonging to the families Fomitopsidaceae, Gleophyllaceae, Meruliaceae, Polyporaceae and Sparassidaceae. Selection of this taxon implies a chemotaxonomic relationship with G. lucidum, which exerts various immunomodulatory activities. All tested species were native to Central Europe. We used in vitro chemiluminescence assays to determine their effects on the phagocytic response (respiratory burst and phagocytosis) of human neutrophil granulocytes. Cytotoxicity in intestinal epithelial cells model was assayed to provide a preliminary assessment of their safety and therapeutic potential in immune-related disorders of the gastrointestinal tract. Moreover, the extracts were characterized in terms of their total phenolic and total β-glucan content.
Materials and methods
Biological material
The 27 chosen extracts to be investigated originated from the sample repository of the ADINACO Research Group (Faculty of Pharmacy, Charles University, Prague, Czech Republic). All mushroom species used had been previously characterized in terms of their antioxidant activities determined by the DPPH free-radical scavenging and hydrogen peroxide-induced luminol chemiluminescence assay, seven of them showing antioxidant potential (Macakova et al. Citation2010). For our study, the following species were selected: Abortiporus biennis (Bull.) Singer; Aurantiporus fissilis (Berk. & M.A. Curtis) H. Jahn ex Ryvarden; Bjerkandera adusta (Willd.) P. Karst.; Coriolopsis gallica (Fr.) Ryvarden; Daedalea quercina (L.) Pers.; Daedaleopsis confragosa (Bolton) J. Schröt.; Fomitopsis pinicola (Sw.) P. Karst.; Gloeophyllum odoratum (Wulfen) Imazeki; Hapalopilus nidulans (Fr.) P. Karst.; Laetiporus sulphureus (Bull.) Murrill; Lentinus tigrinus (Bull.) Fr.; Merulius tremellosus Schrad.; Neolentinus lepideus (Fr.) Redhead & Ginns; Panus conchatus (Bull.) Fr.; Phaeolus schweinitzii (Fr.) Pat.; Piptoporus betulinus (Bull.) P. Karst.; Polyporus brumalis (Pers.) Fr.; Polyporus lipsiensis (Batsch) E.H.L. Krause; Polyporus squamosus (Huds.) Fr.; Polyporus umbellatus (Pers.) Fr.; Postia ptychogaster (F. Ludw.) Vesterh.; Postia stiptica (Pers.) Jülich; Pycnoporus cinnabarinus (Jacq.) P. Karst.; Royoporus badius (Pers.) A.B. De; Sparassis crispa (Wulfen) Fr.; Trametes gibbosa (Pers.) Fr.; Trametes versicolor (L.) Lloyd. Current valid species names from Index Fungorum (http://www.indexfungorum.org/) were used throughout the study.
Fruiting bodies were collected from the forests of the Hradec Kralove district, the Czech Republic. The fruiting bodies were identified based on their morphology by Dr. Vera Samkova (Museum of East Bohemia, Hradec Kralove, Czech Republic); voucher specimens were deposited in the Herbarium of the Museum of East Bohemia. Dried Ganoderma lucidum was purchased from an herbal product manufacturer in Vietnam (Thien Huong Dalat, Vietnam). Dried Shiitake (Lentinula edodes (Berk.) Pegler) fruiting bodies were obtained from a Vietnamese food retailer. Both additional samples were identified based on the morphology by Dr. Jaroslav Havlik (Department of Microbiology, Nutrition and Dietetics, Czech University of Life Sciences Prague). Samples were stored at −80 °C until required. A detailed overview of the uses, and taxonomical relations of the mushrooms species that we investigated are summarized in .
Chemicals and reagents
Ethanol and dimethyl sulphoxide (DMSO) were purchased from Lach-Ner (Neratovice, Czech Republic). Ficoll-Paque PLUS was obtained from GE Healthcare Bio-Sciences (Piscataway, NJ). HISTOPAQUE 1077, Türk’s solution, zymosan, lipopolysaccharide (LPS) from Escherichia coli 0111:B4, Dulbecco’s modified Eagle’s medium (DMEM), foetal bovine serum, penicillin–streptomycin mixture, sodium bicarbonate, sodium pyruvate, nonessential amino acids, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and gallic acid were from Sigma-Aldrich (St. Louis, MO). The luminol reagent used for chemiluminescence was purchased from Fluka Analytical (St. Louis, MO). The Mushroom and Yeast Glucan Assay Kit was from Megazyme (Bray, Ireland).
Folin–Ciocalteu reagent and Na2CO3 were from Penta (Chrudim, Czech Republic). According to general recommendations, lysis buffer [8.29 g/L NH4Cl, 790.6 mg/L NH4HCO3 (Lach-Ner, Neratovice, Czech Republic), 37.22 mg/L EDTA (Penta)] and sodium potassium combo-buffered saline [pH 7.4, 0.2 g/L KCl, 0.24 g/L KH2PO4, 8 g/L NaCl, 1.15 g/L anhydrous Na2HPO4 (Lach-Ner, Neratovice, Czech Republic)] were prepared. Fresh blood samples (buffy coats) were supplied by a blood donor centre (Thomayer Hospital, Prague, Czech Republic), with informed consent from donors and institutional ethical approval. The human colon adenocarcinoma cell lines Caco-2 and HT-29 were a kind gift from Dr. Zuzana Zakostelska (Institute of Microbiology, v.v.i., Academy of Sciences of the Czech Republic). White flat-bottomed NUNC 96-well plates and flat-bottomed NUNCLON 96-well plates were purchased from Thermo Fisher Scientific (Waltham, MA).
Extract preparation
Mushroom extracts were prepared as previously described by Macakova et al. (Citation2010). Fruiting bodies were cleaned, frozen in liquid nitrogen and kept at −20 °C in closed containers. Before extraction, they were cut into small pieces and homogenized in 70% (v/v) ethanol with a hand blender. Homogenates were then sonicated for 30 min in an ultrasonic bath at room temperature (Sonorex SP10, sonication grade 10; Bandelin Electronic GmbH & Co. KC, Berlin, Germany). Filtrates were evaporated and freeze-dried. Powdered extracts were kept at −80 °C under argon until tested. Ethanol extracts from L. edodes and G. lucidum were not included in the previous study, but were prepared as described above. Before the commencement of experiments, extracts were diluted in DMSO to 50 mg/mL and subsequently diluted in phosphate-buffered saline (PBS). A G. lucidum water extract was prepared by boiling 5 g of G. lucidum fruiting bodies in water for 5 min, followed by filtration and freeze-drying of the samples.
Isolation of granulocytes
Thirty millilitres of 20% buffy coat were diluted in PBS and carefully transferred to 10 mL of Ficoll-Paque PLUS or HISTOPAQUE-1077. Samples were centrifuged (400 × g, 30 min, 22 °C) and the supernatants were discarded. Pellets, comprising of erythrocytes and granulocytes, were mixed with 30 mL of lysis buffer. The solution was mixed thoroughly and centrifuged (300 × g, 15 min). The lysis step was repeated until the supernatant was opaque. The pellet was then washed with PBS and carefully resuspended in 3 mL of PBS. To standardize the granulocyte solution, an aliquot of cells was mixed with Türk’s solution and counted using a Bürker chamber. Cell suspensions were further diluted in PBS to achieve a final concentration of 3 × 106 cells/mL.
Caco-2 and HT-29 cell cultures
Caco-2 and HT-29 cells were maintained in DMEM supplemented with 10% foetal bovine serum, 1% penicillin–streptomycin solution, 1% sodium bicarbonate, 1% sodium pyruvate and 1% nonessential amino acids. Cultures were incubated at 37 °C/5% CO2 and media were replenished every 2–3 d, with passaging after every 7 d.
Chemiluminescence assays
Immunomodulatory activity was measured according to the zymosan-induced luminol-dependent chemiluminescence method published by Wagner and Jurcic (Citation1991). Opsonized zymosan (2.5 mg/mL) was prepared by incubating in the serum obtained after centrifuging the buffy coats (1:10 dilution) at 37 °C for 1 h. The particles were washed later on and resuspended in 1.5 mL of PBS. Opsonized zymosan particles were counted in a Bürker chamber and diluted in PBS to achieve a final concentration of 3 × 107 particles/mL.
Triplicate serial dilutions of extracts (31–250 μg/mL) were added to the microplate wells (100 μL). This concentration range was selected based on preliminary measurements for four samples across a wider concentration range (6.25–1024 μg/mL). Active Postia ptychogaster and P. betulinus exhibit peak activity within 31–250 μg/mL. Other researchers have reported a similar concentration range for extract activities on neutrophil respiratory burst (Shamtsyan et al. Citation2004). The cell suspension (50 μL, 3 × 106 cells), luminol solution (50 μL, 0.5 mg/mL) and standardized opsonized zymosan solution (50 μL) was added to the wells to give a final volume of 250 μL.
The G. lucidum water extracts were prepared in triplicate over four dilutions and used as internal standards on each plate as a measure of an inter-day reproducibility. Samples were normalized to G. lucidum inter-day activity, which varied by no more than 30%. Furthermore, negative (no LPS) and positive (LPS added) controls were included in each plate. All samples, including controls, used opsonized zymosan particles for the induction of phagocytosis. Five mushroom samples were tested in each experimental run. Reference samples of ethanol extracts from L. edodes and G. lucidum were used to enable comparisons with other studies.
Plates were inserted in a Tecan M200 multiplate reader (Tecan, Grödig, Austria) with a luminescence module. Luminescence due to phagocytic respiratory burst was measured at 37 °C over 2 min cycles for 3 h. The capacity of the extract to enhance phagocytosis of neutrophils was calculated based on the slope of a tangential line to the initial curve phase as compared with an untreated control, which was considered 100%. This value was designated as the phagocytic index (PIslope). The concentration at which the extracts showed the highest PIslope was referred to as Concmax (). Some of the extracts suppressed luminescence, probably owing to negative effects on cell viability. For these extracts, the PIslope and Concmax values corresponded to the lowest concentrations. The statistical significance of the effects was also assessed.
Table 2. Cytotoxic properties of 27 polypore mushrooms extracts and their effect of phagocytic response in isolated neutrophils.
MTT assay
Cell viability was measured using the MTT assay originally developed by Mosman (Citation1983). Caco-2 and HT-29 cells were pre-incubated in a 96-well plate at a density of 2.5 × 103 cells/well for 24 h at 37 °C/5% CO2. Cells were then treated with two-fold serially diluted extracts (1.56–500 μg/mL) for 72 h. Subsequently, MTT reagent (1 mg/mL) in DMEM was added to each well, and plates were incubated for an additional 2 h at 37 °C. Culture supernatants were aspirated and 100 μL of DMSO added to each well. The absorbance was then measured at 555 nm. The % mortality for each extract concentration was plotted and used to determine the 50% inhibitory concentration (IC50).
Total phenolic content (TPC)
The TPC was determined using a method previously described by Singleton et al. (Citation1999) with some modifications. Each sample (100 μL) was added to 96-well microtitre plates to yield a range of concentrations (21–341 μg/mL). We then added 25 μL of Folin–Ciocalteu reagent to each well. Plates were placed in an orbital shaker and shaken (125 × g, 10 min). Reactions were initiated by the addition of 75 μL of 12% (w/v) Na2CO3 and incubated in dark at 37 °C for 2 h. The absorbance at 760 nm was determined using an Infinite M200 reader (Tecan, Grödig, Austria). Results were expressed as gallic acid equivalents (GAE; μg GAE/mg of extract).
Determination of β-glucan content
β-Glucan content was measured using a Beta Glucan Assay Kit (Megazyme, Bray, Ireland) according to the instructions of the manufacturer, with modifications to accommodate the lower sample volumes. The amount of β-glucan was calculated as follows: β-glucan = total glucan (%, w/w) – α-glucan (%, w/w).
To determine the total glucan content, 10 mg of sample in a glass tube was suspended in 0.15 mL of 36% HCl and incubated in a water bath at 30 °C for 45 min. The hydrolysates were then placed in a boiling water bath for 2 h. The pH was adjusted to neutral with 2 M KOH, and the samples were then centrifuged (1500 × g, 10 min). We then added 0.1 mL of exo-1,3-β-glucanase (20 U/mL) and β-glucosidase (4 U/mL) in 200 mM sodium acetate buffer (pH 5.0) to 1.0 mL of the supernatant. Samples were incubated at 40 °C for 1 h followed by the addition of 2 mL of glucose oxidase/peroxidase mixture (GOPOD).
For α-glucan content, 10 mg of the sample was resuspended in 0.2 mL of 2 M KOH and incubated at 4 °C for 20 min. The pH was adjusted to neutral with 1.2 M sodium acetate buffer (pH 3.8), followed by the addition of 0.2 mL of amyloglucosidase (1630 U/mL) and invertase (500 U/mL). The mixture was then incubated at 40 °C for 30 min and the digests were centrifuged (1500 × g, 10 min). We then mixed 0.1 mL of supernatant with 2 mL of GOPOD reagent and incubated the samples at 40 °C for 20 min. The absorbance was measured at 510 nm using an Infinite M200 reader (Tecan, Grödig, Austria).
Statistical analysis
All tests for phagocytic activity were conducted in triplicate by using two independent inter-day measurements. For cytotoxic activity, measurements were carried out using three independent tests conducted in duplicate. Chemical analyses were done in duplicate. All results are presented as the mean ± the standard deviation of the mean (SD). For phagocytic activities, one-way analysis of variance (ANOVA) followed by Dunnett’s test was performed to determine significant differences between extracts and controls (p < 0.01 or p < 0.05; ). The SDs were calculated using MS Excel (Microsoft Office 2007) while ANOVA with post-hoc testing was conducted with GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA). The IC50 values and curve fittings were obtained using Tecan Magellan software v.7.2 (Tecan, Grödig, Austria).
Results
Optimization of assays
The relative response of zymosan-induced phagocytosis in granulocytes to different concentrations of G. lucidum water extract was evaluated. The extract was added at four concentrations (12–95 μg/mL) to the assay mixture with varying zymosan to cell ratios (2.5:1, 5:1, 10:1, 20:1 and 40:1). Therefore, there were 1.5 × 106, 3 × 106, 6 × 106, 1.2 × 107 and 2.4 × 107 zymosan particles in the assay (250 μL), while the number of cells remained constant (6 × 105 cells). The highest response for all four Ganoderma extract concentrations was observed for a zymosan:cell ratio of 10:1. Higher ratios did not lead to a significant difference, and lower ratios resulted in lower signal:noise ratios. This ratio of 10:1 was similar to those reported previously (Kournikakis & Simpson Citation1995; Hasegawa et al. Citation1997). The optimum signal:noise ratio was observed when we used 3 × 106 cells in each assay.
The slope of a line tangential to the initial part of the chemiluminescence curve was found to be the best parameter to describe the phagocytic effects. We also optimized the volume and concentration of serum needed for opsonization of zymosan and the number of granulocytes in the assay mixture.
Extracts affected granulocyte phagocytosis
Out of the 27 samples tested, 17 mushroom extracts affected phagocytosis (). However, in most cases, these effects were not significantly different from those in controls when considering the variation between inter-day replicates. This variation may be due to inter-individual differences as each day a different donor's blood sample used.
Significant (p < 0.05) positive effects on granulocyte phagocytosis were observed for five samples compared to non-treated controls. These five extracts were from A. fissilis, Trametes gibbosa, P. betulinus, N. lepideus and Polyporus squamosus and exhibited increase in phagocytic activity by 205, 181, 158, 155 and 141%, respectively, compared with untreated controls.
Extracts from other mushrooms, such as Postia stiptica, Panus conchatus and Polyporus brumalis, had positive effects on phagocytosis, which were not statistically significant (p < 0.05). All these prospective extracts exhibited maximum phagocytic activity in concentrations ranging from 125 to 250 μg/mL.
Some extracts suppressed phagocytosis, as indicated by a concentration-dependent decrease of luminescence. The effects were statistically significant (p < 0.01) compared with non-treated control and were most pronounced for P. schweinitzii, S. crispa, F. pinicola, P. lipsiensis and G. odoratum extracts. Examples of luminescence curves showing stimulatory and suppressive activities of extracts are displayed in .
Figure 1. Typical relative luminescence assay curves, showing activation (in case of Trametes gibbosa, Aurantiporus fissilis and Neolentinus lepideus) or suppression (Sparassis crispa) of neutrophil phagocytic response by selected polypore mushroom extracts at various concentrations. RLU, relative luminescence units.
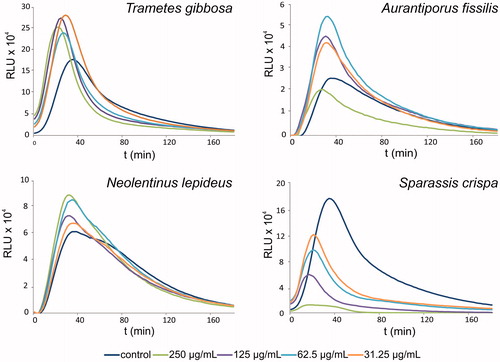
From the extracts used as reference or inter-day controls, ethanol extract of L. edodes reduced phagocytic activity by 44%. Ethanol extracts of G. lucidum did not appear to affect phagocytosis; however, its water extract was significantly more active, and increased phagocytic activity to 190% (p < 0.01) of the control. LPS alone used at 3–25 ng/mL resulted in a 16% increase in phagocytic activity.
Extracts affected cell viability
The effects of the extracts on Caco-2 and HT-29 cell viability are summarized in . Extracts of Daedalea quercina, M. tremellosus, P. betulinus, P. cinnabarinus and S. crispa were cytotoxic to HT-29 cells, with IC50 values of 60, 186, 73, 31 and 107 μg/mL, respectively. The extract of P cinnabarinus exhibited high levels of cytotoxicity against Caco-2 (IC50 value 31 ± 4 μg/mL) and HT-29 cells. LPS alone did not appear to exert any cytotoxic effects on either cell line within the concentration range tested. In most samples, a higher phagocytic index was accompanied by zero or low cytotoxicity against both cell lines, except in case of the P. betulinus extract.
Chemical analyses
All ethanol extracts that were screened contained β-glucans (0.3–120.2 mg/g) (). Extracts from G. odoratum and S. crispa were outliers, with their β-glucan content at the upper limits of the range while the extract of Postia stiptica contained trace amounts of β-glucan. Ethanol extracts were originally obtained for antioxidant activity screening, therefore, their β-glucan was expected to be low. Extracts with the highest effect on phagocytosis had a β-glucan content of 43–64 mg/g.
Table 3. Content of total β-glucans and total phenolics contents of ethanol extracts of selected polypore mushrooms.
Results from TPC assays () revealed the highest phenolic content in the F. pinicola extract (1982.3 μmol GAE/g of extract). Phenolic content of equal or above 500 μmol GAE/g of extract was associated with suppressed phagocytic response, as seen for G. odoratum, P. schweinitzii and P. lipsiensis extracts. Extracts with highest effect on phagocytosis had a TPC of 134–190 μmol GAE/g.
Discussion
Our findings revealed in vitro immunomodulatory activities for some Central European polypore species, which might be of interest for follow-up mycochemical research and to dietary supplement producers or the pharmaceutical industry. Polypore mushrooms represent a large pool of mycochemicals with prospective pharmaceutical properties, among them, polysaccharides, polysaccharide-protein complexes, triterpenoids, flavonoids and various pigments (Grienke et al. Citation2014).
Yet, many of these species are part of some food supplement compositions available globally, often based on evidence of their traditional use or presumable chemotaxonomic relations to more popular species, such as those used in traditional Chinese medicine. Many formulations are based on a simple ethanol extract. Ethanol-extractable constituents such as triterpenoids and flavonoids or their mixtures are often reported to have potent antioxidant (Macakova et al. Citation2010), anti-inflammatory (Yoshikawa et al. Citation2005), and immunomodulatory (Quang et al. Citation2006; Cyranka et al. Citation2011) activities.
Ethanol extracts from N. lepideus, P. betulinus, Polyporus squamosus, Trametes gibbosa and A. fissilis were found to be among the most potent in our study.
Neolentinus lepideus extract at the concentration of 250 μg/mL in the assay mixture caused a 50% increase in chemiluminescence. The extract of this edible mushroom (Jung et al. Citation2008) did not cause any toxicity to epithelial cells. Our proximate mycochemical analysis showed a higher content of β-glucans and relatively low total polyphenolic content. The exopolysaccharide extracted from the fruiting body has been previously reported to increase the expression of various cytokines and thus enhanced the immune response (Jin et al. Citation2003a; Choi et al. Citation2006) and increased lymphocyte proliferation (Jin et al. Citation2003b; Jung et al. Citation2008). Although ethanol extracts are relatively low in the polysaccharide fraction, and the use of this solvent may induce conformational changes in the polysaccharide complexes, it is likely that even such low content was recognized by the specific receptors on neutrophils resulting in increased chemiluminescence. Surprisingly, the related species L. edodes, which was used as a reference sample in our study, did not stimulate the phagocytosis but suppressed it even at concentrations as low as 62 μg/mL. As this species is widely recognized as an immune-regulating drug, this suppression might be ascribed to the nature of the solvent and presence of bioactive ethanol-extractable secondary metabolites. Polysaccharide from this mushroom is known to activate phagocytic response in macrophages both in vitro (Abel et al. Citation1989; Zheng et al. Citation2005) and in vivo (Vetvicka & Vetvickova Citation2014).
A positive effect on phagocytic response was also observed for the extract of P. betulinus, which increased the luminescence response by 58%. This extract was mildly toxic to HT-29 cell line while showing no toxicity below 500 μg/g to Caco-2 cells. The HT-29 cell line often appears more sensitive to secondary metabolites in toxicity tests according to our own results and published literature (Li et al. Citation2014; Doskočil et al. Citation2015). The ethnopharmacological importance of this mushroom has been highlighted after it was found among the equipment carried by the 5300-year-old body of the ice man Ötzi (Lemieszek et al. Citation2009), which led to speculations about its medicinal value and antimicrobial and immunomodulatory properties valued in prehistoric times. The effect of the polysaccharide fraction from this mushroom in a similar luminol-induced chemiluminescence assay conducted by Shamtsyan et al. (Citation2004) was in a comparable range; however, in their screening, this mushroom was among the least potent species. Phagocytosis is a complex process and the results obtained from this assay reflect the role of the constituents in chemotaxis, recognition by receptors on the phagocyte surface, effect on reactive oxygen species formation during respiratory burst and toxicity (Smith Citation1994). Complex mixtures may influence all these processes to different extents. The extracts of P. betulinus are rich in cytotoxic, antimicrobial and anti-inflammatory triterpenoids, lanostanes, and their esters (Lemieszek et al. Citation2009; Cyranka et al. Citation2011; Grienke et al. Citation2014) that might be responsible for mild toxicity against HT-29 cell line.
Polyporus squamosus is edible when young and soft. Injection of it mycelial mass alleviated allergic responses and reduced the proliferation of monocytes in vitro, indicating a potential immunosuppressive activity (Babakhin et al. Citation1997) and showed radical-scavenging properties (Elmastas et al. Citation2007). This mushroom contains lectins (Mo et al. Citation2000). To the best of our knowledge, no reports on further characterization of its β-glucan fraction and its properties that may contribute to this activity were found. Similarly, evidence of the presence of specific secondary metabolites is scarce. Even the polyphenol content was very low as determined by us, by 41.6% μmol GAE/g. The ethanol extract of this mushroom stimulated zymosan-induced phagocytosis in our assay by 41% without showing toxicity to epithelial cancer cells.
Trametes gibbosa was another potent species identified in the current study, which significantly increased zymosan-induced phagocytosis in our test by 81%. The ethanol extract analysis of this species revealed an average level of β-glucans (42.7 mg/g) and an average content of total polyphenols of 133 μmol GAE/g of extract. Literature suggests that injection of the polysaccharide fraction of this mushroom reduced carrageenan-induced inflammation and vessel permeability, while increasing the number of neutrophils and eosinophils and decreasing the number of lymphocytes in the blood (Czarnecki & Grzybek Citation1995).
The A. fissilis extract increased phagocytic activity to the greatest extent by 205% of the control at the concentration of 62 μg/mL and was superior to the water extract of G. lucidum. The extract showed potency at all concentrations tested and did not show any marked toxicity to Caco-2 or HT-29 cell line, with an IC50 value> 500 μg/mL. Both β-glucan- and polyphenol contents were in average values and thus, the quantity itself cannot explain the activity. Various lanostane triterpenoids have been isolated from this species and found to inhibit NO production in macrophages (Quang et al Citation2006). To the best of our knowledge, there are no data on immunomodulatory potency of this mushroom.
It appears that the most potent extracts with a positive effect on phagocytosis were among the weakest antioxidants reported in the previous screening conducted by part of our team (Macakova et al. Citation2010). Compounds in complex mixtures, such as crude ethanol extracts, might be involved in various interactions. For example, antioxidants or nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitors might inhibit the release of reactive oxygen species during the phagocytic response to external stimuli (Busse et al. Citation1984; Kanashiro et al. Citation2004), leading to false-negative results, whereas weak antioxidant activity led to a clear positive effect on phagocytosis. High radical-scavenging activity is likely to explain the suppression of phagocytosis in C. gallica, L. sulphureus, G. odoratum, D. confragosa, P lipsiensis and P. schweinitzii extracts. The latter three were among the most potent antioxidant extracts in the study of Macakova et al. (Citation2010) while in our assays, these extracts suppressed neutrophil phagocytosis by 15–74%. They were still virtually non-toxic to Caco-2 and HT-29 cells, with IC50 value> 500 μg/mL. Most of them were also relatively high in TPC. We suggest that these species should be further examined in the search for novel pharmacologically active NADPH oxidase inhibitors or antioxidants; however, simple toxicity in the neutrophil granulocytes used cannot be disproved.
Our findings contribute to the understanding of the complex mode of action of these species in immune-modulation. The neutrophil granulocytes used in our assays are motile cells capable of phagocytosis, killing and digesting invading micro-organisms and play a critical role in the intestinal immune system. In gastrointestinal disorders such as Crohn's disease and ulcerative colitis, neutrophil failure probably plays a role in the etiopathogenesis, as their respiratory burst activity in patients with these conditions is significantly below those in controls (Levine & Segal Citation2013). Although the causal role has not been established, this might be a possible experimental target.
Caco-2 cell culture is one of the most relevant in vitro models to study differentiation and regulation of intestinal functions and is commonly used to assess intestinal toxicity (Jumarie & Malo Citation1991; Twiss et al. Citation1999). The model is of high physiological relevance as intestinal cells are in contact with parent nutrients or xenobiotics present in the intestinal lumen, avoiding the need for considering their metabolic fate. Additionally, HT-29 cell line with a lower degree of differentiation was used, as this cell line is often used in intestinal models alongside with Caco-2 (Volštátová et al. Citation2015) and shows higher sensitivity. In our study, we found several extracts with potent immune function that simultaneously exhibit low or no toxicity in the intestinal cells ().
A relationship between the total β-glucan content and TPC was not observed in this study, suggesting that the content of constituents vary in structure and activity. As indicated above, the solvents used for extraction determine the content of active ingredients as observed in the reference extracts of G. lucidum. The results show an expectedly superior effect of β-glucan-rich water extracts on phagocytosis, when compared with ethanol extracts. The content of total β-glucans in this reference extract was approximately three-times higher than that in the ethanol extract.
The screening we have presented here is a preliminary report on the immunomodulatory activities of ethanol extracts of Central European polypores. Some of them potentiated the phagocytosis of neutrophils (N. lepideus, Polyporus squamosus, Trametes gibbosa, and A. fissilis), with low levels of toxicity to intestinal cells and might be candidates for further research in treatment of autoimmune disorders of gastrointestinal tract.
Conclusion
In conclusion, a systematic screening of the immunomodulatory properties of Central European polypores was conducted, which expands our previous work on Central European Basidiomycota. The results might be of interest to research concerning inflammatory bowel disease and may provide a basis for their use as food supplements.
Funding information
This research was supported by the Internal Grant Agency of the Czech University of Life Sciences Prague (Projects nos. CIGA 20142012 and CIGA 20132035) and by the COST CZ (Project no. LD14070) funded by the Ministry of Education, Youth, and Sports (Czech Republic).
Disclosure statement
All authors declare that they have no financial or personal relationships with other people or organizations that could inappropriately influence their work.
References
- Abel G, Szollosi J, Chihara G, Fachet J. 1989. Effect of lentinan and mannan on phagocytosis of fluorescent latex microbeads by mouse peritoneal macrophages: a flow cytometric study. Int J Immunopharmacol. 11:615–621.
- Babakhin AA, Majoul LA, Vedernikov AA, Leskov VP, Pisarev VM, Babakhin AA, Logina NYu, Gushchin IS, Nolte H, DuBuske LM. 1997. In vivo and in vitro immunomodulation induced by the extract of the mycelium fungus Polyporus squamosus. Allergy Asthma Proc. 18:301–310.
- Barros L, Calhelha RC, Vaz JA, Ferreira ICFR, Baptista P, Estevinho LM. 2007. Antimicrobial activity and bioactive compounds of Portuguese wild edible mushrooms methanolic extracts. Eur Food Res Technol. 225:151–156.
- Brown GD, Gordon S. 2003. Fungal beta-glucans and mammalian immunity. Immunity. 19:311–315.
- Busse WW, Kopp DE, Elliott M. 1984. Flavonoid modulation of human neutrophil function. J Allergy Clin Immunol. 73:801–809.
- Choi JJ, Jin MR, Lee JK, Lee WY, Park YI, Han YN, Kim S. 2006. Control of cytokine gene expression by PG101, a water-soluble extract prepared from Lentinus lepideus. Biochem Biophys Res Commun. 339:880–887.
- Cyranka M, Graz M, Kaczor J, Kandefer-Szerszen M, Walczak K, Kapka-Skrzypczak K, Rzeski W. 2011. Investigation of antiproliferative effect of ether and ethanol extracts of birch polypore medicinal mushroom, Piptoporus betulinus (Bull.: Fr.) P. Karst. (higher Basidiomycetes) in vitro grown mycelium. Bull.: Fr.). Karst (Basidiomycetes in vitro mycelium. Int J Med Mushrooms. 13:525–533.
- Czarnecki R, Grzybek J. 1995. Antiinflammatory and vasoprotective activities of polysaccharides isolated from fruit bodies of higher Fungi P. 1. polysaccharides from Trametes gibbosa (Pers.: Fr) Fr. (Polyporaceae). Phytother Res. 9:123–127.
- Doskočil I, Hošťálková A, Šafratová M, Benešová N, Havlík J, Havelek R, Kuneš J, Královec K, Chlebek J, Cahlíková L. 2015. Cytotoxic activities of Amaryllidaceae alkaloids against gastrointestinal cancer cells. Phytochem Lett. 13:394–398.
- Elmastas M, Isildak O, Turkekul I, Temur N. 2007. Determination of antioxidant activity and antioxidant compounds in wild edible mushrooms. J Food Compost Anal. 20:337–345.
- Grienke U, Zöll M, Peintner U, Rollinger JM. 2014. European medicinal polypores – a modern view on traditional uses. J Ethnopharmacol. 154:564–583.
- Hanaoka R, Ueno Y, Tanaka S, Nagai K, Onitake T, Yoshioka K, Chayama K. 2011. The water-soluble extract from cultured medium of Ganoderma lucidum (Reishi) mycelia (designated as MAK) ameliorates murine colitis induced by trinitrobenzene sulphonic acid. Scand J Immunol. 74:454–462.
- Hasegawa H, Suzuki K, Nakaji S, Sugawara K. 1997. Analysis and assessment of the capacity of neutrophils to produce reactive oxygen species in a 96-well microplate format using lucigenin-and luminol-dependent chemiluminescence. J Immunol Methods. 210:1–10.
- Jin M, Jung HJ, Choi JJ, Jeon H, Oh JH, Kim B, Shin SS, Lee JK, Yoon K, Kim S. 2003a. Activation of selective transcription factors and cytokines by water-soluble extract from Lentinus lepideus. Exp Biol Med (Maywood). 228:749–758.
- Jin M, Jung HJ, Shin SS, Jeon H, Choi JJ, Kim S. 2003b. Extract of Lentinus lepideus and composition comprising the same having immune enhancing activity. U.S.Patent Application 10,383,773.
- Jumarie C, Malo C. 1991. Caco-2 cells cultured in serum-free medium as a model for the study of enterocytic differentiation in vitro. J Cell Physiol. 149:24–33.
- Jung YS, Yang BK, Jeong YT, Islam R, Kim SM, Song CH. 2008. Immunomodulating activities of water-soluble exopolysaccharides obtained from submerged culture of Lentinus lepideus. J Microbiol Biotechnol. 18:1431–1438.
- Kanashiro A, Kabeya LM, Polizello ACM, Lopes NP, Lopes JL, Lucisano-Valim YM. 2004. Inhibitory activity of flavonoids from Lychnophora sp. on generation of reactive oxygen species by neutrophils upon stimulation by immune complexes. Phytother Res. 18:61–65.
- Kashimoto N, Ishii S, Myojin Y, Ushijima M, Hayama M, Watanabe H. 2010. A water-soluble extract from cultured medium of Ganoderma lucidum (Reishi) mycelia attenuates the small intestinal injury induced by anti-cancer drugs. Oncol Lett. 1:63–68.
- Ko KM, Leung HY. 2007. Enhancement of ATP generation capacity, antioxidant activity and immunomodulatory activities by Chinese Yang and Yin tonifying herbs. Chin Med. 2:3.
- Kournikakis B, Simpson M. 1995. Optimization of a phagocyte microplate chemiluminescent assay. J Biolumin Chemilumin. 10:63–67.
- Lemieszek MK, Langner E, Kaczor J, Kandefer-Szerszen M, Sanecka B, Mazurkiewicz W, Rzeski W. 2009. Anticancer effect of fraction isolated from medicinal birch polypore mushroom, Piptoporus betulinus (Bull.: Fr.) P. Karst.(Aphyllophoromycetideae): in vitro studies. Int J Med Mushrooms. 11:351–364.
- Levine AP, Segal AW. 2013. What is wrong with granulocytes in inflammatory bowel diseases? Dig Dis. 31:321–327.
- Li G, Yu K, Li F, Xu K, Li J, He S, Cao S, Tan G. 2014. Anticancer potential of Hericium erinaceus extracts against human gastrointestinal cancers. J Ethnopharmacol. 153:521–530.
- Macakova K, Opletal L, Polasek M, Samková V. 2010. Free-radical scavenging activity of some European Polyporales. Nat Prod Commun. 5:923–926.
- Macakova K, Opletal L, Polasek M, Samková V, Jahodár L. 2009. Free-radical scavenging activity of some European Boletales. Nat Prod Commun. 4:261–264.
- Mo H, Winter HC, Goldstein IJ. 2000. Purification and characterization of a Neu5Acalpha2-6Galbeta1-4Glc/GlcNAc-specific lectin from the fruiting body of the polypore mushroom Polyporus squamosus. J Biol Chem. 275:10623–10629.
- Mosman T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 65:55–63.
- Quang DN, Harinantenaina L, Nishizawa T, Hasimoto T, Kohchi Ch, Soma GI, Asakawa Y. 2006. Inhibitory activity of nitric oxide production in RAW 264.7 cells of daldinals A–C from the fungus Daldinia childiae and other metabolites isolated from inedible mushrooms. J Nat Med. 60:303–307.
- Shamtsyan M, Konusova V, Maksimova Y, Goloshchev A, Panchenko A, Simbirtsev A, Petrishchev N, Denisova N. 2004. Immunomodulating and anti-tumor action of extracts of several mushrooms. J Biotechnol. 113:77–83.
- Singleton VL, Orthofer R, Lamuela-Raventos RM. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 299:152–178.
- Smith JA. 1994. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol. 56:672–686.
- Tomasi S. 2008. Mushroom secondary metabolites and cancer. In: Watson RR, Preedy VR, editors. Botanical medicine in clinical practice. Wallingford: CABI. pp. 301–320.
- Twiss IM, Pas O, Ramp‐Koopmanschap W, Den Hartigh J, Vermeij P. 1999. The effects of nitrogen-containing bisphosphonates on human epithelial (Caco-2) cells, an in vitro model for intestinal epithelium. J Bone Miner Res. 14:784–791.
- Tzianabos AO. 2000. Polysaccharide immunomodulators as therapeutic agents: structural aspects and biologic function. Clin Microbiol Rev. 13:523–533.
- Vetvicka V, Vetvickova J. 2010. β1,3-Glucan: silver bullet or hot air? Open Glycosci. 3:1–6.
- Vetvicka V, Vetvickova J. 2014. Immune-enhancing effects of Maitake (Grifola frondosa) and Shiitake (Lentinula edodes) extracts. Ann Transl Med. 2:14–16.
- Volštátová T, Havlík J, Doskočil I, Geigerovaacute M, Rada V. 2015. Effect of hydrolyzed milk on the adhesion of lactobacilli to intestinal cells. Sci Agric Bohem. 46:21–25.
- Wagner H, Jurcic K. 1991. Assays for immunomodulation and effects on mediators of inflammation. In: Hostettmann K, editor. Methods in plant biochemistry: vol. 6. Assays for bioactivity. London: Academic Press Ltd. p. 117–126.
- Wasser S. 2002. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol. 60:258–274.
- Yoshikawa K, Inoue M, Matsumoto C, Sakakibara C, Miyataka H, Matsumoto H, Arihara S. 2005. Lanostane tritepenoids and triterpene glycosides from the fruit body of Fomitopsis pinicola and their inhibitory activity against COX-1 and COX-2. J Nat Prod. 68:69–73.
- Zheng R, Jie S, Hanchuan D, Moucheng W. 2005. Characterization and immunomodulating activities of polysaccharide from Lentinus edodes. Int Immunopharmacol. 5:811–820.
