Abstract
Context: Potentilla fulgens Wall. ex Hook (Rosaceae) is a potent medicinal plant of the Western Himalayas, where its roots are traditionally used by the local people of Uttaranchal (India) to treat wounds and tiger bites.
Objective: The present study scientifically evaluates the wound healing activity of P. fulgens ethanol root extract (EPF) and its ethyl acetate fraction (PFEA) on experimental rats.
Materials and methods: Wounds were inflicted on animals by using both excision and incision models. The wounded animals were treated for 16 days with EPF (oral: 200–400 mg/kg and topical: 5–10% w/w) and PFEA (oral: 75 mg/kg; topical: 1.75% w/w). Various physical (wound contraction, epithelialization rate, tensile strength) and biochemical parameters (hydroxyproline, hexosamine, proteins, DNA) were examined during the study. Oxidant product (lipidperoxidase), antioxidant enzymes (catalase, superoxide-dismutase) and reduced glutathione were determined. Morphological and histopathological studies of the skin tissues were monitored.
Results: A significant (p < 0.05) wound healing property was observed when the animals were treated topically with EPF (10% w/w) and PFEA (1.75% w/w). A significantly (p < 0.05) increased in the levels of hydroxyproline, hexosamine, protein and DNA up to 59.22, 70.42, 61.01 and 60.00% was observed, respectively. This effect was further demonstrated by the morphological and histopathological representation, thus showing significant (p < 0.05) re-epethelialization on the healing area. EPF and PFEA also showed significant (p < 0.05) antioxidant activity.
Conclusions: The present study provided the scientific evidence, where P. fulgens rich in polyphenolic components possess remarkable wound healing activities, thereby supporting the traditional claims.
Introduction
A wound is characterized by the loss of epithelial integrity, disruption of normal structure and function of the skin and its underlying tissues (Senthil et al. Citation2006; Friedman Citation2011). Specific immune responses of the body are responsible for the healing of wound, involving inflammatory cells, cytokines and extracellular matrix components. The whole process of wound healing can be categorized into three overlapping phases, viz., inflammation (0–3 days), cellular proliferation (3–12 days) and tissue remodelling (3–6 months) (Karodi et al. Citation2009). The inflammatory phase involves recruitment of leukocytes (neutrophils and macrophages) at the site of injury. The proliferative phase is characterized by the migration and proliferation of keratinocytes and fibroblasts, collagen deposition, angiogenesis, epithelialization, tissue granulation and wound contraction. Remodelling phase involves the degradation of excess collagen in the wound by several proteolytic enzymes, leading to the completion of tissue repair (Emami-Razavi et al. Citation2006; Kumar et al. Citation2006). Any agent which accelerates the above processes is regarded as a promoter of wound healing process. Plant products are considered to be potential agents for healing of wounds and this kind of natural therapy is preferred owing to their widespread availability, reduced toxicity on dermal cells, ease of administration and effectiveness even as crude preparations (Nunes et al. Citation2014). In addition, approximately one-third of all the medicines used for the treatment of wounds and skin disorders were obtained from plant sources, while only 1–3% was obtained synthetically (Mantle et al. Citation2001). Hence, such evidence signifies a clear indication of the essential role of medicinal plants as therapeutic alternatives to conventional medicine.
Potentilla fulgens Wall. ex Hook (Rosaceae) is a short, slender herb which is commonly used as medicine by various tribes of the Northern and North-Eastern states of India. It is commonly called ‘Himalayan Cinquefoil’ (English), ‘Bajradanti’ (Assamese and Hindi) and ‘Lynniangbru’ (Khasi tribes of Meghalaya). The roots are used traditionally to treat various diseases, ailments such as gastric ulcer, mouth ulcers, diabetes, diarrhea, gingivitis and also to cure mouth and gum problems (Laloo, Prasad, et al. Citation2013). In northern parts of India (Uttarakhand), the roots of the plant are commonly used to treat wounds and tiger bites (Kaul et al. Citation2011). Hence, based on the evidence of such traditional practice as medicine, the present study was designed to scientifically validate the wound healing efficacy of topical and oral administration of P. fulgens root extract and its polyphenolic rich fraction in experimental rats.
Materials and methods
Plant extract, fractionation and chemical standardization
The plant material (root portion) was collected during daytime in the month of May–July 2010 from the Shillong region of the East Khasi Hills District, Meghalaya, Northeast India. The botanical authentication of the specimen was done by Dr. N. Odyuo (Scientist C), Botanical Survey of India, Shillong, Meghalaya (Letter no.: BSI/ERC/2010/Plant identification/281). The extraction procedure and chemical standardization (by HPTLC) of P. fulgens ethanol root extract (EPF) was performed in our previous study (Laloo, Prasad, et al., Citation2013). Extraction of the crude drug (1 kg) was done exhaustively with 95% ethanol (3.0 L) using Soxhlet apparatus (Sigma Aldrich Ltd., Mumbai, India) for 72 h. The percentage yield of the ethanol extract (EPF) after concentrated under reduced pressure was found to be 32.9% w/w. The qualitative and quantitative phytochemical evaluation of EPF extract has been reported in our previous study (Laloo, Kumar, et al. Citation2013).
EPF was subjected to fractionation, by suspending the EPF (100 g) in water (1.0 L) and partitioned with ethyl acetate (1.0 L), thereby yielding the ethyl acetate soluble fraction (PFEA). The resulting PFEA was filtered, concentrated under reduced pressure using a rotary evaporator (Perfit Pvt. Ltd., Haryana, India) and kept in desiccator until used. Preliminary phytochemical screening (Trease & Evans Citation2002) of PFEA and quantification of total phenolics and tannins content (Makkar Citation2000) were also determined.
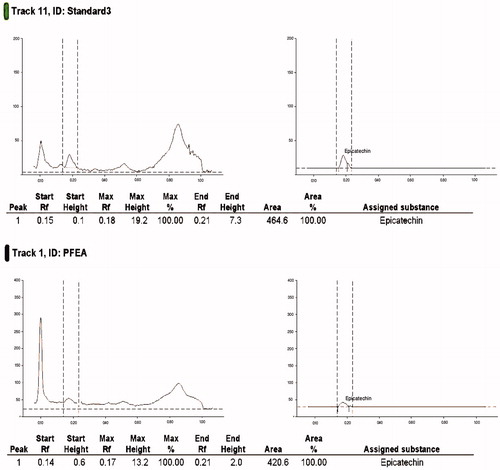
Chemical standardization of EPF for (−)-epicatechin was reported in our previous study using HPTLC technique, where the amount of (−)-epicatechin present in the EPF was reported to be 9.52% w/w (Laloo, Prasad, et al. Citation2013). In the present study, the standardization of the PFEA, rich in polyphenolic components was also examined by HPTLC using (−)-epicatechin as the chemical marker. Stock solutions of both PFEA and standard (−)-epicatechin were prepared in methanol in concentrations of 2.0 and 0.2 mg/mL, respectively. The mobile phase of developing the chromatogram was composed of the mixture of chloroform, acetone and formic acid in the ratio of 130:53:17 (v/v/v). The study was carried out using a Camag HPTLC instrumentation (Anchrom Pvt. Ltd., Mumbai, India) equipped with a Linomat V sample applicator, Camag TLC scanner 3, Camag TLC visualizer and WINCATS 4 software for data interpretation. The Rf values were recorded and the developed plate was screened and photo documented at wavelength (λmax) of 366 nm.
Experimental setup and drug treatment protocol
Inbred Charles-Foster albino rats of either sex (170 ± 10 g) were used for the study after receiving approval from the Institutional Animal Ethical Committee, Institute of Medical Sciences, Banaras Hindu University (Approval letter no. Dean/13-14/CAEA/313). The rats were housed under standard laboratory conditions and were fed with commercial rat feed (Amrut Pvt. Ltd., Pune, India) and water ad libitum. The animals were divided into 12 groups with six animals in each group (n = 6) as shown below. All the standard and tested drugs were administered to the animal for a period of 16 days (Prasad et al. Citation2010). Carboxy methyl cellulose (CMC; 0.5% w/v, p.o.) was used as a suspending agent for oral administration, whereas ointment base was used for topical administration of the tested extract, fractions and reference drug. Animals were fasted for 18 h (with free access to water ad libitum) prior to the commencement of the experiment.
Group I: animals served as a positive control group (untreated group).
Group II: animals (CMC treated group) received 0.5% w/v (p.o.) CMC devoid of extract/fraction.
Group III: animals (ointment base treated group) received 1 g topical administration of the ointment base devoid of extract/fraction.
Group IV: animals (standard control group, VTC 200) received oral administration of vitamin C (Sigma Aldrich Ltd., Mumbai, India) at a dose of 200 mg/kg, suspended in 0.5% w/v CMC (Kamer et al. Citation2010).
Group V: animals (EPF 200) received oral administration of EPF at a dose of 200 mg/kg, suspended in 0.5% w/v CMC.
Group VI: animals (EPF 400) received oral administration of EPF at a dose of 400 mg/kg, suspended in 0.5% w/v CMC.
Group VII: animals (PFEA 75) received oral administration of PFEA at a dose of 75 mg/kg, suspended in 0.5% w/v CMC.
Group VIII: animals (VTC 10%) received 1 g topical administration of vitamin C 10% (Lima et al. Citation2009).
Group IX: animals (EPF 5%) received 1 g topical administration of the EPF ointment (5% w/w).
Group X: animals (EPF 10%) received 1 g topical administration of the EPF ointment (10% w/w).
Group XI: animals (PFEA 1.75%) received 1 g topical administration of the PFEA ointment (1.75% w/w).
Group XII: animals served as a normal control group.
Acute oral toxicity and acute dermal toxicity studies
Based on the results reported in our previous study, the toxicity profile of the EPF in experimental rats has been performed, and was found to be safe up to 4000 mg/kg, p.o. (Laloo, Prasad, et al. Citation2013). In the present study, the acute oral toxicity study of PFEA was again performed following the methods of the Organization for Economic Co-operation and Development guideline no. 425 (Acute oral toxicity Citation2008). A single dose administration of PFEA (2000 mg/kg, p.o., suspended in 0.5% w/v CMC) was given to the overnight fasted rats. The toxicity signs and symptoms or any abnormalities associated with the administration of PFEA were observed at 0, 30, 60, 120, 180 and 240 min and then once a day for the next 14 days. The number of rats that survived was recorded at the end of the study period.
The acute dermal toxicity testing of the tested drugs was performed by applying the ointments containing EPF (10%) and PFEA (5%), on the shaved back of the rats. The animals were then monitored for any sign of dermal toxicity as per the methods described in OCED guideline no. 402 (Acute dermal toxicity Citation1987).
Dose optimization and preparation of suspension and ointment base
In the present investigation, the wound healing activity in rats was evaluated by both oral and topical administration of EPF extract and its ethyl acetate fraction rich in polyphenolics (PFEA). The dose selection for evaluating the wound healing activity of EPF was examined at 200 and 400 mg/kg, p.o., based from the previous data reported on acute oral toxicity and a pilot study (Laloo, Prasad, et al. Citation2013). In our previous study, we have successfully evaluated the potential of EPF in the treatment of gastric ulcer wounds and it is well known that gastric ulcer is a type of deep wound that involves epithelium, connective tissue and smooth muscle (Chai Citation2011). Therefore, in the present study, an attempt was designed to evaluate the effects of EPF and its ethyl acetate fraction (EAF) on an external wound in experimental rats. For the oral dose optimization of PFEA fraction, the dose was calculated based on the individual percentage yield of PFEA fraction (35.67% w/w) calculated in terms of the minimum effective dose of EPF extract (200 mg/kg, p.o.) that produced significant ulcer-wound healing property. Hence, in the present study, the oral administration of all the tested drugs was prepared by suspending EPF (200 and 400 mg/kg, p.o.), PFEA fraction (75 mg/kg, p.o.) and reference drug vitamin C (200 mg/kg, p.o.) in an aqueous suspension of 0.5% w/v CMC. However, for topical application, all the tested drugs (EPF, PFEA and standard reference) were applied externally in the form of ointments. Ointment base (100 g) was prepared as per the methods described in the British Pharmacopoeia (British Pharmacopoeia Citation1980), by melting and mixing the ingredients (wool fat 5 g, hard paraffin 5 g, cetostearyl alcohol 5 g and soft white paraffin 85 g) in a beaker at 65 °C on water bath. The mixture was removed from the heat, which was gently stirred and homogenized until cold. The ointment base was used for the preparation of different formulations containing the active ingredients separately composing of standard drug reference, EPF extract and its polyphenolic rich fraction. Using the ointment base, EPF extract (5% and 10%, w/w), PFEA fraction (1.75% w/w) and reference drug, vitamin C (10% w/w) was accessed for the wound healing study.
Wound healing activity
Excision wound model
The animals were divided into 12 groups with six animals in each group (n = 6) and were anaesthetized by open mask method with 3–5% light ether anaesthesia, before the creation of the wound. The particular skin area was shaved 1 day prior to the experiment. An excision wound was inflicted by cutting away the skin measuring 300 mm2 full thickness and 2 mm depth from a predetermined shaved area (Nayak et al. Citation2007). The wounds were left undressed to the open environment and the animals were carefully observed for any infection and those that showed any sign of infection were separated, excluded from the study and replaced. Haemostasis was achieved by blotting the wound with a cotton swab soaked in normal saline solution. The wounded animals were housed separately in different cages. Wound area was measured instantly by placing a transparent tracing paper over the wound and tracing it out (Nayak et al. Citation2007; Suntar et al. Citation2010). The tracing paper was then placed on a 1-mm2 graph sheet and traced out. The wound area was measured out on respective days (0th, 4th, 8th and 12th and 16th day) and the percentage wound contraction was calculated as shown below.
The period of epithelialization was calculated as the number of days required for falling off of the dead tissue remnants without any residual raw wound (Gutierrez & Vargas Citation2006). For the determination of epithelialization period, animals (n = 6) were divided into 12 separate groups in a similar way as shown above in the excision wound model.
Incision wound model
In this model, all the animals were grouped in the same manner as per the excision wound model describe above. The animals were anaesthetized with ketamine hydrochloride (100 mg/kg) prior to and during the creation of experimental wounds. The dorsal fur of the animals was properly shaved and a paravertebral long incision of 4 cm length was made through the skin at a distance of about 1.5 cm from the midline on each side of the depilated back of the animals. After the incision, the wounded skin was closed by means of interrupted sutures at intervals of 0.5 cm using surgical thread (No. 000) and curved needle (No. 11). The wounds were then left undressed. Sutures were removed on seventh post-wounding day and the treatment was continued (Perez et al. Citation2005; Krishnaveni et al. Citation2009). The wound breaking strength was measured on the 10th day as per the methods of Mukherjee et al. (Citation2003). The breaking strength is defined as the strength of a healing wound which is measured by the amount of force required to disrupt it
Biochemical estimations
All the biochemical parameters were estimated on the 16th post-wounded day by obtaining the granulation tissue samples from the excision wound model. Approximately 250 mg of the wet granulation tissue was dried at 50 °C for 24 h. The dried tissues were weighed and kept in glass stoppered test tubes. Dried tissue was subsequently used for the estimation of various important biochemical parameters such as protein (Lowry et al. Citation1951), DNA (Burton Citation1956) and connective tissue parameters such as hexosamine (Dische & Borenfreund Citation1950) and collagen (hydroxyproline) content. The collagen content was estimated as per the methods of Neuman and Logan (Citation1950). About 30 mg of the dried tissue was hydrolysed in 6 N HCl for 24 h at 110 °C in sealed glass tubes. The hydrolysate was neutralized to pH 7.0. The hydrolysed samples were mixed properly with 1 mL of 0.01 M CuSO4 followed by the addition of 1 mL of 2.5 N NaOH and 1 mL of 6% H2O2. The solution was mixed and shaken occasionally for 5 min. All the tubes were incubated at 80 °C for 5 min with frequent vigorous shaking. Upon cooling, 4 mL of 3 N H2SO4 was added with agitation. Finally, 2 mL of 5% p-dimethylaminobenzaldehyde was added. The samples were incubated at 70 °C for 15 min, cooled by placing the tubes in water at 20 °C and the absorbance was measured at 500 nm using a UV–vis spectrophotometer (Shimadzu Pvt., Ltd., Mumbai, India). The standard calibration curve was plotted for hydroxyproline and was used for the estimation of the test samples.
In the present study, evaluation of the antioxidant activity was also determined on the granulation tissue of the excision wound animals. Tissue was homogenized for 30 s in phosphate buffer solution, which was centrifuged initially at 800g for 10 min and later at 12,000g for 15 min. The LPO level was estimated in terms of malondialdehyde (MDA) which was determined as per the methods of Ohkawa et al. (Citation1979). SOD was determined as per the methods of Kakkar et al. (Citation1984). The results were expressed as units (U) of SOD activity/g tissue. The decomposition of hydrogen peroxide (H2O2) in the presence of catalase enzyme was estimated as per the methods of Beers and Sizer (Citation1952). The results obtained are expressed as units (U) of CAT activity/g of tissue. GSH level was estimated following the method of Sedlak and Lindsay (Citation1968). Reduced glutathione was used for generating the standard calibration curve. The results were expressed as μg of GSH/g of tissue.
Histopathological studies
On 16th post-operative day, a fraction of the excision skin tissues from all the normal, control, standard and treated animals were processed for the histopathological examination. All the samples were fixed in 10% buffered formalin, blocked with paraffin and then sectioned into 5 μm thickness. All the selected sections were processed and stained with haematoxylin and eosin (H–E) dye (Murthy et al. Citation2013). The stained tissues were examined and photographed using Nikon digital microscope (Eclipse 200, Nikon Instruments Inc., Melville, NY).
Statistical analysis
All the values of the experimental results were expressed as mean ± standard error of mean (SEM). For the evaluation of wound area from post-operative day 4–16, analysis of variance (ANOVA) was done, statistically by two-way ANOVA followed by Bonferroni’s post-test for multiple comparisons between groups. For the evaluation of biochemical parameters, one-way ANOVA followed by Tukey’s post-test for multiple comparisons between groups was incorporated. GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA) was used for all statistical analysis. A difference in the mean values of p < 0.05 was considered to be statistically significant.
Results
Phytochemical evaluation
Phytochemical evaluation of EPF has been reported by us in our previous study (Laloo, Kumar, et al. Citation2013; Laloo, Prasad, et al. Citation2013) and showed the presence of a maximum content of phenolics (17.7% w/w) and tannins (11.5% w/w) with traces of flavonoids (2.14% w/w), saponins (4.12% w/w) and carbohydrates (5.26% w/w). Fractionation of EPF with ethyl acetate produces PFEA with a yield of 37.56% w/w EPF extract. Phytochemical screening of the PFEA justifies the presence of polyphenolic content, where the quantitative estimation of PFEA showed the presence of phenolics (226.8 ± 10.5 mg/g, tannic acid equivalent) and tannins (127.0 ± 7.4 mg/g, tannic acid equivalent). The HPTLC quantification of (−)-epicatechin in PFEA was analysed and was found to contain 13.00% w/w ().
Acute oral toxicity and acute dermal toxicity studies
Oral administration of PFEA at 2000 mg/kg, p.o. did not elicit any behavioural signs or symptoms of toxicity up to 14 days. Hence, the drug was found to be safe up to 2000 mg/kg, p.o. However, topical application of EPF and PFEA for acute dermal toxicity testing showed the safety profile of the tested drugs, where there was no any visible sign of skin irritation, inflammation, swelling or any other change on the skin was observed. Therefore, both the oral and topical applications of EPF and PFEA were found to be safe for the present study.
Wound healing activity
Groups treated with simple ointment base (topical) and 0.5% w/v CMC (oral) showed no significant difference (p < 0.05) in the wound contraction area when compared to the positive control group (untreated group). However, treatment of the animals orally with VTC 200, EPF 200, EPF 400 and PFEA 75 on day 4, day 8, day 12 and day 16 depicted a significant (p < 0.05) reduction in the wounded area when compared to their respective untreated control group (positive control, CMC control and ointment base control). The similar but improved healing effect was also observed when the wounded rats were topically treated with VTC 10%, EPF 5%, EPF 10% and PFEA 1.75% ointment. However, when the percentage wound contraction was taken into account, it was observed that there was a marked contraction in the wounded area from day 12 to day 16, with EPF 10% and VTC 10% showing improved healing property. The rate of epithelialization (in days) was also examined where the rats treated topically with EPF 10% w/w, showed significant (p < 0.05) reformation of epithelial cells as compared to other tested groups. The wounded area (in mm2), percentage wound contraction and epithelialization period are represented in and .
Figure 2. Morphological representation of wound contraction on control, standard and tested groups treated orally and topically with vitamin C, EPF and PFEA from 0th to 16th day excision wound model.
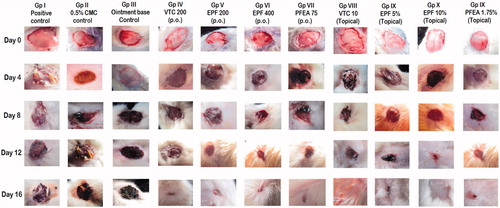
Table 1. Effect of oral and topical treatment of EPF, PFEA and standard VTC on wound contraction in 16 days excision wound model.
In incision wounded model, rats treated orally (VTC 200 and EPF 400) and topically (VTC 10%, EPF 5%, EPF 10% and PFEA 1.75%) showed significant (p < 0.05) increased on the tensile strength expressed in terms of gram, whereas no effect was observed with oral treatment of EPF 200 and PFEA 75. The percentage increase in tensile strength is represented in , signifying that the topically treated rats showed better activity as compared to the orally treated groups.
Table 2. Effect of oral and topical treatment of EPF, PFEA and standard VTC on the tensile strength of incision wound model.
Biochemical estimations
Oral administration of the VTC 200, EPF 400 and PFEA 75 for 16 days when compared to the positive control group showed significant (p < 0.05) increase in the level of hydroxyproline by 44.37%, 45.09% and 36.12%, respectively. No significant effect was observed when the rats were treated with EPF 200 (p.o.). Topical treatment of standard drug (VTC 10%) and tested ointments (EPF 10%, EPF 5% and PFEA 1.75%) for 16 days when compared to the positive control group showed a twofold increase in the level of hydroxyproline. The levels of hexosamine were also found to be increased on the 16th day with oral (VTC 200 and EPF 400) and topical treated groups (VTC 10%, EPF 5%, EPF 10% and PFEA 1.75%). However, oral administration of EPF 200 and PFEA 75 was found to be ineffective ().
Table 3. Effect of oral and topical treatment of EPF, PFEA and standard VTC on levels of hydroxyproline, hexosamine, protein and DNA in 16 days excision wound model.
A significantly (p < 0.05) increase in the protein level was observed with the oral and topical tested groups. In addition to the protein levels, a two- to three-fold increase in the level of DNA was also observed with oral (VTC 200, EPF 400) and topical treated groups (VTC 10%, EPF 10% and PFEA 1.75%), whereas EPF 200 (p.o.) and PFEA 75 (p.o.) produced no significant effect on the DNA levels of the tested animals ().
A significantly (p < 0.05) increase in LPO levels (58.82%) and a significantly (p < 0.05) decrease in SOD (73.97%), CAT (64.66%), GSH (45.68%) levels were observed in the positive control group when compared to the normal control group. Treatment of the animals with VTC 200, EPF 400 and PFEA 75 for 16 days when compared to the positive control group showed significant (p < 0.05) restoration in the levels of oxidant substance (LPO) and antioxidant enzymes (SOD, CAT) and GSH. However, the lower dose of EPF 200 (p.o.) was found to be ineffective. In contrast to the oral application, animal treated topically with standard VTC 10%, EPF 5%, EPF 10% and PFEA 1.75% showed even better results in the restoration of the oxidant and antioxidant parameters with the significant (p < 0.05) antioxidant effect as seen with EPF 10% ().
Table 4. Effect of oral and topical treatment of EPF, PFEA and standard VTC on the levels of LPO, SOD, CAT and GSH in 16 days excision wound model.
Histopathological studies
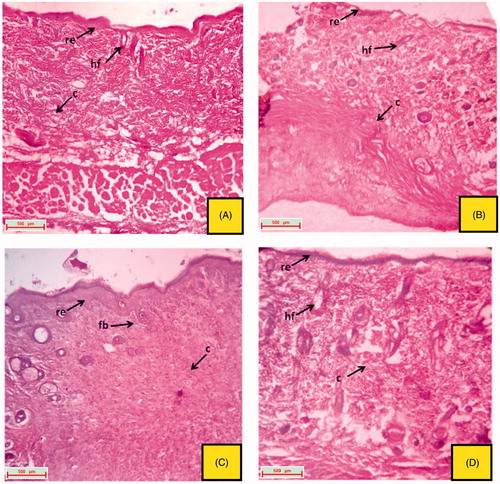
The excised tissue obtained from the normal control group depicted a well organized epithelial cells having well characterize outer epidermis. Normal formation of fibroblast cells and collagen fibres was also observed (). The excision tissue of the animals obtained from the 16th day, untreated groups (positive control, CMC control and ointment base control) showed extreme damage to the epidermis and its epithelial cells with the destructive formation of collagen fibres and fibroblast (). Rats treated with reference drug, vitamin C (oral and topical) produces a skin with significant healing property, followed by re-generation, re-epithelialization and improved reformation of collagen fibres and hair follicles ( and ). Oral and topical treatment of the rats with EPF and its polyphenolic rich fraction (PFEA) on 16th day also showed significant (p < 0.05) healing activity which can be due to the regeneration of epithelial cells and collagen fibres ().
Figure 3. Histological section of the skin tissue obtained from the 16th day excision wound model. (A) Normal control group; (B) positive control group (untreated); (C) group treated with CMC 0.5% w/v, p.o.; (D) group treated with an ointment base (topical) (indications of arrow marks: e: epithelial layer; Fb: fibroblast cells; c: collagen; re: re-epithelialization; hf: hair follicle).
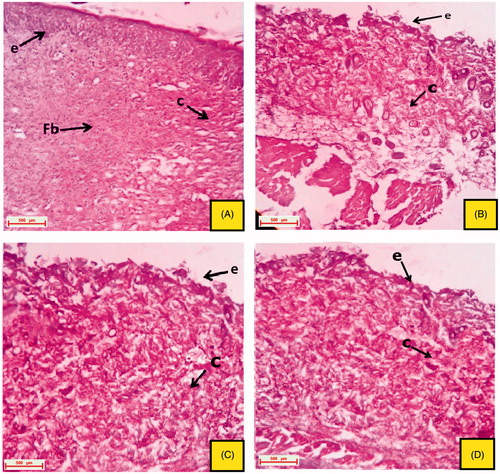
Figure 4. Histological section of the skin tissue obtained from oral treated groups on 16th day excision wound model. (A) Group treated with VTC 200 mg/kg, p.o.; (B) group treated with EPF 200 mg/kg, p.o.; (C) group treated with EPF 400 mg/kg, p.o. and (D) group treated with PFEA 75 mg/kg, p.o. (indications of arrow marks: re: re-epithelialization; fb: fibroblast cells; c: collagen; hf: hair follicle; bc, blood capillaries).
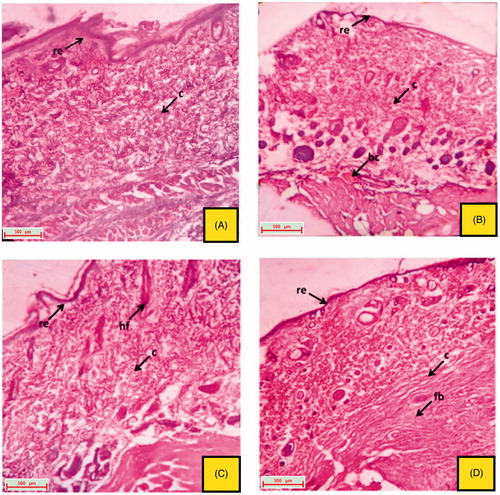
Figure 5. Histological section of the skin tissue obtained from topical treated groups on 16th day excision wound model. (A) group treated with topical VTC 10%; (B) group treated with topical EPF 5%; (C) group treated with topical EPF 10%; (D) group treated with topical PFEA 1.75% (indications of arrow marks: re: re-epithelization; fb: fibroblast cells; c: collagen; hf: hair follicle).
Discussion
Wound healing is a complicated and dynamic process which is a response to injury designed at reconstructing damaged tissue and requires precise coordination of connective tissue repair, re-epithelialization and angiogenesis (Wu et al. Citation2012). Epithelialization is a process which involves the proliferation and migration of epithelial cells across the wounded bed (Esimone et al. Citation2008). Wound contraction is one of the essential parameters that facilitate re-epithelialization of the wounded skin, probably by enhancing the wound closure and by restoration of functional barrier (Chattopadhyay et al. Citation2002). It has been reported that the wound healing activity of any tested drugs was attributed mainly to the shorter epithelialization period and the enhanced wound contraction (Suntar et al. Citation2010). Reduction in the epithelialization period was also observed in the present study when the rats were treated orally and topically with EPF and its polyphenolic rich fraction (PFEA). However, it was observed that the wounded rats topically treated with EPF ointment showed improved and better healing efficacy as compared to that of the oral treated groups. According to MacKay and Miller (Citation2003) and Odimegwu et al. (Citation2008), this effect was in proper corroboration, where the wound healing property enhances if the medicinal plant extract is applied by the topical route probably when formulated in the form of ointment base. Furthermore, rats treated topically with EPF and PFEA showed significant increase in the tensile strength of the incision wounded rats when compared to the positive control group. This signifies that there might be an increase formation of collagen concentration and stabilization of fibres, since tensile strength is an indication of better wound healing (Swamy et al. Citation2007).
In the present study, an attempt was also made to explore the mechanistic effects of EPF and PFEA by estimating the biochemical parameters related to the healing of wounds. The biochemical parameters of all the treated groups were tested and compared against the positive control group. Various biomarkers such as levels of collagen, hexosamine, proteins and DNA along with the histopathological representation of the wounded skin (from 0th day to 16th day) were evaluated in the present study. Vitamin C (ascorbic acid), a natural antioxidant was selected as reference drug, since it elicits improved wound healing property in both preclinical and clinical studies. Vitamin C is a cofactor in the hydroxylation of proline and lysine for procollagen formation, which leads to the generation of collagen. In addition, vitamin C also possesses potent antioxidant and bactericidal activity (Burns et al. Citation2003). Collagen is a constituent responsible for the cell growth in healing tissues, which can be measured by estimating the hydroxyproline concentration. Thus, the higher the concentration of hydroxyproline, faster will be the rate of wound healing, which is a reflection of increased cellular proliferation (DNA) and epithelialization (Roy et al. Citation2012). Hexosamine is also another important biomarker which is responsible for the active synthesis of the ground substance (mucopolysaccharide) by fibroblast, and also in the stabilization of the collagen molecules (Pattanayak & Sunita Citation2008). Additionally, wounded tissue with increase proteins and DNA levels (cell proliferation) also showed accelerated wound healing property. From the results represented in , it was observed that there is a significant restoration in all the levels of these biochemical markers in both the treated and untreated rats. All the rats treated topically with VTC 10%, EPF 5%, EPF 10%, PFEA 1.75% and an oral dose of EPF 400 and VTC 200 showed significant effect in increasing the hydroxyproline, hexosamine, proteins and DNA levels. This signifies that the roots of P. fulgens might have possible wound healing effects by means of tissue remodelling, collagen deposition and epithelial repair which leads to proliferation, mobilization, migration and differentiation of the newly formed cells and tissues. Moreover, to further support the wound healing effect of P. fulgens on such biomarkers, we have examined the morphological and histopathological studies on the rat tissues obtained on the 16th day of the study as represented in .
According to Tomczyk and Latte (Citation2009), phenolics and tannins have been reported to be the important constituents of almost all Potentilla species. They promote wound healing mainly due to their astringent, antimicrobial and free radical scavenging properties (Lopes et al. Citation2005; Deshmukh et al. Citation2009). Eventually, polyphenolic components such as flavonoids can promote excellent healing of wounds probably by means of antimicrobial and antioxidative property, thereby inhibiting the lipid peroxidation, which led to the prevention of cell damage and increase in the viability of collagen fibrils (Getie et al. Citation2002; Shetty et al. Citation2008). P. fulgens produces significant antioxidant property by restoring the imbalance between the oxidant and antioxidant substance, thereby justifying its wound healing efficacy. Qualitative and quantitative phytochemical reports depicted that the roots of P. fulgens contain a considerably good amount of polyphenolics component. (−)-Epicatechin, a polyphenolic component was also found to be present in a considerable amount in both EPF extract and PFEA fraction as per the HPTLC analysis. A report on the wound healing activity of catechins particularly epicatechin gallate (ECG) was also evaluated, where ECG was found to promote wound healing effect probably, via. acceleration of angiogenic response and anti-inflammatory effect (Kapoor et al. Citation2004). Thus, from the present study, it may be inferred that the wound healing effect of P. fulgens might be attributed to the presence of such components. In addition, apart from the major polyphenolic component, the EAF obtained from the roots of P. fulgens was also recently reported to contain terpenoid acids, viz., hyptadienic acid, tormentic acid, rosamultic acid, 2α,19α-dihydroxy-3-oxo-12-ursen-28-oic acid-β-d-glucopyranoside ester and kajiichigoside (Kumar et al. Citation2013). The presence of such terpenoid acids might also contribute to the wound healing property, since terpenoids promote healing effects, via astringent and antimicrobial properties which led to the wound contraction and formation of epithelial layers of the skin (Nayak et al. Citation2009).
Conclusion
The present study for the first time demonstrated that the P. fulgens ERE and its EAF rich in polyphenolics showed significant wound healing activity in rats which may be attributed specifically to their ability to promote cellular re-epithelialization, cell proliferation, collagen deposition and also as potent antioxidants. Hence, the study scientifically validates the traditional claims of P. fulgens as a folk medicinal plant in wound healing.
Acknowledgements
The Botanical survey of India, Shillong, Meghalaya, North Eastern India is gratefully acknowledged for the identification of the plant material. The authors are thankful to Dr. H. Carehome Pakyntien (President of the Jaintia Indigenous medicine association, Meghalaya, India) for providing ethnomedicinal information regarding the usage of the plant in curing external wounds.
Disclosure statement
The authors report no declaration of interest.
References
- Acute dermal toxicity [Internet]. 1987. OECD Guideline for testing of chemicals No. 402. Paris, France; [cited 1987 Feb 24]. Available from: http://www.oecd-ilibrary.org/environment/test-no-402-acute-dermal-toxicity_9789264070585-en
- Acute oral toxicity – up and down procedure (UDP) [Internet]. 2008. OECD guideline for testing of chemicals No. 425. Paris, France; [cited 2008 Oct 3]. Available from: http://iccvam.niehs.nih.gov/SuppDocs/FedDocs/OECD/OECDtg425.pdf
- Beers RF, Sizer IW. 1952. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 195:133–140.
- British Pharmacopoeia. 1980. Vol. 2. United Kingdom: Her Majesty’s Stationary Office Publishers. p. 1096.
- Burns JL, Mancoll JS, Phillips LG. 2003. Impairments to wound healing. Clin Plast Surg. 30:47–56.
- Burton K. 1956. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 62:315–323.
- Chai J, editor. 2011. Gastric ulcer healing – role of serum response factor, peptic ulcer disease. InTech [Internet]; [cited 2011 November 04]. Available from: http://www.intechopen.com/books/peptic-ulcer-disease/gastric-ulcer-healing-role-ofse rum response-factor
- Chattopadhyay D, Arunachalam G, Mandal AB, Sur TK, Mandal SC, Bhattacharya SK. 2002. Antimicrobial and anti-inflammatory activity of folklore: Mallotus peltatus leaf extract. J Ethnopharmacol. 82:229–237.
- Deshmukh PT, Fernandes J, Akarte A, Toppo E. 2009. Wound healing activity of Calotropis gigantea root bark in rats. J Ethnopharmacol. 125:178–181.
- Dische Z, Borenfreund E. 1950. A spectrophotometric method for the microdetermination of hexosamines. J Biol Chem. 184:517–522.
- Emami-Razavi SH, Esmaeili N, Forouzannia SK, Amanpour S, Rabbani S, Alizadeh AM, Mohagheghi MA. 2006. Effect of bentonite on skin wound healing: experimental study in the rat model. Acta Med Iran. 44:235–240.
- Esimone C, Nworu C, Jackson C. 2008. Cutaneous wound healing activity of a herbal ointment containing the leaf extract of Jatropha curcas L. (Euphorbiaceae). Int J Appl Res Nat Prod. 1:1–4.
- Friedman A. 2011. Wound healing: from basic science to clinical practice and beyond. J Drugs Dermatol. 10:427–433.
- Getie MG, Mariam T, Reitz R, Neubert RH. 2002. Evaluation of the release profiles of flavonoids from topical formulations of the crude extract of the leaves of Dodonea viscose (Sapindaceae). Pharmazie. 57:320–322.
- Gutierrez R, Vargas S. 2006. Evaluation of the wound healing properties of Acalypha langiana in diabetic rats. Fitoterapia. 77:286–289.
- Kakkar P, Das B, Viswanathan PN. 1984. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 21:130–132.
- Kamer E, Unalp HR, Gundogan O, Diniz G, Ortac R, Olukman M, Derici H, Ali Onal M. 2010. Effect of ascorbic acid on incisional wound healing in streptozotocin-induced diabetic rats. Wounds. 22:27–31.
- Kapoor M, Howard R, Hall I, Appleton I. 2004. Effects of epicatechin gallate on wound healing and scar formation in a full thickness incision wound healing model in rats. Am J Pathol. 165:299–307.
- Karodi R, Jadhav M, Rub R, Bafna A. 2009. Evaluation of the wound healing activity of a crude extract of Rubia cordifolia L. (Indian madder) in mice. Int J Appl Res Nat Prod. 2:12–18.
- Kaul K, Jaitak V, Kaul VK. 2011. Review on pharmaceutical properties and conservation measures of Potentilla fulgens Wall. ex Hook.: a medicinal endangered herb of higher Himalaya. Indian J Nat Prod Res. 2:298–306.
- Krishnaveni B, Neeharika V, Venkatesh S, Madhava RB. 2009. Wound healing activity of Carallia brachiata bark. Indian J Pharm Sci. 71:576–578.
- Kumar D, Ghosh R, Pal BC. 2013. α-Glucosidase inhibitory terpenoids from Potentilla fulgens and their quantitative estimation by validated HPLC method. J Funct Foods. 5:1135–1141.
- Kumar R, Katoch SS, Sharma S. 2006. Beta-adrenoceptor agonist treatment reverses denervation atrophy with augmentation of collagen proliferation in denervated mice gastrocnemius muscle. Indian J Exp Biol. 44:371–376.
- Laloo D, Kumar M, Prasad SK, Hemalatha S. 2013. Quality control standardization of the roots of Potentilla fulgens Wall.: a potent medicinal plants of the Western Himalayas and Northeastern India. Pharmacog J. 5:97–103.
- Laloo D, Prasad SK, Sairam K, Hemalatha S. 2013. Gastroprotective activity of ethanolic root extract of Potentilla fulgens Wall. ex Hook. J Ethnopharmacol. 146:505–514.
- Lima CC, Pereira APC, Silva JR, Oliveira LS, Resck MC, Grechi CO, Bernardes MT, OlÚmpio FM, Santos AM, Incerpi EK, et al. 2009. Ascorbic acid for the healing of skin wounds in rats. Braz J Biol. 69:1195–1201.
- Lopes GC, Sanches ACC, Nakamura CV, Dias-Filho BP, Hernandes L, de Mello JC. 2005. Influence of extracts of Stryphnodendron polyphyllum Mart. and Stryphnodendron obovatum Benth. on the cicatrisation of cutaneous wounds in rats. J Ethnopharmacol. 959:265–272.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem. 193:265–275.
- MacKay D, Miller A. 2003. Nutritional support for wound healing. Altern Med Rev. 8:359–377.
- Makkar HPS. 2000. Quantification of tannins in tree foliage: a laboratory manual for the FAO/IAEA co-ordinated research project on ‘Use of nuclear and related techniques to develop simple tannin assays for predicting and improving the safety and efficiency of feeding ruminants of tanniniferous tree foliage’. Joint FAO/IAEA of Nuclear Techniques in Food and Agriculture. Animal Production and Health Sub-programme. FAO/IAEA Working Document, Vienna. p. 3–5.
- Mantle D, Gok MA, Lennard TWJ. 2001. Adverse and beneficial effects of plant extracts on skin and skin disorders. Adverse Drug React Toxicol Rev. 20:89–103.
- Mukherjee P, Mukherjee K, Kumar RM, Rajesh Kumar M, Pal M, Saha BP. 2003. Evaluation of wound healing activity of some herbal formulations. Phytother Res. 17:265–268.
- Murthy S, Gautam MK, Goel S, Purihit V, Sharma H, Goel RK. 2013. Evaluation of in vivo wound healing activity of Bacopa monniera on different wound model in rats. Biomed Res Int. 2013:972028.
- Nayak BS, Anderson M, Pinto Pereira LM. 2007. Evaluation of wound-healing potential of Catharanthus roseus leaf extract in rats. Fitoterapia. 78:540–544.
- Nayak BS, Sandiford S, Maxwell A. 2009. Evaluation of the wound-healing activity of ethanolic extract of Morinda citrifolia L. leaf. Evid Based Complement Alternat Med. 6:351–356.
- Neuman RE, Logan MA. 1950. The determination of hydroxyproline. J Biol Chem. 184:299–306.
- Nunes R, Rodrigues S, Pasko P, Tyszka-Czochara M, Grenha A, de Carvalho IS. 2014. Effect of Erica australis extract on Caco-2 cells, fibroblasts and selected pathogenic bacteria responsible for wound infection. Ind Crops Prod. 52:99–104.
- Odimegwu DC, Ibezim EC, Esimone CO, Nworu CS, Okoye FBC. 2008. Wound healing and antibacterial activities of the extract of Dissotis theifolia (Melastomataceae) stem formulated in a simple ointment base. J Med Plants Res. 2:11–16.
- Ohkawa H, Ohishi N, Yagi K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 95:351–358.
- Pattanayak SP, Sunita P. 2008. Wound healing, anti-microbial and antioxidant potential of Dendrophthoe falcate (L.f) Ettingsh. J Ethnopharmacol. 120:241–247.
- Perez GRM, Vargas SR, Ortiz HYD. 2005. Wound healing properties of Hylocereus undatus on diabetic rats. Phytother Res. 19:665–668.
- Prasad SK, Kumar R, Patel DK, Hemalatha S. 2010. Wound healing activity of Withania coagulans in streptozotocin-induced diabetic rats. Pharm Biol. 48:1397–1404.
- Roy P, Amdekar S, Kumar A, Singh R, Sharma P, Singh V. 2012. In vivo antioxidative property, antimicrobial and wound healing activity of flower extracts of Pyrostegia venusta (Ker Gawl) Miers. J Ethnopharmacol. 140:186–192.
- Sedlak J, Lindsay RH. 1968. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 25:192–205.
- Senthil KM, Sripriya R, Vijaya RH, Sehgal PK. 2006. Wound healing potential of Cassia fistula on infected albino rat model. J Surg Res. 131:283–289.
- Shetty S, Udupa S, Udupa L. 2008. Evaluation of antioxidant and wound healing effects of alcoholic and aqueous extract of Ocimum sanctum Linn. in rats. Evid Based Complement Alternat Med. 5:95–101.
- Suntar IP, Akkol EK, Yilmazer D, Baykal T, Kirmizibekmez H, Alper M, Yesilada E. 2010. Investigations on the in vivo wound healing potential of Hypericum perforatum L. J Ethnopharmacol. 127:468–477.
- Swamy H, Krishna V, Shankarmurthy K, Abdul RB, Mankani KL, Mahadevan KM, Harish BG, Raja NH. 2007. Wound healing activity of embelin isolated from the ethanol extract of leaves of Embelia ribes Burm. J Ethnopharmacol. 109:529–534.
- Tomczyk M, Latte KP. 2009. Potentilla – a review of its phytochemical and pharmacological profile. J Ethnopharmacol. 122:184–204.
- Trease GE, Evans WC. 2002. Pharmacognosy. 15th ed. London: W.B. Saunders Company Limited.
- Wu X, Luo X, Gu S, Xu JH. 2012. The effects of Polygonum cuspidatum extract on wound healing in rats. J Ethnopharmacol. 141:934–937.