Abstract
Context: Bacopa monnieri L. Pennell (Scrophulariaceae) is one of the most important plants in the system of Indian medicine (Ayurveda).
Objective: This paper studies the optimal growth of B. monnieri for effective accumulation of metabolites. Biomass growth of this plant could be accomplished in liquid cultures on Murashige & Skoog medium.
Materials and methods: Powdered shoots of in vitro cultures of B. monnieri were extracted by methanol for indole compounds, phenolic compounds and bacosides for RP-HPLC analysis. Fatty acid analysis was performed via gas chromatography. Anti-inflammatory effect of B. monnieri extracts was evaluated in the A549 cells. COX-2 and cPGES expression was analyzed using Western blots.
Results: l-Tryptophan and serotonin were found in biomass from in vitro cultures of B. monnieri on MS medium and in biomass from the MS mediums enriched with the different additions such as of 0.1 g/L magnesium sulphate, 0.1 g/L zinc hydroaspartate, 0.1 g/L l-tryptophan, 0.25 g/L serine, 0.5 g/L serine and 0.5 mg/L anthranilic acid. The content of l-tryptophan and serotonin compounds was significant in biomass from medium with the addition of 0.1 g/L zinc hydroaspartate (0.72 mg/g dry weight and 1.19, respectively). Phenolic compounds identified in biomass from the same variants of MS medium were chlorogenic acid (ranging from 0.20 to 0.70 mg/g dry weight), neochlorogenic acid (ranging from 0.11 to 0.40 mg/g dry weight) and caffeic acid (ranging from 0.01 to 0.04 mg/g dry weight). The main group of fatty acids in biomass was saturated fatty acids (53.4%). The predominant fatty acid was palmitic acid. A significant decrease of COX-2 and cPGES expression was observed in the A549 cells activated with LPS and treated with B. monnieri extracts.
Discussion and conclusions: As far as we know, this is the first analysis of indole compounds and phenolic acids in this plant. The multi-therapeutic effect of B. monnieri is expressed by the activity of bacosides. Information about the presence of indole and phenolic compounds, and fatty acids in this plant is limited, but the content of these compounds might participate in the physiological activity of B. monnieri.
Introduction
Bacopa monnieri (L.) Pennell Scrophulariaceae (Water hyssop), known locally in India as Brahmi or Jalanimba, is one of the most important plants in the system of Indian medicine called Ayurveda. Bacopa monnieri has been used in India for 5000 years to treat epilepsy and insomnia, and as a sedative and anxiety reducing herb (Anonymous Citation2001; Gohil & Patel Citation2010). Indian Materia Medica (Bhavaprakasha Nighantu AD 1500) recommends this plant for improving memory and concentration (Anonymous Citation1999). Commercially available preparations of B. monnieri improve brain function, increase the ability to concentrate and improve memory in both young and older people. Clinical studies confirm the positive effects of this material in the reconstruction of mental functions in children suffering from attention deficit hyperactivity disorder (ADHD), and to improve cognitive functions in patients after strokes and in epilepsy (Jyoti & Sharma Citation2006; Calabrese et al. Citation2008; Kamkaew et al. Citation2013). Based on previous studies, these effects are due to the ability of the plant extracts to modulate the cholinergic system (Peth-nui et al. Citation2012). Compounds which are attributed to the above activities are bacosides and triterpenoids belonging to the saponins (Rastogi et al. Citation2012). This plant is also used in the treatment of neurodegenerative diseases (e.g., Alzheimer’s disease or Parkinson’s). It also exhibits antioxidant, anti-inflammatory, antipyretic, antiulcer, cardioprotective, cooling, laxative and adsorbing effects (Biswas et al. Citation2012). It is used in cases of dermatitis, anemia, diabetes, cough, swelling, fever, arthritis, anorexia and dyspepsia. Hydro-alcoholic and alcoholic extracts activate detoxification processes, support processes of the renewal and regeneration of tissues, intensify the processes of protein synthesis, stabilize the structure of cell membranes and prevent the overgrowth of the prostate. Bacopa monnieri is an herb that also acts as a diuretic and cardio toner, and strengthens and stimulates menstruation (Shah et al. Citation2012; Wasnik et al. Citation2012). In the bloodstream, this herb functions as a metal chelating agent able to remove any excess of toxic metals. It is also used in phytoremediation for the disposal of heavy metals such as cadmium and chromium. Also, B. monnieri is used in the treatment of depression (Jyoti et al. Citation2007; Madhavi et al. Citation2013). This plant is an adaptogenic plant, it helps the body adapt to adverse environmental conditions and has a normalizing effect on the human body. B monnieri is also a good source of elements (Łojewski et al. Citation2014). The currently used, standardized extract of Bacopa, is CDRI-08 (Singh Citation2013).
This paper presents a study of the optimal culture medium for the effective enhancement of the production of metabolites in B. monnieri. Presently, basic research in the field of the specialized cultivation of the biomass of B. monnieri supplemented with organic compounds and elements is especially important for an explanation of the mechanisms of their accumulation and distribution in in vitro cultures. Also, in this work, the connection between the composition of culture medium and biomass growth is presented. In vitro culture enables constant access to the material independent of environmental conditions; this allows research to be conducted under controlled conditions and the possibility of the supplementation of biomass with the desired micronutrients and organic compounds in order to obtain potentially better pharmaceutical material. Additionally, the content of indole compounds, phenolic compounds, sterols and fatty acids was determined. Bacopa monnieri has a multi-therapeutic effect, which is explained by the activity of bacosides. Information about the presence of compounds from other groups, for example, indole and phenolic compounds, fatty acids and sterols, in this plant, is limited, but the content of these compounds might participate in the physiological activity of B. monnieri. Indole compounds are involved in the regulation of sleep, mood; it coordinates the work of the biological clock controlling circadian rhythm, exhibits anti-cancer properties, and plays a neuroprotective role. Indole derivatives are also compounds with anti-inflammatory and analgesic properties (Wurst et al. Citation2004; Keszthelyi et al. Citation2009; Esposito & Cuzzocrea Citation2010; Singhal et al. Citation2012). Biological activity of phenolic acids is related to their antioxidant activity that protects the vital structures such as chromosomal DNA, structural proteins, enzymes, and LDL and lipids of cellular membranes from oxidative damage (Yeh et al. Citation2009). This compounds and fatty acids exhibit also anti-inflammatory activity. Hence, the purpose of the current study was to analyze biologically active compounds such as indolic and phenolic compounds, fatty acids and to determine anti-inflammatory properties of B. monnieri. As far as we know, this is the first analysis of indole compounds and phenolic acids in this plant by the HPLC method.
Materials and methods
Reagents
All the chemicals used were of analytical grade. Magnesium sulphate, chloroform and ammonium acetate were purchased from POCh (Gliwice, Poland); and zinc hydroaspartate was from Farmapol (Poznań, Poland). The growth regulators 1-naphthaleneacetic acid (NAA), 6-benzylaminopurine (BAP) and nicotinic acid were from Sigma-Aldrich (St. Louis, MO). Anthranilic acid and serine (purity ≥ 98% by HPLC); standards of indole compounds, i.e., l-tryptophan, 5-OH-l-tryptophan, 5-CH3-tryptophan, serotonin, melatonin, tryptamine, 5-CH3-tryptamine, kynurenine sulphate, 3-indoleacetic acid, 3-indoleacetonitrile, indole and indole-3-acetamide, bacoside A were also from Sigma-Aldrich (St Louis, MO) and these were all of HPLC grade. Phenolic acid standards, i.e., p-coumaric, ferulic, p-hydroxybenzoic, vanillic acids and myo-inositol were from Fluka (Chemie AG, Berlin, Germany), and those of caffeic, chlorogenic, neochlorogenic, cinnamic, o-coumaric, protocatechuic, sinapic and syringic acids were from Sigma-Aldrich (St. Louis, MO). Sterol standards ergosterol and ergocalciferol were obtained from Fluka (Chemie AG, Berlin, Germany) and fatty acid standards were from Supelco (Poznan, Poland). Vitamin B1 was from TEVA Pharmaceuticals (Tikva, Israel). Methanol, acetic acid, petroleum ether and dichloromethane were from Merck (Darmstadt, Germany). Quadruple-distilled water with a conductivity of less than 1 μS cm−1 was obtained using an S2-97A2 distillation apparatus (ChemLand, Stargard Szczecin, Poland).
Plant material
The in vitro cultures were established from commercially available in vitro cultures of Bacopa monnieri from IVPLANT (representative samples of B. monnieri in vitro cultures were deposited at the Department of Pharmaceutical Botany, Jagiellonian University Collegium Medicum, Kraków, Poland). This material was identified by the botanist Bożena Muszyńska. Shoots were cut into small pieces and placed in Erlenmeyer flasks with liquid medium according to Murashige and Skoog (Citation1962) with our modifications, which consisted of the addition of nicotinic acid (0.5 mg/L), myo-inositol (100 mg/L), vitamin B1 (4.0 mg/L) and the growth regulators 6-benzylaminopurine (BAP) 1.0 mg/L and 1-naphthaleneacetic acid (NAA) 0.2 mg/L, pH adjusted to 5.7–5.8 before autoclaving. This was the basal medium (MS1) and shoots were passaged every four weeks.
Experimental in vitro culture of Bacopa monnieri
To the basal medium of Murashige and Skoog (MS1), the following compounds were added: 0.1 g/L magnesium sulphate; 0.1 g/L zinc hydroaspartate; 0.1 g/L l-tryptophan; 0.25 g/L and 0.5 g/L serine; and 0.5 mg/L anthranilic acid. Cultures were grown under constant artificial light (4W/m2, LF-40W lamp, daylight, Piła) at 25 ± 2 °C for 4 weeks. After this period, the fresh biomass was frozen and lyophilized (lyophilizer Freezone 4.5, Labconco, Kansas City, MO; temp: −40 °C).
High performance liquid chromatography analysis of indole compounds
The lyophilized biomass (5 g of each) was ground in a mortar and then subjected to extraction with petroleum ether to remove lipids (Muszyńska et al. Citation2009). Defatted material was extracted in a glass percolator by methanol. The resultant extract was evaporated to dryness (Buchi evaporator, Buchi, Krefeld, Germany) under a pressure of 200 mBa at 40 °C. The concentrated analyte was dissolved in methanol transferred through a Whatman No. 3 filter paper. The extracts were quantitatively dissolved in 1.5 mL of solvent system (methanol/water/ammonium acetate 15:14:1 v/v/v) and subjected to separation by HPLC, employing a Hitachi HPLC (Merck, Tokyo, Japan) equipped with a type L-7100 pump. The Purospher® RP-18 (4 mm × 200 mm, 5 μm) column (Merck, Tokyo, Japan) was kept at 25 °C and the UV detector operated at λ = 280 nm. The liquid phase used was a mixture of methanol/water/ammonium acetate (15:14:1 v/v/v) at a flow rate of 1 mL/min. The quantitative analyses of indole compounds were performed using a calibration curve with the assumption of the linear size of the area-under-the-peak and the concentration of the reference standard. The results are expressed in mg/g of D.W.
Validation of RP-HPLC for the determined indole compounds (l-tryptophan and serotonin) was conducted. Accuracy, precision, linearity range, detection limit and quantification limit were calculated ().
Table 1. Validation parameters of RP-HPLC methods for L-tryptophan and serotonin.
Accuracy
The accuracy of the method was assessed by calculating the percentage recovery for l-tryptophan and serotonin. The test was performed by the addition of a precisely determined dose of the standard substance in the amount of from 80% to 120% of the declared l-tryptophan and serotonin content. The determination was performed both before and after the addition of the standard substance.
Precision
Precision was determined by using a model solution prepared by dissolving the substances under analysis in methanol. Determination was carried out five times for each sample ().
Linear range
Linear range was estimated as the dependance of the area-under-the-peak on the concentration of model substances in the range from 10 to 50 μg/mL. The linear range of determined compounds is shown as equations describing the curves and linear correlation coefficient values (r).
Limit of detection (LOD) and limit of quantification (LOQ)
Using the standard deviation and the angular coefficient of the slope, the LOD and the LOQ were determined.
Detection limit and quantification limit were calculated with the formulae LOD = 3.3 × Sy/a and LOQ = 10 × Sy/a, in which Sy is the standard estimation error, a is the slope ().
HPLC analysis of phenolic compounds
The lyophilized biomass (5 g of each) was ground in a mortar and then subjected to extraction with petroleum ether to remove lipids (Muszyńska & Sułkowska-Ziaja Citation2012). These oil fractions were discarded and the remaining biomass was dried and extracted again with methanol in a percolator. Methanolic extract was evaporated to dryness (Buchi evaporator, Buchi, Krefeld, Germany) under a pressure of 200 mBa at 40 °C. The residues were quantitatively dissolved in methanol (1.5 mL), filtered through a Millipore Millex-GP (Millipore Inc., Billerica, MA), 0.22 μm and were subjected to HPLC analysis.
The resultant extracts were analyzed for their contents of phenolic acids by the HPLC method. These analyses were carried out according to the procedure developed by Ellnain-Wojtaszek and Zgórka (Citation1999), with some modifications. HPLC analyses were conducted using an HPLC VWR Hitachi-Merck apparatus (Darmstadt, Germany): L-2200 autosampler, L-2130 pump, RP-18e LiChrospher (4 mm × 250 mm, 5 μm) column thermostated at 25 °C, L-2350 column oven, L-2455 diode array detector at the UV range 200–400 nm. The mobile phase consisted of solvent A: methanol/0.5% acetic acid 1:4 (v/v), and solvent B: methanol. The gradient was as follows: 100:0 for 0-25 min; 70:30 for 35 min; 50:50 for 45 min; 0:100 for 50–55 min; 100:0 for 57–67 min. The comparison of UV spectra and retention times with standard compounds enabled the identification of phenolic acids present in analysis samples. The quantitative analysis of free phenolic acids was performed with the use of a calibration curve with the assumption of the linear size of the area-under-the-peak and the concentration of the reference standard. The results are expressed in mg/g of D.W.
Analysis of fatty acids
Powdered biomass of in vitro cultures (1 g) was extracted with chloroform-methanol solution (2:1 v/v). Fatty acid methyl esters (FAME) were synthesized using 20% BF3 in methanol at 100 °C. FAME analyses were performed using gas chromatography (GC) on an Agilent 6890N (Agilent Inc., Santa Clara, CA) with a J and W DB-23 capillary column (60 m, ID 0.25 mm, 0.25 μm) and FID detector. GC parameters: FID 260 °C, injector 250 °C, split ratio 50:1, oven 140 °C for 5 min, ramped from 140 °C to 190 °C at 4 °C/min, 190 °C for 15 min, and ramped from 190 °C to 240 °C at 2.75 °C/min, 240 °C for 4 min, carrier gas – helium. For the identification of fatty acids, retention times of FAME standards were used. Peak areas were measured using an integrator function (ChemStation). The results for the fatty acid composition, total saturated fatty acids (SFA), monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA) of the samples are expressed as the relative % of total fatty acids. Validation of GC for determined fatty acids was carried out. Accuracy, precision, linearity range, detection limit and quantification limit were calculated ().
Table 2. Validation parameters of GC-FID methods for heptadecanoic acid.
Analysis of sterols
Five grams of each powdered biomass was mixed with 100 mL of a 75:25 (v/v) mixture of methanol and dichloromethane, followed by sonification for 10 min and centrifugation at 1200g for 5 min. Merged extracts (300 mL) were concentrated to dryness using a rotary vacuum evaporator at 22 ± 2 °C. The HPLC method was performed according to the procedure developed by Yuan et al. (Citation2008). The mobile phase consisted of solvent A: methanol/water 20:80 (v/v), and solvent B: methanol/dichloromethane 75:25 (v/v). A gradient procedure was used as follows: starting at sample injection, 60% of B for 5 min; a linear gradient from 60% to 100% of B for 10 min; and 100% of B for 10 min. The flow rate was 1.0 mL/min. The chromatographic peaks were recorded at a wavelength of 280 nm and 266 nm.
RP-HPLC analysis of bacosides
The powdered biomass from an in vitro shoot culture of B. monnieri was subjected to extraction with methanol in a percolator. Methanolic extract was left at room temperature to dry. The residues were quantitatively dissolved in methanol (14 mL), filtered through a Millipore Millex-GP (Millipore Inc., Billerica, MA), 0.22 μm and were subject to RP-HPLC analysis. RP-HPLC analyses were conducted according to procedures described elsewhere (Pratibha Citation2012) with our modifications on a Merck-Hitachi liquid chromatograph (LaChrom Elite, Merck, Darmstadt, Germany) equipped with a L-2455 DAD detector and RP-18e Purospher ® (250 × 4 mm, 5 μm) column. Analyses were carried out at 25 °C, with a mobile phase consisting of A – acetonitrile, B – acetonitrile: 0.5% phosphoric acid 0.01 mol/L 35: 65 (v/v), gradient elution: A (0:100%), B (100:0%) at a flow rate 1 mL/min, λ = 205 nm. Identification was performed by comparison of the retention times of the peaks with an authentic reference compound and co-chromatography with a standard. Quantification was conducted by measurement of the peak area with reference to the standard of bacoside A (bacoside A3, bacopaside II, bacopaside X, bacopasaponin C) curve derived from five concentrations (0.1875–3 mg/mL).
Cell cultures
Human Lung Carcinoma Epithelial Cells A549 (CCL-185, ATTC, Mannasas, VA) were cultured as described previously (Gdula-Argasińska et al. Citation2015). Cells were activated by LPS 1 μg/mL for 24 h. After activation, 10 μL or 25 μL of B. monnieri methanolic extracts (100 μg/mL) were added for cells and incubated for 24 h.
Western blot
Cell lysates were prepared and subjected to 10% SDS-polyacrylamide gel electrophoresis as described earlier (Gdula-Argasińska et al. Citation2015). We used primary antibodies: anti-cyclooxygenase 2 (COX-2), anti-cytosolic prostaglandin E synthase (cPGES) and anti-β-actin (GeneTex Inc., Irvine, CA), diluted 1:1000 and secondary antibody Easy Blot anti-rabbit IgG (HRP) diluted 1:2000 in Signal + for Western Blot (GeneTex, Irvine, CA). The integrated optical density of the bands was quantified using Chemi Doc Camera with Image Lab software (Bio-Rad, Hercules, CA).
Statistical analysis
For each of the samples, three repetitions were used for the determination of every compound and all the analyses were conducted in three repetitions. The results are expressed as mean values and standard deviations (SD). Statistical analyses were conducted using Microsoft Excel 2010 and the commercially available package GraphPad Prism v3.02 (GraphPad Software, San Diego, CA) using one-way ANOVA with Tukey’s post hoc test. Values were considered significantly different at p<0.05.
Results
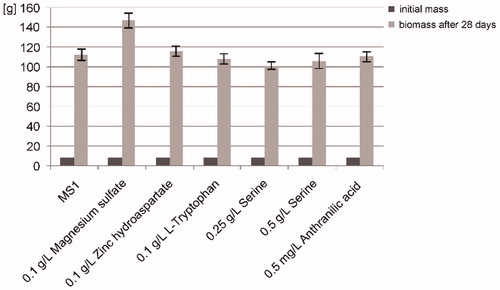
It was established that biomass growth of B. monnieri could be obtained in stationary liquid cultures on MS1 medium at 25 ± 2 °C under a 4 W/m2 for 24 h. A 12–14-fold growth in liquid cultures was obtained within a typical 28-d growth cycle. In the case of MS1 medium with the addition of 0.1 g/L magnesium sulphate, the increase was 18-fold. The biomass growth in the initiated cultures averaged 12 g dry weight (DW)/L of medium. It was found that MS medium without growth regulators did not determine optimal growth. According to other researchers (Ceasar et al. Citation2010; Ahire et al. Citation2013), optimal growth is obtained on MS medium supplemented with growth regulators such as BAP and NAA. The increases in biomass are shown in .
Figure 1. Bacopa monnieri biomass growth within 28 d on Murashige & Skoog (MS1) liquid medium and on the same medium but with the addition of 0.1 g/L magnesium sulphate; 0.1 g/L zinc hydroaspartate; 0.1 g/L l-tryptophan; 0.25 g/L serine; 0.5 g/L serine and 0.5 mg/L anthranilic acid.
Indole compounds
The organic additives used did not influence the biomass growth. l-Tryptophan and serotonin were found in biomass from in vitro cultures on MS1 medium of B. monnieri and in biomass from the same medium, but with the addition of 0.1 g/L magnesium sulphate; 0.1 g/L zinc hydroaspartate; 0.1 g/L l-tryptophan; 0.25 g/L serine; 0.5 g/L serine and 0.5 mg/L anthranilic acid.
The highest amount of these metabolites was observed in cultures with the addition of zinc hydroaspartate. An increase in the amount of l-tryptophan and serotonin was also observed on MS1 medium with the addition of 0.1 g/L magnesium sulphate, 0.5 g/L serine and 0.5 mg/L anthranilic acid. Both serine and anthranilic acid are precursors of indole compounds. The content of l-tryptophan and serotonin compounds was significant in biomass from MS1 medium with the addition of 0.1 g/L zinc hydroaspartate and this was at least four times higher (0.72 mg/g DW and 1.19 mg/g DW, respectively) than in biomass from MS1 medium (control) (0.11 mg/g DW and 0.29 mg/g DW). All the results obtained are given in . On MS1 medium supplemented with 0.5 g/L serine and with 0.5 mg/L anthranilic acid, there was a two-fold increase in the amount of l-tryptophan and serotonin increase compared with the control. An example chromatogram of serotonin and l-tryptophan for B. monnieri biomass from in vitro culture on MS1 medium with 0.1 g/L of zinc hydroaspartate addition is shown in .
Figure 2. RP-HPLC chromatogram of indole compounds in methanolic extract of Bacopa monnieri biomass from in vitro culture on MS (liquid medium with 1.0 mg/L BAP and 0.2 mg/L NAA) with the addition of 0.1 g/L zinc hydroaspartate (serotonin – RT = 2.08 min.; l-tryptophan – RT = 3.31 min.).
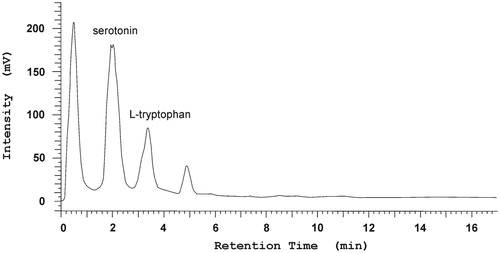
Table 3. The content of indole compounds in biomass from an in vitro culture of Bacopa monnieri.
Phenolic compounds
Phenolic compounds were identified in biomass from in vitro cultures of B. monnieri from the same variants of MS1 medium as in the case of the indole compound analysis. The examined phenolic compounds were chlorogenic acid (ranging from 0.20 mg/g DW to 0.70 mg/g DW), neochlorogenic acid (ranging from 0.11 mg/g DW to 0.40 mg/g DW) and caffeic acid (ranging from 0.01 mg/g DW to 0.04 mg/g DW). The highest increase in the total content (approximately three-fold) of phenolic compounds was observed in biomass from in vitro cultures on MS1 medium with the addition of 0.5 g/L serine and this amounted to 1.13 mg/g DW. In contrast, the increase in biomass from MS1 medium without additions was 0.36 mg/g D.W. All results are summarized in . The material from MS1 medium with the addition of 0.1 g/L magnesium sulphate and 0.5 mg/L anthranilic acid showed the same level of accumulation of phenolic compounds (0.86 mg/g DW and 0.79 mg/g DW, respectively). An example chromatogram of phenolic compounds for B. monnieri biomass from in vitro culture on MS1 medium with 0.5 g/L of serine addition is shown in .
Figure 3. RP-HPLC chromatogram of phenolic compounds in methanolic extract of Bacopa monnieri biomass from in vitro culture on MS1 (MS liquid medium with 1.0 mg/L BAP and 0.2 mg/L NAA) with the addition of 0.5 g/L serine (neochlorogenic acid – RT = 7.28 min.; chlorogenic acid – RT = 12.41; caffeic acid – RT = 15.95 min.).
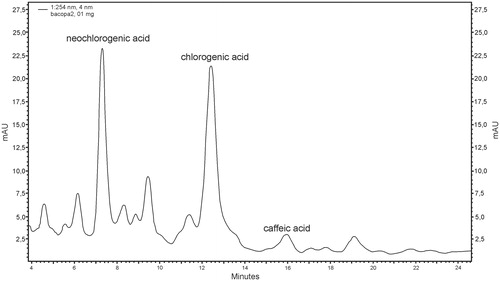
Table 4. The content of free phenolic compounds in biomass from an in vitro culture of Bacopa monnieri.
Fatty acids
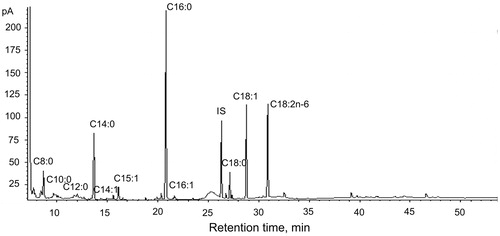
Fatty acids were examined only in the MS1 group, because the additives used had no influence on the content of fatty acids (). The main group of fatty acids in biomass from in vitro cultures of B. monnieri were saturated fatty acids (53.4%). The predominant fatty acid from this group was palmitic acid (32.6%). A total content of unsaturated fatty acids slightly lower than that of saturated fatty acids was determined and this amounted to 46.5%. Linoleic acid, oleic acid and myristoleic acid were present in the highest levels (17.0%, 15.7% and 12.6%, respectively). cis-10-Pentadecanoic acid and palmitoleic acid were found only in small amounts: 0.8% and 0.4%, respectively.
Table 5. The percentage of fatty acids in B. monnieri in vitro cultures on a liquid MS medium with 1.0 mg/L BAP and 0.2 mg/L NAA (MS1).
An example GC chromatogram of fatty acids for B. monnieri biomass from in vitro culture on MS medium with 1.0 mg/L BAP and 0.2 mg/L NAA is shown in .
Sterol compounds
In the current study, we did not find any free sterols in biomass from in vitro cultures of B. monnieri. These results are in contrast to those from Chatterji et al. (Citation1963) who observed stigmasterol, stigmastanol and β-sitosterol. The difference between these and the current analyses was as follows: plant material used (naturally grown versus in vitro culture) and method of determination (melting point versus HPLC).
Bacosides
The analysis of bacoside accumulation in the biomass of the experimental in vitro cultures showed that only the addition of anthranilic acid to the medium results in a higher accumulation of them in comparison with the control (respectively, 28.9 mg/g DW and 26.8 mg/g DW). Other additives used in the present work generate lower contents of bacosides in the biomass compared to the control. Results are shown in .
Table 6. The content of bacosides in biomass from in vitro culture of Bacopa monnieri.
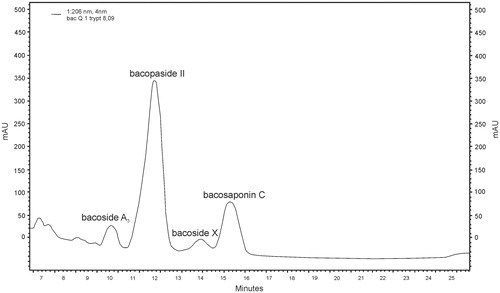
An example chromatogram of bacoside A for B. monnieri standard is shown in ; for B. monnieri, control (MS medium) is shown in , and for B. monnieri, biomass from in vitro culture on MS1 medium with 0.1 mg/L l-tryptophan is shown in .
Figure 5. RP-HPLC chromatogram of bacoside A standard (bacoside A3 – RT = 10.02; bacopaside II – RT = 12.12; bacopaside X – RT = 14.03; bacopasaponin C – RT = 15.49).
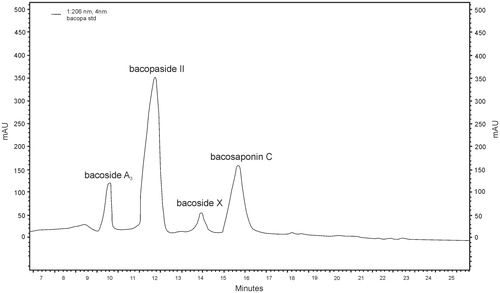
Figure 6. RP-HPLC chromatogram of bacoside A control (bacoside A3 – RT = 10.02; bacopaside II – RT = 12.12; bacopaside X – RT = 14.03; bacopasaponin C – RT = 15.49).
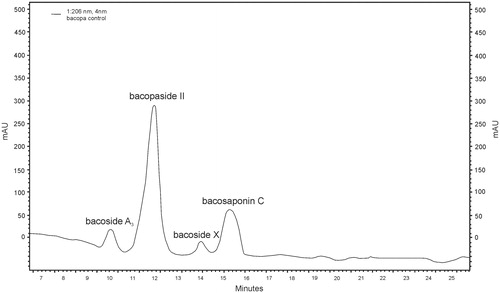
Figure 7. RP-HPLC chromatogram of bacoside A (bacoside A3 – RT = 10.02; bacopaside II – RT = 12.12; bacopaside X – RT = 14.03; bacopasaponin C – RT = 15.49) in methanolic extract of Bacopa monnieri biomass from in vitro culture on MS1 medium (liquid medium with 1.0 mg/L BAP and 0.2 mg/L NAA) with addition of 0.1 mg/L l-tryptophan.
COX-2 and cPGES protein expression
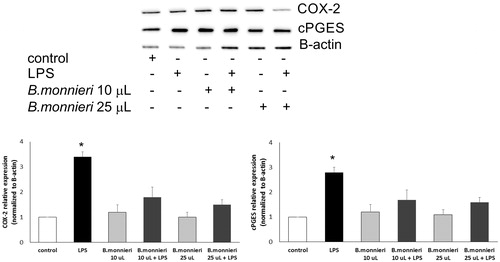
No cytotoxic effects were observed in the A549 cells treated with B. monnieri extracts. In the cells incubated with LPS and B. monnieri, significant decrease of COX-2 and cPGES expression was observed when compared with the LPS activated cells ().
Discussion
The obtained biomass increments and dynamics of shoot growth did not differ from the results that have previously been obtained by other researchers (Szopa & Ekiert Citation2014). The methods of extraction and HPLC analysis used here proved able to provide the optimal conditions for the qualitative determination of indole compounds, and phenolic compounds in the test material. As a result of the current experiment, the levels of release of indole compounds (l-tryptophan and serotonin), phenolic compounds (chlorogenic acid, neochlorogenic acid and caffeic acid) and fatty acids were detected.
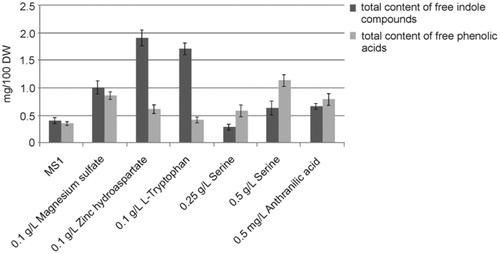
As a result of previous research, the highest total contents of the examined compounds were determined in biomass from in vitro cultures of B. monnieri on MS1 medium with the addition of 0.1 g/L zinc hydroaspartate and 0.1 g/L magnesium sulphate ().
Figure 9. The total content of indole and phenolic compounds in biomass from an in vitro culture of Bacopa monnieri on Murashige and Skoog (MS1) liquid medium and on the same medium with the addition of 0.1 g/L magnesium sulphate; 0.1 g/L zinc hydroaspartate; 0.1 g/L l-tryptophan; 0.25 g/L serine; 0.5 g/L serine and 0.5 mg/L anthranilic acid.
Indole compounds
l-Tryptophan is an essential amino acid for humans; therefore, it needs to be delivered to the body with food. In the central nervous system of the human body, l-tryptophan is modified to serotonin and melatonin. The hypnotic and antidepressant activities (it crosses the blood–brain barrier and is metabolized to serotonin) of this amino acid have been known for a long time (Stone et al. Citation2003; Buczko et al. Citation2005). l-Tryptophan is sometimes combined with other antidepressants in depression therapy. It is also contained in some dietary supplements used in the treatment of depression, stress and sleep disorders.
Serotonin has a wide spectrum of pharmacological activity and it acts as a neurotransmitter in the central nervous system and, together with melatonin, is a regulator of the daily rhythm. Moreover, it is produced not only in the brain but mainly by enterochromaffin-like cells in the duodenum and it is involved in the contraction of smooth muscles, regulates gastrointestinal motility, affects blood pressure, participates in blood clotting and acts as an antioxidant (Turner et al. Citation2006; Kaneez & Arshad Citation2007: Keszthelyi et al. Citation2009). Serotonin produced endogenously in the brain is involved in the regulation of sleep, anxiety, aggression, body temperature, mood, maturation process, regeneration and reduction of cell-aging processes which lead to a general improvement of the immunological system of the body. It is also one of the agents that regulates the contraction and relaxation of blood vessels (Ouchi et al. Citation2009). Due to the range of serotonin activities, there are seven types of serotonin receptors (5-HT) and several subtypes of each type.
Labelled indole compounds from biomass of B. monnieri are l-tryptophan and serotonin. The highest (excluding results with the addition of 0.1 g/L of l-tryptophan) amount of l-tryptophan and serotonin was achieved in biomass with the addition of 0.1 g/L zinc hydroaspartate and this was 0.72 mg/g DW and 1.19 mg/g DW, respectively. Compared with the control, these concentrations are more than six (for l-tryptophan) or four times (for serotonin) higher. The second best result was on medium containing 0.1 g/L magnesium sulphate, i.e., 0.44 mg/g DW and 0.56 mg/g DW, respectively. In the case of l-tryptophan addition to the medium, its influence was only observed on l-tryptophan level in the biomass (an almost 10-fold increase). This suggests that the added compound passed to the cultured explants. However, the mere fact of the prevalence of indole compounds in the plant might suggest that these secondary metabolites can support the antidepressant effect of bacosides. Zinc and magnesium are activators of enzymes which may explain the increase of the metabolites. It should be noted that the levels of l-tryptophan and serotonin are high when compared with garlic (approx. 0.1 mg/g DW l-tryptophan) and Hypericum perforatum from in vitro cultures (Muszyńska et al. Citation2014) or an edible mushroom Leccinum scabrum (approx. 0.27 mg/g DW l-tryptophan and 0.2 mg/g DW serotonin) (Muszyńska et al. Citation2013).
Phenolic compounds
Phenolic acids exhibit a wide spectrum of biological activities which have been attributed to their strong antioxidant power and their ability to protect important cellular structures such as cell membranes, structural proteins, enzymes, membrane lipids or nucleic acids against oxidative damage. The strongest antioxidant properties and capability of cell protection against hydrogen peroxide were found for vanillic acid and, among cinnamic acid derivatives, for caffeic acid. Caffeic acid also shows immunomodulatory and anti-inflammatory activity. Phenolic compounds are the most abundant antioxidants in the human diet (Scalbert et al. Citation2005; Terpinc & Abramovic Citation2010). Chlorogenic acid has a modest but significant blood pressure lowering effect (Onakpoya et al. Citation2015). Due to their physiological activity, phenolic acids are generally identified in biomass from in vitro cultures of medicinal plants (Szopa & Ekiert Citation2014; Bastos et al. Citation2015).
Phenolic compounds were examined during the process of phytochemical screening by Subashri and Koilpillai (Citation2012) and Shah et al. (Citation2012) who only reported that these kinds of secondary metabolites are found in B. monnieri. Both assigned the antioxidant properties of this plant to flavonoids and phenolic compounds. We found three phenolic compounds in B. monnieri. The highest concentrations of chlorogenic acid (0.70 mg/g DW) and neochlorogenic acid (0.40 mg/g DW) were found in material supplemented with 0.5 g/L serine, and caffeic acid (0.04 mg/g DW) in biomass supplemented with 0.1 g/L l-tryptophan. In in vtiro shoot culture of Exacum affine, the content of chlorogenic acid was lower (0.12 mg/g DW) than in biomass of under study material (Skrzypczak-Pietraszek et al. Citation2014). The content of the examined phenols in B. monneri is high for plants, e.g., in Ribes nigrum leaves, the average amount of chlorogenic acid is 0.1 mg/g DW and neochlorogenic acid is 0.14 mg/g D.W. (Vagiri et al. Citation2015). In seeds of Setaria italica, the levels are as follows: chlorogenic acid 0.1 mg/g DW and caffeic acid 0.04 mg/g DW (Zhang & Liu Citation2015).
Fatty acids
The largest percentage of the labelled saturated fatty acid is palmitic acid (32.6%), which is a common fatty acid found in animals, plants and microorganisms. Stearic acid (11.7%) is the next most common of the examined saturated fatty acids in B. monnieri. Also, in nature, it is one of the most common after palmitic acid. In the case of the examined unsaturated fatty acids, linoleic acid (17.0%) has the greatest content. This is an essential fatty acid and it is used in the biosynthesis of arachidonic acid. It belongs to the omega-6 group. The second most common unsaturated fatty acid is oleic acid (15.7%). Oleic acid may hinder the progression of adrenoleukodystrophy, a disease that affects the brain and adrenal glands (Rizzo et al. Citation1986). Oleic acid may be responsible for the hypotensive (blood pressure reducing) effects of olive oil (Teres et al. Citation2008). The third most common unsaturated fatty acid is myristoleic acid (12.6%). Unlike the previous two, this is rare in nature. One of the major sources of this fatty acid is the seed oil from plants of the Myristicaceae, comprising up to 30% of the oil in some species. It is a constituent of Serenoa or Saw palmetto, and appears to have activity against LNCaP prostate-cancer cells (Iguchi et al. Citation2001).
Bacosides
The most effective medium for bacoside A accumulation in the B. monnieri biomass from in vitro culture (28.9 mg/g DW) was the MS medium with the addition of 0.1 mg/g anthranilic acid. The lowest accumulation rate was estimated in the biomass from the medium with addition of l-tryptophan (15.5 mg/g DW). However, even this value is much higher than in the case of the application of methyl jasmonate (8.81 mg/g DW bacosides) (Sharma et al. Citation2013) or sodium chloride (1.32 mg/g DW bacosides) (Ahire et al. Citation2013) into the medium for B. monnieri in vitro culture. For the combination of methyl jasmonate and salicylic acid, the content of bacoside A in biomass of B. monnieri was 59.18 mg/g DW (Largia et al. Citation2015).
Anti-inflammatory properties of Bacopa monnieri extract
A significant repression of COX-2 and cPGES proteins was noted both in the cells treated with B. monnieri extracts alone and in the cells activated with LPS which may be due to synergistic anti-inflammatory effects of indolic, phenolic, fatty acids, bacoside A and other compounds in B. monnieri extract. Cyclooxygenase-2 is a key enzyme in the inflammatory response, decreasing the expression of this protein by some compounds may be regarded as an anti-inflammatory characteristic. COX-2 inhibition significantly decreased prostaglandin E2 production and also repressed cPGES activity (Gdula-Argasińska et al. Citation2015). The anti-inflammatory properties of B. monnieri metabolites thus provide enable further research.
Conclusion
Our proposed method of in vitro cultures proved that the essential micronutrients (zinc and magnesium) and organic compounds (anthranilic acid, l-tryptophan and serine) had an influence on biomass growth and the levels of metabolites. These studies may enable us to obtain biomass with improved therapeutic properties. Hence, the possibility to transfer the results we obtained on the basis of technologically fortified varieties of selected elements and micronutrients is considered to be very real. This suggests that the next step should be to estimate the release and bioavailability of elements and metabolites from in vitro cultured plants in in vitro and in vivo conditions.
Disclosure statement
The authors report that they have no conflicts of interest.
References
- Ahire ML, Walunj PR, Kishor PBK, Nikam TD. 2013. Effect of sodium chloride-induced stress on growth, proline, glycine betaine accumulation, antioxidative defense and bacoside A content in in vitro regenerated shoots of Bacopa monnieri (L.) Pennell. Acta Physiol Plant. 35:1943–1953.
- Anonymous. 1999. The Ayurvedic Pharmacopoeia of India, Part-I, vol. II, 1st ed. New Delhi: Ministry of Health and Family Welfare, Govt. of India.
- Anonymous. 2001. The Ayurvedic Pharmacopoeia of India. Part-I, vol. III. New Delhi: Ministry of Health and Family Welfare, Govt. of India.
- Bastos C, Barros L, Dueñas M, Calhelha RC, Queiroz MJ, Santos-Buelga C, Ferreira IC. 2015. Chemical characterisation and bioactive properties of Prunus avium L.: the widely studied fruits and the unexplored stems. Food Chem. 173:1045–1053.
- Biswas SK, Das J, Chowdhury A, Karmakar UK, Hossain H. 2012. Evaluation of antinociceptive and antioxidant activities of whole plant extract of Bacopa monniera. Res J Med Plant. 6:607–614.
- Buczko P, Cylwik D, Stokowska W. 2005. Metabolism of tryptophan via the kynurenine pathway in saliva. Post Hig Med Doświad. 59:283–289 (in Polish).
- Calabrese C, Gregory WL, Leo M, Kraemer D, Bone K, Oken B. 2008. Effects of a standardized Bacopa monnieri extract on cognitive performance, anxiety, and depression in the elderly: a randomized, double-blind, placebo-controlled trial. J Altern Complement Med. 14:707–713.
- Ceasar SA, Maxwell SL, Prasad KB, Karthigan M, Ignacimuthu S. 2010. Highly efficient shoot regeneration of Bacopa monnieri (L.) using a two-stage culture procedure and assessment of genetic integrity of micropropagated plants by RAPD. Acta Physiol Plant. 32:443–452.
- Chatterji N, Rastogi RP, Dhar ML. 1963. Chemical examination of Bacopa monniera Wenst.: Part I – isolation of chemical constituents. Int J Chem. 1:212–215.
- Ellnain-Wojtaszek M, Zgórka G. 1999. High-performance liquid chromatography and thin-layer chromatography of phenolic acids from Ginkgo biloba L. leaves collected within vegetative period. J Liq Chrom Rel Tech. 22:1459–1471.
- Esposito E, Cuzzocrea S. 2010. Antiinflammatory activity of melatonin in central nervous system. Curr Neuropharmacol. 8:228–242.
- Gdula-Argasińska J, Czepiel J, Woźniakiewicz A, Wojtoń K, Grzywacz A, Woźniakiewicz M, Jurczyszyn A, Perucki W, Librowski T. 2015. n-3 Fatty acids as resolvents of inflammation in the A549 cells. Pharmacol Rep. 6:610–615.
- Gohil KJ, Patel JA. 2010. A review on Bacopa monniera: current research and future prospects. Int J Green Pharm. 4:1–9.
- Iguchi K, Okumura N, Usui S, Sajiki H, Hirota K, Hirano K. 2001. Myristoleic acid, a cytotoxic component in the extract from Serenoa repens, induces apoptosis and necrosis in human prostatic LNCaP cells. Prostate. 47:59–65.
- Jyoti A, Sharma D. 2006. Neuroprotective role of Bacopa monniera extract against aluminium-induced oxidative stress in the hippocampus of rat brain. NeuroToxicol. 27:457.
- Jyoti A, Sethi P, Sharma D. 2007. Bacopa monniera prevents from aluminium neurotoxicity in the cerebral cortex of rat brain. J Ethnopharmacol. 111:56–62.
- Kamkaew N, Scholfield CN, Ingkaninan K, Taepavarapruk N, Chootip K. 2013. Bacopa monnieri increases cerebral blood flow in rat independent of blood pressure. Phytother Res. 27:135–138.
- Kaneez FS, Arshad SS. 2007. The metabolism of serotonin in neuronal cells in culture and platelets. Exp Brain Res. 183:411–416.
- Keszthelyi D, Troost FJ, Masclee AM. 2009. Understanding the role of tryptophan and serotonin metabolism in gastrointestinal function. Neurogastroenterol Motil. 21:1239–1249.
- Largia MJV, Pothiraj G, Shilpha J, Ramesh M. 2015. Methyl jasmonate and salicylic acid synergism enhances bacoside A content in shoot cultures of Bacopa monnieri (L.). Plant Cell Tiss Organ Cult. 122:9–20
- Łojewski M, Muszyńska B, Smalec A, Reczyński W, Sułkowska-Ziaja K, Opoka W. 2014. Development of optimal medium content for bioelements accumulation in Bacopa monnieri (L.) in vitro culture. Appl Biochem Biotechnol. 174:1535–1540.
- Madhavi T, Mahitha B, Mallikarjuna K, Sushma NJ. 2013. Therapeutic effect of Bacopa monniera against aluminium induced toxicity in Medulla oblongata of albino rat. J Med. Sci. 13:465–470.
- Murashige T, Skoog F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 15:473–497.
- Muszyńska B, Sułkowska-Ziaja K, Ekiert H. 2009. Indole compounds in fruiting bodies of some selected Macromycetes species and in their mycelia cultured in vitro. Pharmazie. 64:479–480.
- Muszyńska B, Sułkowska-Ziaja K. 2012. Analysis of indole compounds in edible Basidiomycota species after thermal processing. Food Chem. 132:455–495
- Muszyńska B, Sułkowska-Ziaja K, Wójcik A. 2013. Levels of physiologically active indole derivatives in the fruiting bodies of some edible mushrooms (Basidiomycota) before and after thermal processing. Mycoscience. 54:321–326.
- Muszyńska B, Ekiert H, Kwiecień I, Maślanka A, Zodi R, Beerhues L. 2014. Comparative analysis of therapeutically important indole compounds in in vitro cultures of Hypericum perforatum cultivars by HPLC and TLC analysis coupled with densitometric detection. NPC. 10:1437–1440.
- Onakpoya IJ, Spencer EA, Thompson MJ, Heneghan CJ. 2015. The effect of chlorogenic acid on blood pressure: a systematic review and meta-analysis of randomized clinical trials. J Hum Hypertens. 29:77–81.
- Ouchi Y, Yoshikawa E, Futatsubachi M, Yagi S, Ueki T, Nakamura K. 2009. Altered brain serotonin transporter and associated glucose metabolism in Alzheimer disease. J Nucl Med. 50:1260–1266.
- Peth-nui T, Wattanathorn J, Muchimapura S, Tong-Un T, Piyavhathkul N, Rangseekajee P, Ingkaninan K, Vitaya-areeku S. 2012. Effects of 12-week Bacopa monnieri consumption on attention, cognitive processing, working memory, and functions of both cholinergic and monoaminergic systems in healthy elderly volunteers. Evidence-Based Complem Alternat Med. 6:81–90.
- Pratibha S. 2012. Stability studies of crude plant material of Bacopa monnieri and quantitative determination of bacopaside I and bacoside A by HPLC. Phytochem Anal. 23:502–507.
- Rastogi M, Ojha RP, Prabu PC, Devi BP, Agrawal A, Dubey GP. 2012. Prevention of age-associated neurodegeneration and promotion of healthy brain ageing in female Wistar rats by long term use of bacosides. Biogerontology. 13:183–195.
- Rizzo WB, Watkins PA, Phillips MW, Cranin D, Campbell B, Avigan J. 1986. Adrenoleukodystrophy: oleic acid lowers fibroblast saturated C22-26 fatty acids. Neurology. 36:357–361.
- Scalbert A, Johnson IT, Saltmarsh M. 2005. Polyphenols: antioxidants and beyond. Am J Clin Nutr. 1:2155–2175.
- Shah M, Behara YR, Jagadeesh B. 2012. Phytochemical screening and in vitro antioxidant activity of aqueous and hydroalcoholic extract of Bacopa monnieri Linn. IJPSR. 3:3418–3424.
- Sharma P, Yadav S, Srivastava A, Shrivastava N. 2013. Methyl jasmonate mediates upregulation of bacoside A production in shoot cultures of Bacopa monnieri. Biotechnol Lett. 35:1121–1125.
- Singhal NK, Srivastava G, Agrawal S, Jain SK, Singh MP. 2012. Melatonin as a neuroprotective agent in the rodent models of Parkinson's disease: is it all set to irrefutable clinical translation? Mol Neurobiol. 45:186–199.
- Singh HK. 2013. Brain enhancing ingredients from Āyurvedic medicine: quintessential example of Bacopa monniera, a narrative review. Nutrients. 5:478–497.
- Skrzypczak-Pietraszek E, Słota J, Pietraszek J. 2014. The influence of L-phenylalanine, methyl jasmonate and sucrose concentration on the accumulation of phenolic acids in Exacum affine Balf. f. ex Regel shoot culture. Acta Biochim Pol. 61:47–53.
- Stone TW, Mackay GM, Forrest CM, Clark CJ, Darlington LG. 2003. Tryptophan metabolites and brain disorders. Clin Chem Lab Med. 41:852–859.
- Subashri B, Koilpillai YJ. 2012. Phytochemical analysis and in vitro antioxidant activity from the extract of Bacopa monnieri (L.) Pennel – a multipurpose medicinal plant. Int J Pharm Bio Sci. 2:698–702.
- Szopa A, Ekiert H. 2014. Production of biologically active phenolic acids in Aronia melanocarpa (Michx.) Elliott in vitro cultures cultivated on different variants of the Murashige and Skoog medium. J Plant Growth Regul. 72:51–58.
- Teres S, Barceló-Coblijn G, Benet M, Alvarez R, Bressani R, Halver JE, Escribá PV. 2008. Oleic acid content is responsible for the reduction in blood pressure induced by olive oil. Proc Natl Acad Sci USA. 105:13811–13816.
- Terpinc P, Abramovic H. 2010. A kinetic approach for evaluation of the antioxidant activity of selected phenolic acids. Food Chem. 121:366–371.
- Turner EH, Loftis JM, Blackwell AD. 2006. Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan. Pharmacol Ther. 109:325–338.
- Vagiri M, Conner S, Stewart D, Andersson SC, Verrall S, Johansson E, Rumpunen K. 2015. Phenolic compounds in blackcurrant (Ribes nigrum L.) leaves relative to leaf position and harvest date. Food Chem. 172:135–142.
- Wasnik U, Singh V, Ali M. 2012. Evaluation of anticonvulsant activity on leaves of alcoholic extract of Bacopa monnieri Linn. Int J Pharm Sci Rev Res. 17:1–5.
- Wurst M, Kysilka R, Flieger M. 2004. Psychoactive tryptamines from basidiomycetes. Folia Microbiol (Praha). 47:3–27.
- Yeh CT, Ching LC, Yen GC. 2009. Inducing gene expression of cardiac antioxidant enzymes by dietary phenolic acids in rats. J Nutr Biochem. 20:163–171.
- Yuan JP, Kuang HC, Wang JH, Liu X. 2008. Evaluation of ergosterol and its esters in the pileus, gill, and stipe tissues of agaric fungi and their relative changes in the comminuted fungal tissues. Appl Microbiol Biotechnol. 80:459–465.
- Zhang LZ, Liu RH. 2015. Phenolic and carotenoid profiles and antiproliferative activity of foxtail millet. Food Chem. 174:495–450.