Abstract
Context: Amitriptyline (AT), one of the tricyclic antidepressants, is still widely used for the treatment of the depression and control of anxiety states and panic disorders in the developing countries.
Objective: This study evaluates the catalytic activities of CYP2D6*1, CYP2D6*2, CYP2D6*10 and 22 novel alleles in Han Chinese population and their effects on the N-demethylation of AT in vitro.
Materials and methods: CYP2D6*1 and 24 CYP2D6 allelic variants were highly expressed in insect cells, and all variants were characterized using AT as a substrate. Reactions were performed at 37 °C with 10–1000 μM substrate for 30 min. We established a HPLC method to quantify the levels of nortriptyline (NT). The kinetic parameters Km, Vmax and intrinsic clearance (Vmax/Km) of NT were calculated.
Results: Among the 24 CYP2D6 variants, all variants exhibited decreased intrinsic clearance values compared with wild-type CYP2D6.1. Kinetic parameters of two CYP2D6 variants (CYP2D6*92, *96) could not be determined because of absent enzyme activities.
Conclusions: The comprehensive in vitro assessment of CYP2D6 variants provides significant insight into allele-specific activity towards AT in vivo.
Introduction
Cytochrome 2D6 (CYP2D6) is one of five large subfamilies on CYP450, playing an important role in human xenobiotic-metabolizing. Although proportion of CYP2D6 in CYP450 is only about 2%, more than 40 drugs are based on the metabolism of isoenzyme CYP2D6, including antiarrhythmics, antidepressants, antipsychotics, β-adrenergic blockers, analgesics (Rendic Citation2002; Funae et al. Citation2003; Marechal et al. Citation2008; Zhou Citation2009) and opioids (Ingelman-Sundberg Citation2005). In a previous study, more than 100 CYP 2D6 allelic variants found in the human hepatic enzymes of which many led to difference in enzyme activity and drugs metabolism (Ingelman-Sundberg et al. Citation2007). In particular, some of tricyclic antidepressants (e.g., amitriptyline [AT] and nortriptyline [NT]) have been shown large inter-individual metabolic variations which proved to be due to genetic polymorphism of enzymes involved in biotransformation (Brosen Citation2004; Grasmader et al. Citation2004).
Although selective serotonin reuptake inhibitors have become the main pharmacotherapeutic tools in clinical setting, AT, one of the tricyclic antidepressants, is still widely used for the treatment of depression (Barbui & Hotopf Citation2001) and control of anxiety states and panic disorders in developing countries. And, recently, some studies have found that it also has certain effect in the adjuvant therapy of tumour (Kulaksiz-Erkmen et al. Citation2013; Gewandter et al. Citation2014). AT has many metabolic paths in the liver, among which can be partially catalysed by CYP2D6 into an active production NT.
In our recent study, we sequenced to analyse all nine exons of the CYP2D6 gene in 2129 unrelated, healthy Chinese individuals from northern and southern regions. As a result, 22 new non-synonymous, mutated sites were found (Qian et al. Citation2013), 12 of them were defined as the novel alleles (*87–*93, *94A, *94B and *95–*98) by the Human CYP Allele Nomenclature Committee. Even though the frequency of genetic polymorphism of enzyme CYP2D6 is very low, based on that China has a population of 1.4 billion. So, it is very urgent to further systematical research. Furthermore, the previous study revealed that 22 new identified variants significantly affected the catalytic activity of CYP2D6 towards dextromethorphan and bufuralol in transfected COS-7 cell (Dai et al. Citation2015), but considering the fact that the low expression of CYP2D6 in a mammalian cell expression system, resulting in the difficulty of the analysis of catalytic activity. In the present study, we highly expressed the wild-type CYP2D6*1 and newly found variants in Sf21 insect cell. Then, we used AT as the probe substrate. Our data indicated that most of 24 alleles displayed significantly decreased intrinsic clearance values compared with the wild type.
Materials and methods
AT and NT were purchased from Sigma-Aldrich (St. Louis, MO). Desipramine was obtained from Toronto Research Chemicals Inc. (Toronto, Ontario, Canada). Baculosomes co-expressing human CYP2D6 and NADPH cytochrome P450 oxidoreductase (OR) or cytochrome b5 and OR were obtained from BD Gentest (Woburn, MA). The NADPH-regenerating system was purchased from Promega (Madison, WI). High-pressure liquid chromatog-Raphy-grade solvents were purchased from Merck (Darmstadt, Germany). All of the other chemicals and solvents were of the highest grade or analytical grade commercially available.
Conditions for enzymatic activity analysis
Insect microsomes expressing CYP2D6*1 and 24 CYP2D6 allelic variants were prepared according to the methods reported before. Reaction mixtures included as follows: 5–10 pmol of CYP2D6 from insect microsomes (5 pmol for CYP2D6*1 or 10 pmol for other variants), 10–20 pmol purified cytochrome B5 (CYP2D6/B5 = 1:1) and the substrate AT 10–1000 μM in 100 mmol/L potassium phosphate buffer (pH 7.4). The reaction preincubated for 5 min at 37 °C. Then, an NADPH regeneration system containing 1.3 mmol/L NADP+, 3.3 mmol/L glucose-6-phosphate, 3.3 mmol/L magnesium chloride and 0.4 U/mL glucose-6-phosphate dehydrogenase was added to start the reaction at 37 °C in a final volume of 200 μL, and the reaction was allowed to proceed for 30 min in a shaking water bath (150 rpm). Then incubation was terminated by adding 50 μL 0.1 M NaOH and 25 ng/μL desipramine as an internal standard. The mixtures were allowed to use 900 μL ethyl acetate for the extraction. After thoroughly vortexing for 2 min, the reaction mixture was centrifuged at 12,000 rpm for 8 min. The organic phase was transferred into a clean tube and evaporated to dryness under a nitrogen stream at 45 °C. The residues were dissolved in 100 μL mobile phase and used for the following analysis of two targeted compound above. The incubations were performed in triplicate, and data are determined as the mean ± SD from three experiments.
Measurement of metabolite by HPLC
Concentrations of AT and NT were determined by the high performance chromatography system (HPLC) with a ultraviolet detector (Agilent Technologies, Santa Clara, CA) at 40 °C. HPLC was equipped with a ZORBAX Eclipse Plus C18 column (Rapid Resolution HD, 3.0 mm × 100 mm, id 1.8 μm; Agilent Technologies) injected Aliquots of samples (1 μL). The mobile phase consisted of 60% H2O with 0.05% trifluoroacetic acid and 40% acetonitrile at a flow rate of 0.3 mL/min. The formation of NT was monitored with ultraviolet detection at excitation wavelength of 240 nm and emission wavelength of 360 nm, respectively. Under these conditions, the retention times of NT, AT and desipramine were 6.17, 6.832 and 5.389 min, respectively. The concentration of the standard curve for targeted compound is 10, 25, 50, 100, 250, 500, 1000 μg/mL. All data were collected and integrated with Agilent Open LAB CDSChemStation Edition software.
Statistical analysis
The values of Km and Vmax were estimated using a software program designed for the nonlinear regression analysis of a hyperbolic Michaelis–Menten equation (Prismversion 5; GraphPad Software Inc., San Diego, CA). The intrinsic clearance (CLint) were presented as the ratio of Vmax/Km. Kinetic data for each variant are determined as the mean ± SD of three microsomal preparations derived from separate transfections, and the results were analysed using one-way analysis of the variance.
Results
The kinetic parameters are summarized in , and Michaelis–Menten plots for all CYP2D6 variants are shown in . Using the baculovirus-insect cell system as a recombinant enzyme source, we previously investigated the catalytic activities of 24 CYP2D6 allelic variants towards the typical substrate, bufuralol (Cai et al. Citation2016). Similar to that, our results displayed all the allelic variants significantly decreased the intrinsic clearance values towards AT compared with CYP2D6*1. As described in , of 24 variants, CYP2D6*92 and CYP2D6*96 had no detectable enzyme activity towards AT. Compared with the wild-type protein CYP2D6*1, other 22 variants (except CYP2D6*92 and *96) showed increased Km values and 18 variants (CYP2D6 *10, *87–*91, *93–*95, *98, R344Q, *97, F164L, R497C, V327M, F219S, R25Q, R440C, E215K) exhibited reduced Vmax values.
Figure 1. Michaelis–Menten curves of the enzymatic activity of the recombinant wild-type CYP2D6 protein and 24 variants towards AT (each point represents the mean ± SD of three or four separate experiments). The variants have been manually arranged into six different groups (A–F) in the order of the designated allele names.
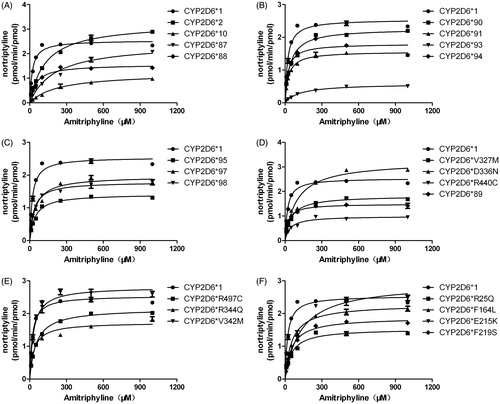
Table 1. Kinetic parameters for catalytic activities of recombinant wild-type and mutant CYP2D6 proteins against AT.
In this study, using AT as a substrate assess the catalytic activities of wild-type CYP2D6 and 24 allelic variants. As described , the relative intrinsic clearance of 24 variants tested could be manually clarified into three different parts: 14 variants (CYP2D6*2, *10, *87, *88, *92, *93, *95, *96, *E215K, *R440C, *V327M, *D336N, *F219S, *R25Q) exhibited values below 30% of counterparts of CYP2D6*1 and regarded as the seriously defective group. Nine variants (CYP2D6*89, 90, 91, 94, 97, 98, R344Q, F164L, R497C) exhibited values ranged from 30% to 60%, which can be classified into the middle defective variant; only one variant (CYP2D6*V327M) showed the largest relative intrinsic value (71.42%) and should be grouped into the mild defective one.
Discussion
Hepatic enzyme CYP2D6 plays an important role in the metabolism of about 25% drugs clinically used (Ingelman-Sundberg Citation2005). CYP2D6 is the most polymorphic enzyme with over 100 variants among all the cytochrome P450 designated by the Human CYP Allele Nomenclature Committee (http://www.cypallleles.ki.se/cyp2-d6.htm). Genetic polymorphism can decrease or enhance the metabolism of homologous drugs, according to which, patients can be classified into four groups: ultrarapid metabolizers (UMs), extensive metabolizers (EMs), intermediate metabolizers (IMs), poor metabolizers (PMs) (Ingelman-Sundberg Citation2004, Citation2005; Zhou Citation2009). Several studies investigated CYP2D6 genetic variation on the metabolism of antidepressants, and found its better response in impaired CYP2D6 activity (Tsai et al. Citation2010) or worse response in UMs (Rau et al. Citation2004; Gex-Fabry et al. Citation2008; Kwadijk-de Gijsel et al. Citation2009). CYP2D6 accounts for only about 2% of the total hepatic enzyme content and the frequencies of rare alleles was less than 1%; however, it is necessary and valuable for us to investigate the novel alleles on effect of drugs in China. It is impossible to study about the novel alleles on the metabolism of humans due to the low frequency in vivo; therefore, it is necessary to perform the experiments in vitro to elucidate the catalytic characters of the recombinant enzymes.
Considering on the fact of expense that the tricyclic antidepressants AT are still common used for the cure for patients with depression and treatment resistance (Crismon et al. Citation1999), NT is the one of its major active metabolites. Although CYP2D6 is not the main enzyme, it also has a certain effect on participating in the metabolism of AT. Now, the amount of CYP2D6 polymorphism was over 100 variants, which would lead to significantly differ in the drugs metabolism. Meanwhile, only just a few allelic variants (*2 and *10) were investigated in vitro using AT as the probe substrate, which displayed the decreased intrinsic clearance values and Vmax compared to CYP2D6*1 (Ramamoorthy et al. Citation2001; Niwa et al. Citation2011). Aside from above, no more other reported CYP2D6 mediated AT evaluated the metabolic characteristics.
In the previous study from our laboratory, we performed a large-scale genetic research of polymorphisms of CYP2D6 in 2129 randomly healthy Chinese Han subjects (Qian et al. Citation2013). Among these novel mutation sites, 22 were nonsynonymous and 12 were named as novel alleles by the Human CYP Allele Nomenclature Committee. Most of these novel allelic CYP2D6 variants exhibited significantly decreased catalytic abilities compared with CYP2D6*1 (Dai et al. Citation2015). When expressed in mammalian cells with probing substrates dextromethorphan and bufuralol. Even though previous reports revealed that either mammalian cells (COS-7) or baculovirus-mediated insect cells (Sf21) was suitable or acceptable for enzymatic activity analysis in vitro, it was extensively limited by the relatively low expression levels of exogenous proteins in mammalian cells, which had an effect on measuring the precision of enzymatic characteristics of CYP proteins with COS-7 expression system. In addition, balculovirus-mediated expression of mammalian CYP has been extensively used for evaluating the enzymatic activity of CYPs in vitro. We previously used CYP2D6 Probe substrate bufuralol to investigate the catalytic activity in insect cell with a high expression level for the wild-type CYP2D6*1 and 24 allelic variants. In the present study, the catalytic characteristics of 22 novel allelic variants found in the Chinese Han population towards another typical CYP2D6 phenotype probe substrate, AT, were further assessed in vitro.
To improve the reliability and suitability of the experiment for functional analysis, two typical defective alleles (*2 and *10) were included, which were the most widely investigated in the past. It was reported that CYP2D6*2 exhibited decreased intrinsic clearance towards dextromethorphan and fluoxetine of N-demetheylation (Yu et al. Citation2002). Our data showed approximately one-fifth intrinsic clearance values with respect to the CYP2D6*1. The previous study showed that CYP2D6*10 had higher Km, lower Vmax and lower intrinsic clearance values for bufuralol, atomoxetine and NT in vitro (Shen et al. Citation2007). As expected, CYP2D6*10 exhibited significantly increased Km (2-fold), nearly 10-fold Vmax and 5-fold intrinsic clearance value compared to the wild-type one. The results were brought into correspondence with the previous study (Ramamoorthy et al. Citation2001; Niwa et al. Citation2011), which showed that Sf21 expression system and metabolic activity analysis system was suitable for evaluating the functional characteristic of the other CYP2D6 allelic variants.
Using the baculovirus-mediated expressing system in insect cell microsomes, we previously assessed the catalytic activities of 24 CYP2D6 allelic variants towards bufuralol by Cai et al. (Citation2016). The data showed that most of allelic variants exhibited decreased intrinsic clearance values compared with the wild-type. In the present study, more allelic variants exhibited decreased intrinsic clearance values than that observed in the metabolism of bufuralol. For instance, 14 variants (CYP2D6*2, *10, *87, *88, *92, *93, *95, *96, *R25Q, *E215K, *F219S, *V327M, *D336N and *R440C) exhibited >30% lower intrinsic clearance values towards AT compared to the wild type CYP2D6*1, whereas only 6 variants (CYP2D6*10, *92, *93, *96, R25Q and E215K) exhibited a similar decreased level towards bufuralol. Moreover, nine variants (CYP2D6*89, -*91, *94, *97, *98, F164L, R497C and R344Q) exhibited moderately decreased clearance values for AT metabolism. Also, nine variants (CYP2D6*87, *88, *91, *95, *97, *98, F219S, D336N and R344Q) exhibited similar values in the metabolism of substrate bufuralol. But only one variant V342M displayed the value above 60%. Moreover, some allelic variants showed a different trend. In our study, CYP2D6*90 exhibited the moderate clearance intrinsic value (43.15%), while the corresponding value was more than 60% in previous studies (Dai et al. Citation2015; Cai et al. Citation2016). And, CYP2D6*2, CYP2D6*87, CYP2D6*88, CYP2D6*95 showed the relative clearance intrinsic values 20.34, 9.53, 25.46, 25.06% classified into the seriously defective group, respectively, whereas in previous research ranged from 30% to 60% (Cai et al. Citation2016) and even were above 60% (Dai et al. Citation2015). We speculated that the substrate-dependent intrinsic characteristics and metabolic path (hydroxylation and demethylation) of the CYP2D6 protein might be responsible for these results, as several groups have reported that a given CYP2D6 allelic variant can exhibit different catalytic activities towards different substrates in vitro and/or in vivo.
One thing must be pointed out that CYP2D6*92 and CYP2D6*96 displayed the absent enzyme activity at any substrate concentration. The previous study showed that two variants had a single nucleotide deletion and would cause a frame shift effect during protein translation, which resulted in the loss of the activity. The other two reported variants CYP2D6*8 and CYP2D6*20 exhibited the similar results (Zhou et al. Citation2009). Meanwhile, some research showed that many amino acid residues in the structure of protein CYP2D6 played an important role in the catalytic activity. The Glu216 was one of them locating in the F helix, thus the replacement of Glu216 could lead to significantly decrease the binding of amine substrates and exhibit reduced or almost abolished catalytic activity (Guengerich et al. Citation2003). Three variants (F215K, 218Frameshift and F219S) mutated in the F helix and obviously decreased the activity of enzyme. In our study, CYP2D6*92 (218Frameshift) had no detectable enzymatic activity, and two other mutants (F219S, E215K) exhibited a decreased catalytic activity approximately CYP2D6*2. In addition, CYP2D6*89 and CYP2D6*93 contained one nucleotide substitution in the cDNA which resulted in one amino acid change from Leu to Ser at position 142 and Thr to Pro at position 249, respectively. The Thr-249 residue was nearly located on the border of CYP2D6 active site cavity of the residues Phe-247 and Leu-248 (Rowland et al. Citation2006). It had been reported that the access channel for substrate entrance consist of three amine acids including Leu-248. So, we guessed that an amino acid changed at position 249 resulting in the transformation of the spatial structure of CYP2D6 protein, which hindered the combination the substrate and enzyme; our data provided evidence and further confirmed this fact. No previous functional evaluation data have demonstrated the importance of residue 142; however, our data indicated that L142S dramatically impaired the catalytic activity of the CYP2D6 protein.
Conclusions
We systematically assessed the functional consequences of 24 CYP2D6 allelic isoforms that existed in the Chinese subjects in vitro using the baculovirus-insect cell system. Our results revealed that all the allelic variants significantly exhibited decreased the enzyme activity towards AT metabolism. These data promoted our understanding of effect of CYP2D6 genetic polymorphism, also provided an important foundation for future use.
Funding information
This work was supported by one grant that was funded by the National Natural Science Foundation of China (No. 31371280) and a grant from the National Health and Family Planning Commission of the People’s Republic China (No. 201302008).
Acknowledgements
We are grateful to the members of the Beijing Institute of Geriatrics of the Ministry of Health and Wang Y for their advice and assistance.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Barbui C, Hotopf M. 2001. Amitriptyline v. the rest: still the leading antidepressant after 40 years of randomised controlled trials. Br J Psychiatry. 178:129–144.
- Brosen K. 2004. Some aspects of genetic polymorphism in the biotransformation of antidepressants. Therapie. 59:5–12.
- Cai J, Dai DP, Geng PW, Wang SH, Wang H, Zhan YY, Huang XX, Hu GX, Cai JP. 2016. Effects of 22 novel CYP2D6 variants found in the Chinese population on the bufuralol and dextromethorphan metabolisms in vitro. Basic Clin Pharmacol Toxicol. 118: 190–199.
- Crismon ML, Trivedi M, Pigott TA, Rush AJ, Hirschfeld RM, Kahn DA, DeBattista C, Nelson JC. 1999. The texas medication algorithm project: report of the texas consensus conference panel on medication treatment of major depressive disorder. J Clin Psychiatry. 60:142–156.
- Dai DP, Geng PW, Wang SH, Cai J, Hu LM, Nie JJ, Hu JH, Hu GX, Cai JP. 2015. In vitro functional assessment of 22 newly identified CYP2D6 allelic variants in the Chinese population. Basic Clin Pharmacol Toxicol. 117:39–43.
- Funae Y, Kishimoto W, Cho T, Niwa T, Hiroi T. 2003. CYP2D in the brain. Drug Metab Pharmacokinet. 18:337–349.
- Gewandter JS, Mohile SG, Heckler CE, Ryan JL, Kirshner JJ, Flynn PJ, Hopkins JO, Morrow GR. 2014. A phase III randomized, placebo-controlled study of topical amitriptyline and ketamine for chemotherapy-induced peripheral neuropathy (CIPN): a University of Rochester CCOP study of 462 cancer survivors. Support Care Cancer. 22:1807–1814.
- Gex-Fabry M, Eap CB, Oneda B, Gervasoni N, Aubry JM, Bondolfi G, Bertschy G. 2008. CYP2D6 and ABCB1 genetic variability: influence on paroxetine plasma level and therapeutic response. Ther Drug Monit. 30:474–482.
- Grasmader K, Verwohlt PL, Rietschel M, Dragicevic A, Müller M, Hiemke C, Freymann N, Zobel A, Maier W, Rao ML. 2004. Impact of polymorphisms of cytochrome-P450 isoenzymes 2C9, 2C19 and 2D6 on plasma concentrations and clinical effects of antidepressants in a naturalistic clinical setting. Eur J Clin Pharmacol. 60:329–336.
- Guengerich FP, Hanna IH, Martin MV, Gillam EM. 2003. Role of glutamic acid 216 in cytochrome P450 2D6 substrate binding and catalysis. Biochemistry. 42:1245–1253.
- Ingelman-Sundberg M. 2004. Pharmacogenetics of cytochrome P450 and its applications in drug therapy: the past, present and future. Trends Pharmacol Sci. 25:193–200.
- Ingelman-Sundberg M. 2005. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J. 5:6–13.
- Ingelman-Sundberg M, Sim SC, Gomez A, Rodriguez-Antona C. 2007. Influence of cytochrome P450 polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol Ther. 116:496–526.
- Kulaksiz-Erkmen G, Dalmizrak O, Dincsoy-Tuna G, Dogan A, Ogus IH, Ozer N. 2013. Amitriptyline may have a supportive role in cancer treatment by inhibiting glutathione S-transferase pi (GST-pi) and alpha (GST-alpha). J Enzyme Inhib Med Chem. 28:131–136.
- Kwadijk-De Gijsel S, Bijl MJ, Visser LE, van Schaik RH, Hofman A, Vulto AG, van Gelder T, Ch Stricker BH. 2009. Variation in the CYP2D6 gene is associated with a lower serum sodium concentration in patients on antidepressants. Br J Clin Pharmacol. 68:221–225.
- Marechal JD, Kemp CA, Roberts GC, Paine MJ, Wolf CR, Sutcliffe MJ. 2008. Insights into drug metabolism by cytochromes P450 from modelling studies of CYP2D6-drug interactions. Br J Pharmacol. 153:S82–S89.
- Niwa T, Murayama N, Yamazaki H. 2011. Comparison of cytochrome P450 2D6 and variants in terms of drug oxidation rates and substrate inhibition. Curr Drug Metab. 12:412–435.
- Qian JC, Xu XM, Hu GX, Dai DP, Xu RA, Hu LM, Li FH, Zhang XH, Yang JF, Cai JP. 2013. Genetic variations of human CYP2D6 in the Chinese Han population. Pharmacogenomics. 14:1731–1743.
- Ramamoorthy Y, Tyndale RF, Sellers EM. 2001. Cytochrome P450 2D6.1 and cytochrome P450 2D6.10 differ in catalytic activity for multiple substrates. Pharmacogenetics. 11:477–487.
- Rau T, Wohlleben G, Wuttke H, Thuerauf N, Lunkenheimer J, Lanczik M, Eschenhagen T. 2004. CYP2D6 genotype: impact on adverse effects and nonresponse during treatment with antidepressants – a pilot study. Clin Pharmacol Ther. 75:386–393.
- Rendic S. 2002. Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 34:83–448.
- Rowland P, Blaney FE, Smyth MG, Jones JJ, Leydon VR, Oxbrow AK, Lewis CJ, Tennant MG, Modi S, Eggleston DS, et al. 2006. Crystal structure of human cytochrome P450 2D6. J Biol Chem. 281:7614–7622.
- Shen H, He MM, Liu H, Wrighton SA, Wang L, Guo B, Li C. 2007. Comparative metabolic capabilities and inhibitory profiles of CYP2D6.1, CYP2D6.10, and CYP2D6.17. Drug Metab Dispos. 35:1292–300.
- Tsai MH, Lin KM, Hsiao MC, Shen WW, Lu ML, Tang HS, Fang CK, Wu CS, Lu SC, Liu SC, et al. 2010. Genetic polymorphisms of cytochrome P450 enzymes influence metabolism of the antidepressant escitalopram and treatment response. Pharmacogenomics. 11:537–546.
- Yu A, Kneller BM, Rettie AE, Haining RL. 2002. Expression, purification, biochemical characterization, and comparative function of human cytochrome P450 2D6.1, 2D6.2, 2D6.10, and 2D6.17 allelic isoforms. J Pharmacol Exp Ther. 303:1291–1300.
- Zhou SF. 2009. Polymorphism of human cytochrome P450 2D6 and its clinical significance: part I. Clin Pharmacokinet. 48:689–723.
- Zhou SF, Liu JP, Chowbay B. 2009. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev. 41:89–295.
