Abstract
Context: Red algae have been recognized as a rich natural source of compounds possessing interesting biological and pharmacological activities.
Objective: This work investigates anti-inflammatory, analgesic and gastroprotective activities of MeOH/CH2Cl2 crude extract and its fractions F1 (50% MeOH) and F2 (80% MeOH) from the whole alga plant Laurencia obtusa Hudson (Rhodomelaceae).
Materials and methods: Anti-inflammatory activity was evaluated in vitro using cytometric bead array (CBA) technology to follow up the secretion of tumour necrosis factor alpha (TNF-α) in lipopolysaccharide activated THP-1 monocytic cells at doses of 10–250 μg/mL and in vivo using carrageenan-induced paw oedema in Wistar rats at doses of 25, 50, 100 and 200 mg/kg. Crude extract and fractions were tested at the doses of 25, 50, 100 and 200 mg/kg for peripheral and central analgesic activity by acetic acid-induced writhing test and hot-plate method, respectively, in Swiss albino mice. Gastroprotective activity was evaluated using HCl/ethanol-induced gastric ulcer test in rats at doses of 25, 50, 100 and 200 mg/kg.
Results: Crude extract, F1 and F2 showed an interesting inhibition of TNF-α secretion with IC50 values of 25, 52 and 24 μg/mL, respectively, and a significant anti-inflammatory activity in vivo (p < 0.01), 3 h after carrageenan injection, the oedema inhibition was 55.37%, 52.18% and 62.86%, respectively, at the dose of 100 mg/kg. Furthermore, they showed a significant peripheral analgesic activity with 53.79%, 55.92% and 57.37% (p < 0.01) of writhing inhibition, respectively. However, no significant activity was found in the hot-plate test. An interesting gastroprotective effect was observed with crude extract and its fractions F1 and F2 with a gastric ulcer inhibition of 65.48%, 77.42% and 81.29%, respectively, at the dose of 50 mg/kg.
Discussion and conclusion: These results suggest that L. obtusa might be used as a potential source of natural anti-inflammatory and analgesic agents with gastroprotective effect.
Introduction
Inflammation is a physiological response of vascular tissues to harmful stimuli such as injury, pathogens or irritants and can be characterized by redness, swelling, pain, heat and dysfunction of the tissue and organs (Lawrence et al. Citation2002). During the process of inflammation, the host activates cellular immune responses that increase production of pro-inflammatory mediators, including the tumour necrosis factor alpha (TNF-α). TNF-α can regulate many cellular and biological processes, such as the production of other pro-inflammatory cytokines, like interleukin-6 (IL-6) and interleukin-1 (IL-1), to mediate and/or amplify their effects in peripheral organs (Cawthorn & Sethi Citation2008). Another effect of this cytokine is the release of pro-inflammatory mediators including bradykinin and histamine at the site of inflammation (Brennan & McInnes Citation2008).
Currently, several anti-inflammatory agents are used to treat different types of pain associated with inflammation. Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used because of their efficacy in reducing pain and inflammation (Laine et al. Citation2008); the pharmacological action of these agents was assigned to inhibit cyclooxygenase enzymes (Vane et al. Citation1998). Although their efficiency in most cases, NSAIDs were proven to induce the secretion of TNF-α especially when they are used chronically. Overproduction of TNF-α believes to play a key role in the onset and progression of various pathologies such as disseminated intravascular coagulation and death in septic choc and cerebral malaria (Medana et al. Citation1997; Murphy et al. Citation1998) and a range of inflammatory diseases including asthma (Björnsdottir & Cypcar Citation1999), dermatitis, inflammatory bowel disease, cystic fibrosis, rheumatoid arthritis and multiples sclerosis (Sekut & Connolly Citation1996); in addition, TNF-α has been shown to be a crucial mediator of NSAIDs-induced gastric mucosal injury (Santucci et al. Citation1994). In spite of enormous efforts, the only available drugs to inhibit TNF-α activity, in clinics, are proteins (Etanercept, Infliximab, Adalimumab and Anakinra) that display adverse effects such as aplastic anaemia, pancytopenia, vasculitis, demyelination and congestive heart failure (Desai & Furst Citation2006). Therefore, there is a continuing interest in the search of potent anti-inflammatory natural products that can block TNF-α signalling without side effects.
Red algae were reported to contain active secondary metabolites that may inhibit inflammation (Kang et al. Citation2008), prevent or treat gastric ulcers and cancers caused by oxidative stress (Gonzalez et al. Citation1999; Yeh et al. Citation2012), inhibit inflammatory activities by suppressing the production of inflammatory mediators (Khan et al. Citation2007, Citation2008) and induce cancer cell apoptosis in colon (Synytsya et al. Citation2010) and stomach (Kwon & Nam Citation2007). Natural compounds derived from the edible algae could be used as anti-inflammatory, analgesic and anti-ulcerogenic therapeutics as they have been taken in alimentation and used in traditional medicines in the last centuries (Dhargalkar & Pereira Citation2005). Among all marine red algal flora, species of the genus Laurencia (Rhodomelaceae) are always expected to be a rich source of novel bioactive secondary metabolites, especially sesquiterpenes, diterpenes and halogenated C15 acetogenins (Kladi et al. Citation2006; Ayyad et al. Citation2011; Angawi et al. Citation2014) with biological and pharmacological activities. In the continuation of our research on seaweeds, we report here the anti-inflammatory and analgesic potential of crude extract and its polar fractions of Laurencia obtusa Hudson with gastroprotective effects.
Materials and methods
Sample collection and extract preparation
L. obtusa was collected during June 2010, from the coastal region of Bizerte (Tunisia) in the Mediterranean Sea at a depth of 1–2 m. The identification was carried out by Dr Rafik Ben Said (National Institute of Marine Sciences and Technologies, Salambo, Tunisia). A voucher specimen was deposited in the Department of Pharmacology, Faculty of Pharmacy, Monastir University. The collected samples were transported to the laboratory and rinsed with sea water and distilled water to remove associated debris and epiphytes. The seaweeds were then air dried in the shade at 30 °C, powdered and finely packed in small bags (5 × 10 cm) of Whatman filter paper No. 1, all bags were sealed and soaked in MeOH/CH2Cl2 (1:1, v/v) bath for 48 h three times. The crude extract was concentrated to solvent free by evaporation in a rotating evaporator (Buchi B-480, Flawil, Switzerland) at low temperature (<40 °C).
Fractionation and purification of the crude extract
In order to localize the active fraction, we used the methods described by Dellai et al. (Citation2012) and Deghrigue et al. (Citation2015). Crude extract of L. obtusa was fractionated and purified, using C18 cartridges (Sep-pak, Supelco Sigma-Aldrich, Saint-Quentin-Fallavie, France), by gradient elution with methanol–water mixture (50% and 80% methanol) to give two fractions (F1 and F2). Methanol solvent was removed from fractions recuperated using rotating evaporator at 40 °C and aqueous phases were lyophilized. Fractions were then, stored at −20 °C until use.
Total phenolic content
Estimation of total phenolic content (TPC) of crude extract and its polar fractions F1 and F2 was carried out according to Folin-Ciocalteu method (Taga et al. Citation1984). The Folin-Ciocalteu reagent determines total phenols, producing blue colour by reducing yellow hetero polyphosphate molybdate tungstate anions. The absorbance of all sample solutions was measured at 720 nm using spectrophotometer (Jenway 6505 UV/Vis, Essex, UK). A calibration curve of gallic acid (GA) (0.03–1 mg/mL) was prepared, and TPC was determined.
Total flavonoid content
Total flavonoidw content (TFC) of crude extract and its polar fractions were determined by aluminium chloride (AlCl3) colorimetric method (Bahorun et al. Citation1996). The absorbance of all sample solutions was measured at 415 nm using spectrophotometer (Jenway 6505 UV/Vis, Essex, UK). The TFC was determined on a standard curve of rutin. The mean of three readings was used and expressed as mg of rutin equivalents per 1 g of sample (mg RE/g sample).
Total condensed tannins content
Condensed tannins content were measured using the modified vanillin assay described by Sun et al. (Citation1998). Four percent methanol vanillin solution (3 mL) and 1.5 mL of concentrated H2SO4 were added to 50 mL of suitably diluted sample. The well mixed solution was incubated at room temperature in the dark for 20 min; the absorbance of the reaction mixture was spectrophotometrically measured at 500 nm. The amount of 4% vanillin was substituted by the same amount of MeOH in blank. (+)-Catechin was used to make the calibration curve and condensed tannins were expressed as mg catechin equivalents per 1 g of sample (mg CE/g sample). The mean value of condensed tannin content was obtained from triplicate experiments.
Animals
All experiments were performed according to the guidelines for Animal Experimentation of Monastir University. Wistar rats (150–200 g) and albino mice (20–25 g) of both sexes purchased from Pasteur Institute (Tunis, Tunisia) were used. They were housed in groups of eight animals in plastic cages at 20–25 °C and maintained on a standard pellet diet with free access to water. Housing conditions and in vivo experiments were approved according to the guidelines established by the European Union on Animal Care (CCE Council 86/609).
Cell culture
Acute monocytic leukaemia cells (THP-1, ATCC® TIB-202) were maintained in RPMI-1640 medium (Life Technologies, Saint Aubin, France) with GlutaMax™, supplemented with 10% (v/v) foetal bovine serum (BioWhittaker, Verviers, Belgium) and 1% (v/v) penicillin-streptomycin (100 units/mL and 100 μg/mL, Life Technologies). Cells were grown in humidified atmosphere with 5% CO2 at 37 °C in 25 and 75 cm2 flasks up to 70–80% confluency prior to treatment. Cells were replicated every 2–3 days and the medium changed once in-between.
Cell viability assay
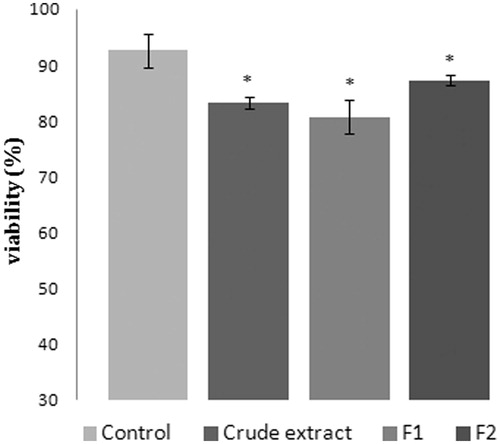
Trypan blue exclusion assays were carried out as previously described (Liu et al. Citation1999). THP-1 cells (at 2 × 105 cells/mL) were dispensed into 24-well microplates, in 1 mL of medium/well and incubated overnight at 37 °C, under 5% CO2. Cells were then treated with crude extract and its polar fractions for 24 h at the final concentration of 200 μg/mL. Control cultures were maintained under the same conditions. At the end of incubation time, six enumerations per sample were performed by immediate microscopic observation, after blue Trypan staining; using a KOVA counting cell (Hycor, VWR, Strasbourg, France). THP-1 cells that were no longer viable, which had damaged membranes allowed entry of the dye, were stained blue. Viability is expressed as the percentage of cells alive after contact with the different extracts.
Cytometric bead array immunoassay
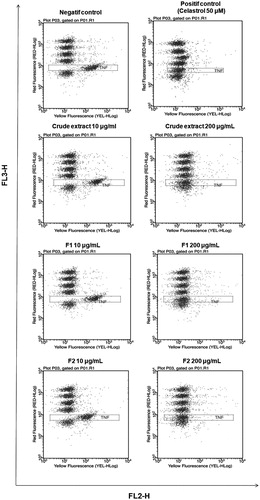
Human Th1/Th2 cytokine cytometric bead array (CBA) kits were obtained from BD Bioscience (Le Pont de Claix, France). This technique uses uniform-size microparticles based flow cytometry to measure a panel of six cytokines (IL-2, IL-4, IL-5, IL-10, IFNγ and TNF-α) simultaneously in a single sample. In our case, only TNF-α secretion could be obtained for PLS-activated THP-1 cells. THP-1 cells were seeded in 96-well plates (3 × 105 cells/mL). Increasing concentrations of samples (10, 20, 50, 100 and 200 μg/mL) dissolved in culture medium were freshly prepared from stock solution prior to each experiment. To appropriate wells containing 100 μL of cells in culture medium, 100 μL of samples in culture medium were added using a multichannel pipette to reach specified final concentrations, then, 2 μL of lipopolysaccharide (LPS) from Salmonella abortus equi (Sigma, L'Isle d'Abeau Chesnes, France) was added to reach a final concentration of 1 μg/mL. Positive and negative controls were included in all TNF-α secretion assays to ensure appropriate gating. Celastrol (50 μM) was used as a positive control for cytokine inhibition since it inhibits pro-inflammatory cytokine secretion in peripheral blood mononuclear cells (PBMC) in vitro (Allison et al. Citation2001; Pinna et al. Citation2004). LPS-stimulated cells, in culture medium without DMSO and compounds were used as a negative control. After treatment, 96-well plates were incubated in CO2 incubator for 6 h. Then, supernatant was separated by centrifugation (500g for 10 min) at 4 °C and stored at −20 °C until analysis was carried out.
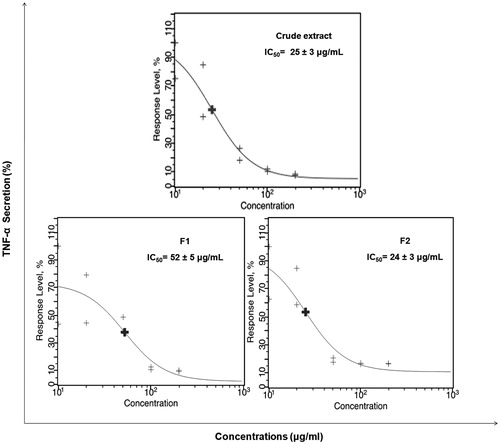
The assay kit was used according to the manufacturer instructions. The mixture was washed with the provided wash buffer and centrifuged at 500g for 5 min and the pellet was resuspended in 300 μL of wash buffer. Microcapillary flow cytometry system (Guava® EasyCyte, Guava Technologies, Hayward, CA) with blue (488 nm) and red (642 nm) laser was used for acquisition of in vitro inflammation assays. The capillary cytometer was calibrated with the setup beads then 3000 events were acquired for each sample. Dose–response curves and the IC50 concentration of compound, which inhibits TNF-α secretion by 50% after 6 h incubation were evaluated under the InCyte software 2.6 (Guava, Merck Millipore, USA). Dot plots and histograms were plotted with InCyte 2.6.
carrageenan-induced rat paw oedema
We determined the anti-inflammatory activity of crude extract and its polar fractions on carrageenan-induced paw oedema assay according to Winter et al. (Citation1962). Animals were divided into groups of six rats each: the control group received intraperitoneally (i.p.) dose of 0.9% saline solution (NaCl 9 g/L, 2.5 mL/kg); standard groups received reference drugs acetylsalicylate of lysine (ASL) (300 mg/kg, i.p.) and Diclofenac (10 mg/kg) and the test groups received the crude extract at the dose of (50, 100 and 200 mg/kg, i.p.) and its polar fractions at the doses of 25, 50 and 100 mg/kg, i.p. Lower and/or higher doses were administered, in order to study doses dependency of the anti-inflammatory activity. Thirty minutes after intraperitoneal administration of different substances, 0.05 mL of 1% carrageenan suspension was injected to all animals in the left hind paw. The paw volume up to the tibiotarsal articulation was measured using a Plethysmometer (model 7150, Ugo Basile, Varese, Italy) at 0 h (V0) before carrageenan injection and at 1, 2, 3, 4 and 5 h after carrageenan injection (Vt). Inhibition was obtained for each group using the following formula:
Analgesic activity
Acetic acid induced writhing in mice
The analgesic activity was performed according to the method of Koster et al. (Citation1959) and assessed by the acetic acid writhing test, a chemical visceral pain model. Swiss albino mice were selected 1 day prior to each test and were divided into groups of six mice each. One group served as control and was pretreated subcutaneous (s.c.) with 10 mL/kg of 0.9% saline; second group was pretreated with the reference drug, ASL at the dose of 200 mg/kg by the same route and this 30 min before intraperitoneal administration of 1% of acetic acid at the dose of 10 mL/kg. The remaining groups were injected i.p. with 10 mL/kg of 1% acetic acid solution 30 min after the administration of the crude extract at the doses of 50, 100 and 200 mg/kg, s.c. and its polar fractions at the doses (25, 50 and 100 mg/kg, s.c.). The number of writhing was recorded during 30 min after the acetic acid injection. A writhe is indicated by abdominal constriction and stretching of at least one hind limb. Analgesic activity was expressed as inhibition percent of the usual number of writhes observed in control animals. The percentages of inhibition were calculated according to the following formula:
Hot-plate method
The hot-plate test is one of the most common tests for evaluating the central analgesic activity of compounds. Central antinociceptive activities of crude extract and its polar fractions F1 and F2 against thermal noxious stimuli were measured by hot-plate test in Swiss albino mice as described by Eddy and Leimback (Citation1953). Mice were placed individually on a hot-plate analgesiameter (Ugo Basile, No. 35100, Varese, Italy), which was maintained at 55 ± 0.5 °C. The time between placement and licking of the paws or jumping was recorded as latency second. In order to minimize variability among animals, mice with latency time greater than 15 s on hot-plate pretesting were excluded from the test analysis (Colucci et al. Citation2008). Animals were divided into groups of six mice each. The control group was pretreated (s.c.) with 10 mL/kg of 0.9% saline; standards groups received reference drugs, ASL at the dose of 200 mg/kg and Tramadol at the dose of 25 and 50 mg/kg by the same route. The remaining groups were injected with 10 mL/kg of the crude extract at the doses of 50, 100 and 200 mg/kg, s.c. and its polar fractions at the doses (25, 50 and 100 mg/kg, s.c.). The latency time was recorded at 30, 60, 90 and 120 min following s.c. treatments.
Gastric lesions induced by HCl/ethanol
The gastroprotective activity of the crude extract and its polar fractions F1 and F2 was assessed using the HCl/ethanol-induced gastric ulcer on rat (Hara & Okabe Citation1985). Rats were divided into groups of six animals and fasted for 24 h prior test to make sure that stomachs of all rats are empty.
Control group received intraperitoneally dose of 0.9% saline (2.5 mL/kg, i.p.); test groups received by the same route crude extract (50, 100 and 200 mg/kg) and its polar fractions at doses (25 and 50 mg/kg) and standard groups received ranitidine (60 mg/kg, i.p.) and omeprazole (30 mg/kg, i.p.) as reference drugs. After 30 min, all groups received 10 mL/kg of HCl/ethanol (60% ethanol in 150 mM) orally, 1 h after the administration of ulcerogenic agent, animals were killed; the stomachs were rinsed under a stream of water and pinned flat on a cork board, the surface was examined for the presence of lesions and the extent of the lesions was measured. The summative length of the lesions along the stomach was recorded (mm) as a lesion index. The mean ulcer index was determined by dividing the total ulcer indices in a group by the total number of animals in that group. The percentage severity of ulceration was determined by dividing the scores of ulcers of each group by the total number of scores in the control group and the result multiplied by 100 (Akah et al. Citation1997).
Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM) and as percentage. Statistical analysis was performed using Student’s t-test. The significance of difference was considered to include values of p < 0.05.
Results
Phenolics, flavonoids and condensed tannins contents
Crude extract and its polar fractions F1 and F2 from the red alga L. obtusa were evaluated for their phenolic contents. Results are shown in . The levels of phenolic in crude extract (41 mg GAE/g dried sample) and F2 (19.21 mg GAE/g dried sample) were higher than levels in F1 with 14.23 mg GAE/g dried sample.
Figure 1. Total phenolics (A), flavonoids (B) and condensed tannins (C) contents of the crude extract and its polar fractions (F1 and F2) from the red alga L. obtusa. Values are expressed as mean ± SEM (n = 3). GAE: gallic-acid equivalent; RE: rutin equivalent.
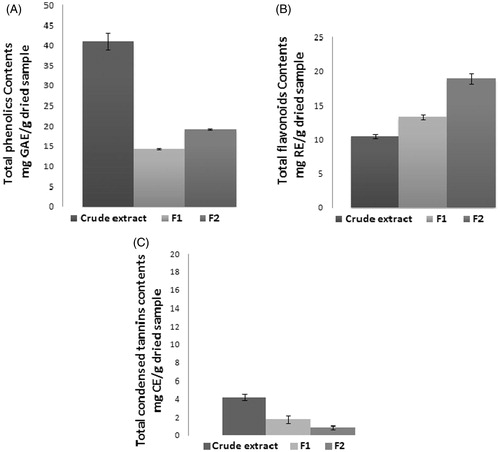
The levels of flavonoids in crude extract and its fractions F1 and F2 are also shown in . Flavonoids form internal complexes, chelate type, with Al3+, the intensity of the yellow colour of the kelate formed by the flavonoids when treated with AlCl3 in acetate buffer was spectrophotometrically determined. The levels of flavonoids in F1 and F2 were 13.33 and 18.93 mg RE/g dried sample, respectively, higher than crude extract with 10.52 mg RE/g dried sample.
Vanillin-concentrated HCl test is a quite reproducible and sensitive assay for the estimation of condensed tannins. The results revealed that crude extract F0 and its polar fractions F1 and F2 presented very low levels of condensed tannins with concentrations of 4.23, 1.78 and 0.87 mg CE/g dried sample ().
Cell viability in human THP-1 cells
After 24 h of incubation of THP-1 cells with 200 μg/mL of crude extract and its polar fractions F1 and F2, the Trypan blue exclusion test showed a cell viability of ≥80% ().
TNF-α secretion inhibitory activity
THP-1 cells were stimulated for 6 h by LPS and incubated with increasing concentrations of our samples (10–200 μg/mL). shows red fluorescence/yellow fluorescence dot plots of TNF-α secretion with negative and positive controls (Celastrol 50 μM), crude extract and its polar fractions in ranging doses (10 and 200 μg/mL). Positive control showed total inhibition of TNF-α secretion as compared to maximum TNF-α secretion. Crude extract and the fraction F2 showed at 200 μg/mL an excellent inhibition of the TNF-α secretion, similar to positive control whereas the fraction F1 showed a moderate inhibition of TNF-α secretion. Dose–response curves and IC50 values are displayed in . IC50 determination of TNF-α secretion was computed for the crude extract and its polar fractions. Crude extract and its polar fraction F2 showed higher in vitro anti-inflammatory activity with significant lower IC50 values of 25 and 24 μg/mL (p < 0.05), respectively, compared to the fraction F1 with IC50 value of 52 μg/mL.
Carrageenan paw oedema test
Results of the carrageenan rat paw oedema test are shown in . Carrageenan is used as a noxious agent inducing a severe inflammatory reaction (Borgi et al. Citation2007). The crude extract and its fractions F1 and F2 produced reduction of the oedema throughout the entire period of observation in a dose-related manner with a maximum at 3 h with, respectively, 52.11%, 50.72 % and 56.06% at the dose of 50 mg/kg and with 55.35%, 52.18% and 62.86%, respectively, at 100 mg/kg. Standard drugs ASL (200 mg/kg) and diclofenac (10 mg/kg) decreased paw oedema by 51.46% and 60.42%, respectively, at the third hour.
Table 1. Anti-inflammatory effect of the intraperitoneal administration of crude extract and its polar fractions (F1 and F2) from the red alga L. obtusa in Carrageenan-induced rat paw oedema test in comparison to control group (0.9% saline, 2.5 mL/kg) and reference drugs (diclofenac and acetylsalicylate of lysine: ASL).
Analgesic activity
Acetic acid induced writhing
On acetic acid-induced writhing in mice, a significant decrease of writhing, in a dose-dependent manner, was observed. After administration of the crude extract at the dose 50, 100 and 200 mg/kg, the writhing inhibition percentage was 40.84%, 53.79%, and 57.74%, respectively, in a 30 min interval period (). Also polar fractions F1 and F2 showed a significant analgesic effect at 25 mg/kg with a writhing inhibition percentage with 48.73 and 50.64%, respectively, at dose of 50 mg/kg, respectively, with 52.88 and 54.80% and at 100 mg/kg with 55.92 and 57.37%, respectively. ASL, the reference drug, inhibited 54.93% of the writhing number elicited by acetic acid at the dose of 200 mg/kg.
Table 2. Effects of the crude extract and its polar fractions (F1 and F2) from the red alga L. obtusa on acetic acid-induced writhing in mice, in comparison to control group (0.9% saline, 10 mL/kg) and reference drug acetylsalicylate of lysine (ASL).
Hot-plate test
The results of hot-plate test are shown in . Subcutaneous pretreatment with Tramadol at 25 and 50 mg/kg induced significant analgesia (p < 0.01), as shown by the delays in reaction time with a peak effect of 30 min post treatment in comparison with saline treated control. In contrast, ASL did not show any antinociceptive activity in this assay after administration. Crude extract and its polar fractions F1 and F2 did not significantly alter the reaction latency at all the tested doses and at all time points (30, 60, 90 and 120 min).
Table 3. Effects of the crude extract and its polar fractions (F1 and F2) from the red alga L. obtusa on hot-plate test in mice, in comparison to control group (0.9% saline, 10 mL/kg) and reference drugs (Tramadol and acetylsalicylate of lysine: ASL).
Gastroprotective effect
The results of antiulcerogenic activity on gastric ulcer induced by HCl/ethanol solution are shown in . Oral administration of the damaging agent to the control group clearly produced a mucosal damage characterized by multiple haemorrhage red bands of different sizes along the long axis of the glandular stomach as described in other studies (Shay et al. Citation1945; Al-Mulla Hummadi et al. Citation1999), the lesion index was 131.33 mm. Pretreatment with crude extract at the doses 50, 100 and 200 mg/kg produced a significant decrease in gastric haemorrhage and the lesion index was inhibited by 65.48, 73.60 and 85.91% at doses of 50, 100 and 200 mg/kg, respectively. A dose-related gastroprotective effect was observed with the polar fractions with highly activity for F2, the lesion index was inhibited by 71.27 and 81.29% at doses of 25 and 50 mg/kg, respectively. The two classical ulcer drugs ranitidine (60 mg/kg) and omeprazole (30 mg/kg) showed a significant activity (p < 0.01) with an inhibition of gastric lesions of 66.96 and 86.67%, respectively.
Table 4. Effects of crude extract and its polar fractions F1 and F2, from the red alga L. obtusa, on gastric ulcer induced by HCl/ethanol in rats, in comparison to control group (0.9% of saline, 2.5 mL/kg) and reference drugs (omeprazole and ranitidine).
Discussion
The carrageenan-induced paw oedema is a well-known experimental animal model applied to the study of acute inflammation. It is known to be sensitive to cyclooxygenase inhibitors and has been used to evaluate the effect of NSAIDs, which primarily inhibit the cycloxygenase enzymes involved in prostaglandin synthesis (Seibert et al. Citation1994). Indeed, inflammation induced by carrageenan is related to phospholipase A2 activation, which releases arachidonic acid from membrane cells, and that in turn is metabolized to prostaglandins. The conversion of arachidonic acid into inflammatory prostaglandin is catalyzed by cyclooxygenase enzyme COX-2. Substances able to inhibit this type of oedema could be inhibitor of COX-2 (Charlier & Michaux Citation2003).
The time course of oedema development induced by carrageenan is believed to be biphasic and at the third hour after carrageenan inoculation the oedema reaches its highest volume (Vinegar et al. Citation1969). The early phase of inflammation (1–2 h) after carrageenan injection is partly attributed to trauma of injection and mediated by histamine, serotonin and kinins components (Crunkhorn & Meacock Citation1971). The last phase is characterized by the release of prostaglandin and mediated by bradykinin and leukotrienes (Brito & Antonio Citation1998). This second phase is sensitive to most clinically valid anti-inflammatory drugs (Vinegar et al. Citation1969). The results of the present study reveal that crude extract and its polar fractions especially F2 significantly inhibited the carrageenan-induced acute inflammation in the third hour of study and was similar to that of the standard drugs diclofenac (10 mg/kg) and ASL (200 mg/kg). So, this anti-inflammatory in vivo activity of L. obtusa may be due to its suppressive action on prostaglandin synthesis by inhibiting the action of cyclooxygenase enzyme. Crude extract and its polar fraction, especially the fraction F2 showed an important anti-inflammatory activity in vitro by inhibiting the secretion of the pro-inflammatory cytokine TNF-α. Crude extract and its polar fraction showed also good cell viability. This further established that their inhibition of TNF-α production was not associated with the cytotoxic effects to THP-1 cells. The anti-inflammatory activity in vivo and in vitro of the crude extract and its polar fractions suggest that they could interfere with some of the mediators, by inhibiting their productions or antagonize their actions.
Further studies, conclusively demonstrated that the pro-inflammatory cytokine TNF-α induces COX-2 expression and consequently, the release of prostaglandin E2 (PGE2) and prostaglandin F2α (PGF2α) (Chen et al. Citation2000; Mark et al. Citation2001), therefore, an inhibition of the secretion of TNF-α induces a decrease in the COX-2 expression. The anti-inflammatory activity observed with the crude extract and its polar fractions may be attributed to the seaweed composition and to a possible molecular mechanism by effectively decreasing the production of the pro-inflammatory cytokines, especially TNF-α and the expression of COX2.
Acetic acid inducing writhing test is a widely used method for the evaluation of peripheral antinociceptive activities. Acetic acid is an irritating agent which stimulates the local peritoneal receptors to induce the release of prostaglandins and sympathomimetic system mediators like PGE2 and PGF2α (Deraedt et al. Citation1980). Hot-plate method is used to detect centrally acting analgesics (Nunez Guillen et al. Citation1997); this test produces two kinds of behavioural response, paw licking and jumping. Both are considered to be supraspinally integrated responses (Chapman et al. Citation1985; Chavan et al. Citation2012).
Crude extract and its polar fractions were shown to possess a significant analgesic effect on acetic acid inducing writhing test when compared to the control group and this in a dose-dependent manner. In contrast, they failed to increase pain threshold in the hot-plate test. This confirms the peripheral analgesic mechanisms of crude extract and its fractions which may be mediated via the inhibition of synthesis and release of prostaglandins. This peripheral analgesic activity may be related to the anti-inflammatory activity of these fractions.
Polyphenols, flavonoids and tannins were reported to inhibit prostaglandin synthesis (Park et al. Citation2001; Rajnarayana et al. Citation2006; Gupta et al. Citation2008, Citation2012; Pan et al. Citation2010). We consider that the presence of polyphenols and flavonoids in the crude extract and its polar fractions could be responsible of their anti-inflammatory and analgesic activities. The low amount of condensed tannins observed in the crude extract and its polar fractions indicated that these classes of compounds didn’t contribute in the observed activities. In other studies, phytochemical investigation of L. obtusa indicates the presence of other secondary metabolites like terpenoids and alkaloids (Demirel et al. Citation2011; Johnson et al. Citation2012). Terpenoids were reported to possess significant analgesic and anti-inflammatory activities (Neukirch et al. Citation2005; Moody et al. Citation2006). A new diterpene compound extracted from the red algae Laurencia glandulifera was reported to possess in vitro and in vivo anti-inflammatory activity (Chatter et al. Citation2011) by the inhibition of NF-κB activation, TNF-α production, NO release and COX-2 expression. Sesquiterpenes have been also shown to have an anti-inflammatory potential by the inhibition of NO and TNF-α production (Jin et al. Citation2006). Moreover, alkaloids have been shown to possess anti-inflammatory activity by inhibiting the action of arachidonic acid metabolism via the cycloxygenase and 5-lipoxygenase pathways (Barik et al. Citation1992; Chao et al. Citation2009) and by inhibiting TNF-α secretion (Yui et al. Citation2001; Yamazaki & Kawano Citation2011). Based in these results, the in vitro and in vivo anti-inflammatory and analgesic activities of the crude extract and its polar fractions from the red algae L. obtusa could be also attributed to the presence of various other secondary metabolites especially terpenoids and alkaloids.
Crude extract and its polar fractions produced a significant inhibition of lesion formation in HCl/EtOH induced gastric ulcer in rat, similar to the gastroprotection effect of the standard drugs ranitidine and omeprazole. Ethanol is commonly used to induce ulcer in experimental rat; there is growing evidence that ethanol leads to intense gastric mucosal damage, directly and indirectly through such mediators as reactive oxygen species (ROS) and cytokines (Abdel-Salam et al. Citation2001) and by depleting cellular oxidative defences in a process triggered by neutrophil activation, causing a sequential ROS-mediated induction of lipid peroxidation and protein oxidation (Rocha et al. Citation2011).
The capacity of the red algae L. obtusa to protect the gastric mucosa may be related to the presence of flavonoids which could interfere with the ulcerogenic mechanism of HCl/EtOH. Indeed, phenolics and flavonoids compounds were reported to possess gastroprotective effects (Alarcon de la Lastra et al. Citation1994; Borrelli & Izzo Citation2000; Gonzalez & Di Stasi Citation2002; Sairam et al. Citation2003; Laloo et al. Citation2013). The most important mechanism of action responsible for the gastroprotective effect is their antioxidant properties, which involves free radical scavenging, transition metal ions chelation, inhibition of oxidizing enzymes, increase of proteic and nonproteic antioxidants and reduction of lipid peroxidation (Mota et al. Citation2009). In addition, flavonoids have been shown to possess an anti-secretory mechanism of action by decreasing histamine levels, as well as preventing the release of histamine from gastric mast cells and inhibiting the gastric H+/K + proton pump, diminishing acid gastric secretion (Park et al. Citation2008).
Conclusion
In the present study, crude extract and its polar fractions showed potent anti-inflammatory properties through not only by inhibition the pro-inflammatory cytokine TNF-α, but also reducing the inflammatory oedema in vivo. They also exhibited strong antinociceptive activity and showed interesting gastroprotective effect. Based on these results we suggest that L. obtusa has the potential to provide a therapeutic approach to inflammatory diseases and to protect from gastrointestinal adverse. Further studies on isolation and characterization of the bioactive compounds from this red alga are underway to determine the bioactive constituents that are responsible for these important activities.
Acknowledgements
The authors acknowledge the “Ministry of Higher Education, Scientific Research and Technology, Tunisia”.
Disclosure statement
The authors declare that they have no conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. The manuscript has been read and approved by all named authors.
References
- Abdel-Salam OME, Czimmer J, Debreceni A, Szolcsányi J, Mózsik G. 2001. Gastric mucosal integrity: gastric mucosal blood flow and microcirculation. J Physiol Paris. 95:105–127.
- Akah PA, Gamaniel KS, Wambebe CN, Shittu A, Kapu SD, Kunle OO. 1997. Studies on gastrointestinal properties of Ficus exasperata. Fitoterapia. 68:17–20.
- Alarcon de la Lastra C, Martin MJ, Motilva V. 1994. Antiulcer and gastroprotective effects of quercetin: a gross and histologic study. Pharmacology. 48:56–62.
- Allison AC, Cacabelos R, Lombardi VR, Alvarez XA, Vigo C. 2001. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 25:1341–1357.
- Al-Mulla Hummadi YM, Najim RA, Farjou IB. 1999. A new in vitro model for ethanol induced gastric mucosal damage. Jpn J Pharmacol Toxicol Methods. 41:167–172.
- Angawi RF, Alarif WM, Hamza RE, Badria FA, Ayyad SN. 2014. New cytotoxic laurene, cuparene- and laurokamurene-type sesquiterpenes from the Red Sea alga Laurencia obtuse. Helv Chim Acta. 97:1388–1395.
- Ayyad SE, Al-Footy KO, Alarif WM, Sobahi TR, Bassaif SA, Makki MS, Asiri AM, AL Halwani AY, Badria AF, Badria FA. 2011. Bioactive C15 acetogenins from the red alga Laurencia obtusa. Chem Pharm Bull (Tokyo). 59:1294–1298.
- Bahorun T, Gressier B, Trotin F, Brunet C, Dine T,Luyckx M, Vasseur J, Cazin M, Cazin JC, Pinkas M. 1996. Oxygen species scavenging activity of phenolic extracts from Hawthorn fresh plant organs and pharmaceutical preparations. Arzneimittel Forsch. 46:1086–1089.
- Barik BR, Bhowmik T, Dey AK, Patra A, Chatterjee A, Joy S, Susan T, Alam M, Kundu AB. 1992. Premnazole and isoxazole alkaloid of Prema integrifolia and Gmelina arborea with anti inflammatory activity. Fitoterapia. 53:295–299.
- Björnsdottir US, Cypcar DM. 1999. Asthma: an inflammatory mediator soup. Allergy. 54:55–61.
- Borgi W, Ghedira K, Chouchane N. 2007. Antiinflammatory and analgesic activities of Zizyphus lotus root barks. Fitoterapia. 78:16–19.
- Borrelli F, Izzo AA. 2000. The plant kingdom as a source of anti-ulcer remedies. Phytother Res. 14:581–591.
- Brennan FM, McInnes IB. 2008. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 118:3537–3545.
- Brito ARMS, Antonio MA. 1998. Oral anti-inflammatory and anti-ulcerogenic activities of a hydroalcoholic extract and partitioned fractions of Turnera ulmifolia (Turneraceae). J Ethnopharmacol. 61:215–228.
- Cawthorn WP, Sethi JK. 2008. TNF-alpha and adipocyte biology. FEBS Lett. 582:117–131.
- Chao J, Lu TC, Liao JW, Huang TH, Lee MS, Cheng HY, Ho LK, Kuo CL, Peng WH. 2009. Analgesic and anti-inflammatory activities of ethanol root extract of Mahonia oiwakensis in mice. J Ethnopharmacol. 125:297–303.
- Charlier C, Michaux C. 2003. Dual inhibition of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) as a new strategy to provide safer non-steroidal anti-inflammatory drugs. Eur J Med Chem. 38:645–659.
- Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE. 1985. Pain measurement: an overview. Pain. 22:1–131.
- Chatter R, Ben Othman R, Rabhi S, Kladi M, Tarhouni S, Vagias C, Roussis V, Guizani-Tabbane L, Kharrat R. 2011. In vivo and in vitro anti-inflammatory activity of neorogioltriol, a new diterpene extracted from the red algae Laurencia glandulifera. Mar drugs. 9:1293–1306.
- Chavan MJ, Kolhe DR, Wakte PS, Shinde DB. 2012. Analgesic and antiinflammatory activity of kaur-16-en-19-oic acid from Annona reticulata L. bark. Phytother Res. 26:273–276.
- Chen CC, Sun YT, Chen JJ, Chiu KT. 2000. TNF-alpha-induced cyclooxygenase-2 expression in human lung epithelial cells: involvement of the phospholipase C-gamma 2, protein kinase C-alpha, tyrosine kinase, NF-kappa B-inducing kinase, and I-kappa B kinase 1/2 pathway. J Immunol. 165:2719–2728.
- Colucci M, Maione F, Bonito MC, Piscopo A, Di Giannuario A, Pieretti S. 2008. New insights of dimethyl sulphoxide effects (DMSO) on experimental in vivo models of nociception and inflammation. Pharmacol Res. 6:419–425.
- Crunkhorn P, Meacock SC. 1971. Mediators of the inflammation induced in the rat paw by carrageenin. Br J Pharmacol. 42:392–402.
- Deghrigue M, Lajili S, Turki M, Eltaief N, Bouraoui A. 2015. Evaluation of anti-inflammatory, analgesic and gastroprotective activities of Eunicella singularis fractions using in vivo assays. Ann Med Biomed Sci. 1:23–28.
- Dellai A, Deghrigue M, Laroche-Clary A, Masour HB, Chouchane N, Robert J, Bouraoui A. 2012. Evaluation of antiproliferative and anti-inflammatory activities of methanol extract and its fractions from the Mediterranean sponge. Cancer Cell Int. 12:18–23.
- Demirel Z, Koz FFY, Yafasoglu NUK, Ozdemir G, Sukatar A. 2011. Antimicrobial and antioxidant activities of solvent extracts and the essential oil composition of Laurencia obtusa and Laurencia obtusa var. pyramidata. Rom Biotechnol Lett. 16:5927–5936.
- Deraedt R, Joughney S, Delevakee F, Flahaut M. 1980. Release of prostaglandin E and F in an algogenic reaction and its inhibition. Eur J Pharmacol. 51:17–24.
- Desai SB, Furst DE. 2006. Problems encountered during anti-tumour necrosis factor therapy. Best Pract Res Clin Rheumatol. 20:757–790.
- Dhargalkar VK, Pereira N. 2005. Seaweed: promising plant of the millennium. Sci Culture. 4:60–66.
- Eddy NB, Leimback D. 1953. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther. 107:385–402.
- Gonzalez FG, Di Stasi LC. 2002. Anti-ulcerogenic and analgesic activities of the leaves of Wilbrandia ebracteata in mice. Phytomedicine. 9:125–134.
- Gonzalez R, Rodriguez S, Romay C, Ancheta O, González A, Armesto J, Remirez D, Merino N. 1999. Anti-inflammatory activity of phycocyanin extract in acetic acid-induced colitis in rats. Pharmacol Res. 39:55–59.
- Gupta M, Sasmal S, Majumdar S, Mukherjee A. 2012. HPLC profiles of standard phenolic compounds present in medicinal plants. Int J Pharmacognosy Phytochem Res. 4:162–167.
- Gupta M, Shaw BP, Mukherjee A. 2008. Studies on antipyretic-analgesic and ulcergenic activity of polyherbal preparation in rats and mice. Int J Pharmacol. 4:88–94.
- Hara N, Okabe S. 1985. Effects of gefernate on acute lesions in rats. Folia Pharmacol Jpn. 85:443–448.
- Jin HZ, Lee D, Lee JH, Hong YS, Choung DH, Kim YH, Lee JJ. 2006. New sesquiterpene dimers from Inula britannica inhibit NF-kappaB activation and NO and TNF-alpha production in LPS-stimulated RAW264.7 cells. Planta Med. 72:40–45.
- Johnson M, Babu A, Janakiraman N, Renisheya JJMT. 2012. Phytochemical studies on Laurencia obtusa (Hudson) Lamouroux. Int J Biomed Adv Res. 3:225–232.
- Kang JY, Khan MNA, Park NH, Cho JY, Lee MC, Fujii H, Hong YK. 2008. Antipyretic, analgesic and anti-inflammatory activities of the seaweed Sargassum fulvellum and Sargassum thunbergii in mice. J Ethnopharmacol. 116:187–190.
- Khan MNA, Cho JY, Lee MC, Kang JY, Park NG, Fujii H, Hong YK. 2007. Isolation of two anti-inflammatory and one pro-inflammatory polyunsaturated fatty acids from the brown seaweed Undaria pinnatifida. J Agric Food Chem. 55:6984–6988.
- Khan MNA, Choi JS, Lee MC, Kim E, Nam TJ, Fujii H, Hong YK. 2008. Antiinflammatory activities of methanol extracts from various seaweed species. J Environ Biol. 29:465–469.
- Kladi M, Xenaki H, Vagias C, Papazafiri P, Roussis V. 2006. New cytotoxic sesquiterpenes from the red algae Laurencia obtusa and Laurencia microcladia. Tetrahedron. 62:182–189.
- Koster R, Anderson M, Beer EJ. 1959. Acetic acid for analgesic screening. Fed Proceed. 18:412–416.
- Kwon MJ, Nam TJ. 2007. A polysaccharide of the marine alga Capsosiphon fulvescens induces apoptosis in AGS gastric cancer cells via an IGF-IR-mediated PI3K/Akt pathway. Cell Biol Int. 31:768–775.
- Laine L, Takeuchi K, Tarnawski A. 2008. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology. 135:41–60.
- Laloo D, Prasad SK, Krishnamurthy S, Hemalatha S. 2013. Gastroprotective activity of ethanolic root extract of Potentilla fulgens Wall. ex Hook. J Ethnopharmacol. 146:505–514.
- Lawrence T, Willoughby DA, Gilroy DW. 2002. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol. 2:787–795.
- Liu Z, Wu XY, Bendayan R. 1999. In vitro investigation of ionic polysaccharide microspheres for simultaneous delivery of chemosensitizer and antineoplastic agent to multidrug-resistant cells. J Pharm Sci. 88:412–418.
- Mark KS, Trickler WJ, Miller DW. 2001. Tumor necrosis factor-alpha induces cyclooxygenase-2 expression and prostaglandin release in brain microvessel endothelial cells. J Pharmacol Exp Ther. 297:1051–1085.
- Medana IM, Hunt NH, Chaudhri G. 1997. Tumor necrosis factor-alpha expression in the brain during fatal murine cerebral malaria: evidence for production by microglia and astrocytes. Am J Pathol. 150:1473–1486.
- Moody JO, Robert VA, Connolly JD, Houghton PJ. 2006. Anti-inflammatory activities of the methanol extracts and an isolated furanoditerpene constituent of Sphenocentrum jollyanum Pierre (Menispermaceae). J Ethnopharmacol. 104:87–91.
- Mota KS, Dias GE, Pinto ME, Luiz-Ferreira A, Souza-Brito AR, Hiruma-Lima CA, Barbosa-Filho JM, Batista LM. 2009. Flavonoids with gastroprotective activity. Molecules. 14:979–1012.
- Murphy K, Haudek SB, Thompson M, Giroir BP. 1998. Molecular biology of septic shock. New Horiz. 6:181–193.
- Neukirch H, D’Ambrosio M, Sosa S, Altinier G, Della Loggia R, Guerriero A. 2005. Improved anti-inflammatory activity of three new terpenoids derived, by systematic chemical modifications, from the abundant triterpenes of the flowery plant Calendula officinalis. Chem Biodivers. 2:657–671.
- Nunez Guillen ME, Emim JA, Souccar C, José Lapa A. 1997. Analgesic and anti-inflammatory activities of the aqueous extract of Plantago major L. Int J Pharmacogn. 35:99–104.
- Pan MH, Lai CS, Ho CT. 2010. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 1:15–31.
- Park HH, Lee S, Son HY, Park SB, Kim MS, Choi EJ, Singh TS, Ha JH, Lee MG, Kim JE, et al. 2008. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch Pharm Res. 31:1303–1311.
- Park JW, Choi YJ, Suh SI, Kwon TK. 2001. Involvement of ERK and protein tyrosine phosphatase signaling pathways in EGCG-induced cyclooxygenase-2 expression in Raw 264.7 cells. Biochem Biophys Res Commun. 286:721–725.
- Pinna GF, Fiorucci M, Reimund JM, Taquet N, Arondel Y, Muller CD. 2004. Celastrol inhibits pro-inflammatory cytokine secretion in Crohn’s disease biopsies. Biochem Biophys Res Commun. 322:778–786.
- Rajnarayana K, Reddy MS, Chaluvadi MR. 2006. Bioflavonoids classification, pharmacological, biochemical effects and therapeutical potential. Ind J Pharm Sci. 68:380–384.
- Rocha NF, Oliveira GV, Araújo FY, Rios ER, Carvalho AM, Vasconcelos LF, Macêdo DS, Soares PM, Sousa DP, Sousa FC. 2011. (−)-α-Bisabolol-induced gastroprotection is associated with reduction in lipid peroxidation, superoxide dismutase activity and neutrophil migration. Eur J Pharm Sci. 44:455–461.
- Sairam K, Priyambada S, Aryya NC, Goel RK. 2003. Gastroduodenal ulcer protective activity of Asparagus racemosus: an experimental, biochemical and histological study. J Ethnopharmacol. 86:1–10.
- Santucci L, Fiorucci S, Giansanti M, Brunori PM, Di Matteo FM, Morelli A. 1994. Pentoxifylline prevents indomethacin-induced acute gastric mucosal damage in rats: role of tumour necrosis factor alpha. Gut. 35:909–915.
- Seibert K, Zhang Y, Leahy K, Hauser S, Masferrer J, Perkins W, Lee L, Isakson P. 1994. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci USA. 91:12013–12017.
- Sekut L, Connolly KM. 1996. Pathophysiology and regulation of TNF-in inflammation. Drug News Perspect. 9:261–269.
- Shay H, Konaror SA, Fels SS, Meranze D, Gruenstein M, Siplet H, Meraze D, Gruentein M, Gruestein M, Feles SS, et al. 1945. A simple method for the uniform production of gastric ulceration in rats. Gastroentoxology. 5:43–61.
- Sun B, Richardo-da-Silvia JM, Spranger I. 1998. Critical factors of vanillin assay for catechins and proanthocyanidins. Agric Food Chem. 46:4267–4274.
- Synytsya A, Kim WJ, Kim SM, Pohl R, Synytsya A, Kvasnička F, Čopíkováa J, Parke YI. 2010. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr Polym. 81:41–48.
- Taga MS, Miller EE, Pratt DE. 1984. Chia seeds as a source of natural liquid antioxidants. J Am Oil Chem Soc. 61:928–931.
- Vane JR, Bakhle YS, Botting RM. 1998. Cyclo-oxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 38:97–120.
- Vinegar R, Schreiber W, Hugo R. 1969. Biphasic development of carrageenin edema in rats. J Pharmacol Exp Ther. 166:96–103.
- Winter CA, Risley EA, Nuss GW. 1962. Carrageenan induced edema hind paw of the rat as an easy for anti-inflammatory drugs. Proc Soc Exp Biol Med. 3:544–547.
- Yamazaki Y, Kawano Y. 2011. Inhibitory effects of herbal alkaloids on the tumor necrosis factor-α and nitric oxide production in lipopolysaccharide-stimulated RAW264 macrophages. Chem Pharm Bull (Tokyo). 59:388–391.
- Yeh CC, Yang JL, Lee JC, Tseng CN, Chan YC, Hseu YC, Tang JY, Chuang LY, Huang HW, Chang FR, et al. 2012. Anti-proliferative effect of methanolic extract of Gracilaria tenuistipitata on oral cancer cells involves apoptosis, DNA damage, and oxidative stress. BMC Complement Altern Med. 12:142.
- Yui S, Mikami M, Mimaki Y, Sashida Y, Yamazaki M. 2001. Inhibition effect of Amaryllidaceae alkaloids, lycorine and lycoricidinol on macrophage TNF-alpha production. Yakugaku Zasshi. 121:167–171.