Abstract
Context: Termitomyces clypeatus (Lyophyllaceae) is a filamentous edible mushroom, having ethnomedicinal uses. However, information about the antioxidant, anticancer and antitumour properties of this mushroom remains to be elucidated.
Objective: The study examines the in vitro antioxidant, anticancer and in vivo antitumour activity of T. clypeatus.
Materials and methods: Antioxidant activity was evaluated with seven in vitro assays. Cytotoxicity of T. clypeatus was tested against a panel of cancer cells lines including U373MG, MDA-MB-468, HepG2, HL-60, A549, U937, OAW-42 and Y-79 using MTT assay. The antitumour activity of aqueous extract was evaluated against Ehrlich ascites carcinoma (EAC) tumour model in Swiss albino mice.
Results: HPLC analysis of aqueous extract revealed the presence of sugar entities. Termitomyces clypeatus showed excellent in vitro antioxidant activity. Termitomyces clypeatus was found cytotoxic against all cancer cells, among which it showed higher activity against U937 (IC50 25 ± 1.02 μg/mL). Treatment of EAC-bearing mice with varied doses of aqueous extract significantly (p < 0.01) reduced tumour volume, viable tumour cell count and improved haemoglobin content, RBC count, mean survival time, tumour inhibition and % increase life span. The enhanced antioxidant status in treated animals was evident from the decline in the levels of lipid peroxidation, increased levels of glutathione, catalase and superoxide dismutase.
Discussion: The analyzed data indicate that the aqueous extract of T. clypeatus exhibits significant antitumour activity, which might be due to the antioxidant effects on EAC bearing hosts.
Conclusion: Termitomyces clypeatus possesses anticancer activity, valuable for application in food and drug products.
Keywords:
Introduction
The search for natural bioactive compounds that can serve as an antioxidant and anticancer agents gained interests both in industry and in scientific research. The harmful nature of reactive oxygen species (ROS) produced during oxidation processes, the harmful nature of synthetic antioxidants such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) and the increasing resistance of the micro-organisms towards synthetic antibiotic drugs have contributed to the increase in search for novel bioactive components from natural origin (Oyetayo Citation2009). It is known that the uncontrolled production of free radical reactive oxygen species such as superoxide anion (O2-), hydroxyl radical (OH•) and hydrogen peroxide (H2O2) can induce DNA damage in humans. Such damage to DNA has been suggested to contribute to aging and various diseases, including cardiovascular, cancer and chronic inflammation (Wickens Citation2001; Valko et al. Citation2006).
Mushrooms are well known to produce a wide range of secondary active metabolites with high therapeutic value (Mizuno Citation1999). Extracts from mushroom have received immense attention based on their safety and records of health promotion. Health-promoting properties such as the antioxidant, anticancer, cholesterol lowering and immunostimulatory effects have been previously documented for some species of mushrooms (Barros et al. Citation2007; Mau et al. Citation2004). Both fruiting bodies and the mycelium-contained compounds have a wide-range of antioxidant and anticancer potential (Ferreira et al. Citation2007).
Termitomyces clypeatus (Lyophyllaceae), is an edible mushroom, having ethnomedicinal uses (Maiti et al. Citation2008; Okhuoya et al. Citation2010), was identified and morphogenized in our laboratory as IICB 411 and has been submitted to Microbial Type Culture Collection (MTCC), Institute of Microbial Technology, Chandigarh, India, and is identified as MTCC 5091. This edible mushroom has been traditionally eaten seasonally by specific groups of local people. Several value added materials of the species T. clypeatus (Khowala & Sengupta Citation1992; Ghorai et al. Citation2011; Banik et al. Citation2012; Pal et al. Citation2013) have been already reported. Some enzymes of high therapeutic values have been reported from T. clypeatus (Pal et al. Citation2010) as well. The fungus contained 31% protein, 32% carbohydrate and 10–14% ascorbic acid (Ghorai et al. Citation2009).
However, until now, no investigation has evaluated the antioxidant and anticancer potential of this edible mushroom. There is an increasing interest in natural antioxidants, i.e., proteins and carbohydrate present in medicinal and dietary mushrooms, which helps to prevent oxidative damage specially in the treatment of cancer (Menshova et al. Citation2015; Han et al. Citation2016). This has prompted us to test the in vitro antioxidant and anticancer activities of the T. clypeatus using MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide] assay, on a panel of cancer cell lines. We also evaluated the antitumour activity of aqueous extract of T. clypeatus with reference to in vivo antioxidant activity.
Materials and methods
Chemicals
1,1-Diphenyl-2-picryl-hydrazyl (DPPH), butylated hydroxy toluene (BHT), curcumin, ascorbic acid, sodium nitroprusside, nicotinamide adenine dinucloetide (NADH), nitrobluetetrazolium (NBT), phenazine methosulphate, sulphanilamide, naphthylethylene diamine dihydrochloride, 2,2′-azinobis-(3-ethyl-benzothiazoline-6-sulphonic acid)-(ABTS), EDTA, α-tocopherol and potassium ferricyanide were purchased from Sigma Chemical Co. Ltd (St. Louis, MO). DMSO was obtained from Merck (Darmstadt, Germany). For HPLC analysis, solvents used were procured from Spectrochem Pvt. Ltd (Mumbai, India). Media ingredients were procured from Himedia, Mumbai, India. All other chemicals and reagents were of analytical grade and purchased from Merck (Darmstadt, Germany) and Sigma (St. Louis, MO).
Preparation of inoculum
Seven-day-old mycelia of T. clypeatus (MTCC-5091) from PDA slants (20% potato extract, 2% dextrose and 1.5% agar) were transferred to 100 mL PGM liquid medium [20% potato extract, 1% malt extract and 5% glucose (w/v)] in 500 mL polypropylene flasks with two 1 cm diameter glass beads and incubated at 28 °C for 48 h under constant shaking in a shaker incubator. The fragmented mycelial culture (2%) was used as inoculums (Ghorai et al. Citation2009).
Biomass preparation of T. clypeatus
The fungal strain of T. clypeatus was cultured in complex medium under submerged condition at 30 °C for 4 d and mycelia were taken as a byproduct. The complex medium (%, w/v) composed of the following ingredients: sucrose 1.0, malt extract 1.0, soya bean meal-1.0, yeast extract-1.0, biopeptone-1.0, boric acid-0.037, KH2PO4-0.87, CaCl2·2H2O-0.034, MgSO4·2H2O-0.05, MnCl2·4H2O-0.0036, NaMoO4·4H2O-0.0032, ZnSO4·7H2O-0.031, FeSO4·7H2O-0.025 and NH4H2PO4-24.63, at pH 4–4.5. Inoculum (2%) was added and growth was continued under submerged condition (150 g) at 30 °C for 4 d. Biomass of T. clypeatus was obtained by filtering the culture medium, and the mycelium was washed thoroughly and repeatedly with distilled water (Ghorai et al. Citation2009). It was kept at 50 ± 5 °C for 48 h and stored in an airtight container for further use.
Preparation of the Extract
Ethanol extract was prepared by macerating the dried mycelium in a homogenizer (IKA Ultra-Turrax T-25 digital, IKA, Baden-Wurttemberg, Germany) in ethanol at 4 °C. The whole content was centrifuged at 12,000 g for 30 min at 4 °C. Then the supernatant was collected. Aqueous extract was prepared by extracting the mycelium with distilled water at 100 °C for 6 h. The resultant extract was centrifuged at 9000 g for 30 min at 4 °C and concentrated. The Sevag method (Qian Citation2014) was used to remove protein. The supernatant was added to absolute ethanol (4 vols) and kept overnight. The water-soluble fraction F1 was separated and the precipitate fraction F2 was collected and washed with absolute ethanol and acetone, then dried under vacuum, yielding a yellowish-brown crude polysaccharide powder.
Protein and carbohydrate assay
Protein was assayed using Coomassie blue (Bradford) protein assay reagent (Sigma, St. Louis, MO) (Bradford Citation1976). The carbohydrate content of the extract was measured by Orcinol–H2SO4 reagent, with glucose as the standard (Brown & Anderson Citation1971).
Monosaccharide composition analysis by HPLC
The HPLC chromatographic separation of the constituents present in the water-soluble fraction F1 of the aqueous extract of the fungus, was carried out on a Waters Alliance 2695 HPLC system (Waters, Milford, MA). Waters Empower 2 chromatography data software (Waters, Milford, MA) was used for the recording and integration of the chromatograms. The detector used was a Refractive Index detector (2414 Waters RI detector). The column used for the analysis was Phenomenex Luna NH2 LC column (250 × 4.6 mm, 5 μm particle size). HPLC analysis was performed at ambient temperature using sample solutions filtered through 0.22 μm membrane (Millipore syringe filter, Waters, Milford, MA) and analyzed (50 μL injected volume) using the Waters HPLC system (Waters, Milford, MA). Isocratic elution was achieved using the mobile-phase acetonitrile: water (50: 50 v/v) at a flow rate of 3 mL/min. Evaluation of the fraction F2 for the isolation and structural elucidation of the polysaccaharides is currently under progress.
Antioxidant assay
The ability of ethanol and aqueous extract of T. clypeatus to act as hydrogen donors or free radical scavengers was tested by conducting a series of in vitro antioxidant assays involving DPPH radical, hydroxyl radical, nitric oxide (NO) radical, superoxide anion radical, metal chelating assay, total antioxidant activity and reducing ability. The results were then compared with that of standard antioxidants, i.e., BHA, BHT, catechin, curcumin and α-tocopherol.
DPPH radical scavenging effect
DPPH radical scavenging effects of the extracts were performed according to the method of Blois (Citation1958). Butylated hydroxy toluene (BHT) was used as a reference drug. The percentage inhibition was evaluated by comparing the absorbance values of the control and experimental samples. It was calculated according to the following equation:
(1)
where A0 is the absorbance of the control (blank, without extract) and A1 is the absorbance in the presence of the extract.
NO radical scavenging effect
NO generated from sodium nitroprusside in aqueous solution at physiological pH interacts with oxygen to produce nitrite ions which were measured by Griess reaction (Green et al. Citation1982; Marcocci et al. Citation1994). Ascorbic acid was used as a standard drug. The percentage inhibition of the NO radical was evaluated by in a similar manner for comparison and calculation as mentioned above (EquationEquation (1)(1) ).
Superoxide anion radical scavenging effect
Superoxide anion scavenging activities of the extracts were evaluated based on the method described by Nishikimi et al. (Citation1972), with slight modification. Nitroblue tetrazolium (NBT) solution (156 μmol/L NBT in 100 mmol/L phosphate buffer, pH 7.4) (1 mL), 1 mL of NADH solution (468 μM in 100 mmol/L phosphate buffer, pH 7.4) and 0.1 mL of sample solution of test extracts (20, 40, 60, 80, 100 and 150 μg/mL) in distilled water were mixed, and the reaction started by adding 100 μL of phenazine methosulphate (PMS) solution (60 μmol/L PMS in 100 mmol/L phosphate buffer, pH 7.4). The reaction mixture was incubated at 25 °C for 5 min, and the absorbance at 560 nm was measured against blank samples. The decreased absorbance of the reaction mixture indicates the increase in superoxide anion scavenging activity. Curcumin was used as a reference compound. The percentage of inhibition was determined by comparing the results of control and test samples.
Hydroxyl radical scavenging activity
The scavenging activity for hydroxyl radicals was measured with Fenton’s reaction (Wenli et al. Citation2004). The hydroxyl radicals scavenging activity was calculated according to the above-described formula.
Ferrous ion-chelating ability
The ferrous ion-chelating (FIC) assay reported by Singh and Rajini (Citation2004) was adopted. EDTA was used as standard. The ability of the extracts to chelate ferrous ions was calculated as per the following equation:
(2)
where AB is the absorbance of the control (blank) and AT is the absorbance of the test sample.
Total antioxidant activity by ABTS radical cation decolorization assay
The total antioxidant activity of Termitomyces clypeatus was measured by the method of ABTS [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)] radical cation decolorization assay (Wolfenden & Willson Citation1982). BHT was used as the standard.
Reducing ability
The reducing power of Termitomyces clypeatus ethanolic and aqueous extracts was determined by the method of Oyaizu (Citation1986). Substances, which have reduction potential, react with potassium ferricyanide (Fe3+) to form potassium ferrocyanide (Fe2+), which then reacts with ferric chloride to form a ferric–ferrous complex that has an absorption maximum at 700 nm. An increase in the reduction of ferric to ferrous ion increases the absorbance indicating the reducing ability of both the extracts of T. clypeatus. The standard α-tocopherol was used for comparison.
Cell culture
Cell lines used (U373MG, MDA-MB-468, HepG2, HL-60, A549, U937, OAW-42 and Y-79) in this study were obtained from the Department of Pharmaceutical Technology, Jadavpur University, Kolkata, India. Cells were grown in DMEM, with 10% FBS, 1% penicillin/streptomycin solution (100 IU/mL penicillin and 100 mg/mL streptomycin) and 2 mmol/L of l-glutamine in a 5% CO2 atmosphere at 37 °C.
In vitro cytotoxicity activity
MTT [(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide)] is a pale yellow substrate that is cleaved by living cells to yield a dark-blue formazan product. This process requires active mitochondria, and even freshly dead cells do not cleave significant amount of MTT. Thus, the amount of MTT cleaved is directly proportional to the number of viable cells present, which is quantified by colorimetric methods. This assay was performed using the standard operating procedures. Briefly, the compounds were dissolved in DMSO and serially diluted with complete medium to get the concentrations a range of test concentration. DMSO concentration was kept <0.1% in all the samples. Human glioblastoma cell line U-373-MG (Hashioka et al. Citation2009), human breast carcinoma cell line MDA-MB-468 (Xu et al. Citation2008), human hepatocellular carcinoma cell line HepG2 (Costantini et al. Citation2013), human promyelocytic leukaemia cell line HL-60 (Kostrzewa-Nowak & Tarasiuk Citation2013), human lung adenocarcinoma epithelial cell line A549 (Eda et al. Citation2011), human leukaemic monocyte lymphoma cell line U937 (Passmore et al. Citation2001), human ovarian cancer cell line OAW-42 (Zaffaroni et al. Citation2001) and human retinoblastoma cell line Y-79 (Kanno et al. Citation2009) were used in this study. Cell lines maintained in minimum essential medium with 10% foetal calf serum at appropriate conditions were seeded in 96-well plates and treated with different concentrations of the test samples and incubated at 37 °C, 5% CO2 for 96 h. MTT reagent was added to the wells and incubated for 4 h, the dark blue formazan product formed by the cells was dissolved in DMSO under a safety cabinet and read at 550 nm. Percentage inhibitions were calculated and plotted against the concentrations used to calculate the IC50 values.
Animals
All experiments were performed after approval of the protocol by the Ethics Committee for Animal Research (367001/C/CPCSEA), of the Jadavpur University, Kolkata, India, and were carried out in accordance with the current guidelines for the care of laboratory animals and the ethical guidelines. Male Swiss albino mice weighing 20 ± 2 g were used in the present investigation. They were housed in clean polypropylene cages and were fed with a standard pellet diet (Hindustan Lever, Kolkata, India) and water ad libitum. The animals were acclimatized to laboratory condition for one week before the start of the experiment.
Acute toxicity study for in vivo study
This was performed for the extract according to the acute toxic classic method of Litchfield and Wilcoxon (Citation1949). The animals were divided into six groups containing 10 animals in each group. The AETC solution was administrated orally in increasing dose up to 2000 mg/kg, bw. These animals were observed for mortality and toxicity for 72 h.
In vivo antitumour activity
Antitumour activity of the AETC was evaluated using ascites tumour models.
Tumour cells
Ehrlich ascites carcinoma (EAC) cells were obtained from the Chittaranjan National Cancer Institute, Kolkata, India. The EAC cells were maintained in vivo in Swiss albino mice, by intraperitoneal (i.p.) transplantation of 2 × 106 cells/mouse after every 10-d interval. EAC cells of 9-d old were mainly used for the screening of aqueous extract of T. clypeatus.
Experimental protocol
Animals were divided into five groups of 10 animals each. All the groups were injected i.p. with 2 × 106 viable EAC cells with ice-cold normal saline (0.9% w/v) (aspirated from EAC-bearing mice at the log-phase 7–8th day) except group I (served as normal control). After 24 h of tumour inoculation, drugs were administered at the doses mentioned below for 14 consecutive days (Gupta et al. Citation2010). Food and water were withheld 18 h before sacrificing the animals. On the 15th day, six mice from each group were sacrificed 24 h after the last dose and the rest were kept with food and water ad libitum to check the increase in the life span of the tumour hosts. 5-Fluorouracil (5-FU) at a dose level of 20 mg/kg body weight was used as standard. After sacrificing the animals, blood was collected to evaluate the haematological and biochemical parameters. The in vivo antioxidant activity was determined in liver tissues. The groups and the design of the experiment were as follows:
Group I: Normal saline [5 mL (0.9%, w/v)/kg]
Group II: EAC (2 × 106 cells/mice) + Normal saline (5 mL/kg)
Group III: EAC (2 × 106 cells/mice) + AETC (200 mg/kg)
Group IV: EAC (2 × 106 cells/mice) + AETC (400 mg/kg)
Group V: EAC (2 × 106 cells/mice) + 5-Flurouracil (20 mg/kg)
Tumour growth response
The effect of AETC on tumour growth was examined by studying the following parameters: tumour volume, tumour cell count and the percentage of the viable and non-viable cell count.
Tumour cell count
The ascitic fluid was taken in an RBC pipette and diluted up to the cells become freely separated in the slide and countable easily. The dilution factor was mentioned and a drop of the diluted cell suspension was placed in the Neubauer counting chamber and the number of cells in the 64 small squares was counted in a compound microscope under 10× magnification.
Viable and non-viable tumour cell count
The cells were taken from ascitic fluid then stained with Trypan blue (0.4% in normal saline) dye. The cells that did not take up the dyes were viable and those, which took the stain, were non-viable. These viable and non-viable cells were counted. The cell count was calculated by using the following equation:
(3)
Haematological studies
The effect of AETC on peripheral blood was investigated. RBC, WBC counts and estimation of haemoglobin was done with standard procedures. Red blood cell count (RBC), haemoglobin content (D'Amour et al. Citation1965) and white blood cell count (WBC) (Wintrobe Citation1962) were measured from the blood obtained intracardially.
In vivo antioxidant status
The antioxidant system was performed on liver and evaluated by measuring the level of lipid peroxidation and the amount of enzymatic and non-enzymatic antioxidant. Microsomal lipid peroxidation was determined by measuring the formation of thiobarbituric acid reactive substances (TBARS) according to the method of Ohkawa et al. (Citation1979). The non-enzymatic antioxidant such as reduced glutathione (GSH) level was measured by Mulder et al. (Citation1995) method. The enzymatic antioxidant such as catalase (CAT) activity and superoxide dismutase (SOD) were assayed by the method of Luck (Citation1963) and Kakkar et al. (Citation1984), respectively.
Processing of tumour cells collected from tumour-transplanted mice
EAC cells were collected from animal’s body after giving the last dose and kept at 37 °C for 1 h for macrophages adherence (Mau et al. Citation2004). Tumour cells isolated were treated with 0.83% ammonium chloride to remove blood cell contamination. Cells were used for assays after four washes with PBS.
Statistical analysis
Each experiment was performed at least three times and the results are presented as the mean ± SD. The statistical analyses were performed using GraphPad Prism 5 software (GraphPad Software Inc, La Jolla, CA). Graphs are prepared using Microsoft Office Excel 2007. The IC50 values were calculated using the linear regression analysis.
Results
The dried extract of the mushroom was subjected to isolation of biologically potent components, which could be responsible for showing potential antioxidant and anticancer activity. It has been reported that polysaccharides are the major active components, so the concentration of the polysaccharides present in the extract were measured. The results of preliminary screening of the ethanol and aqueous extract revealed the presence of carbohydrates and proteins (). Polysaccharide content of the total sugar in the aqueous extract was 18.45%.
Table 1. The percentage yield as well as the protein and carbohydrate concentration of different extract.
Qualitative estimation using HPLC analysis
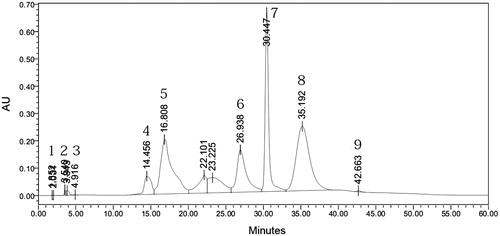
Qualitative estimation of the constituents present in the aqueous fraction F1 of the water extract was determined. The standard solutions of the extract (1 mg/mL) and standard compounds (1 mg/mL) were prepared in DMSO. The identification of sugars was done by comparing retention times of the individual sugars in the reference versus tested solution (qualitative analysis). HPLC analysis showed the presence of ascorbic acid, fructose, sucrose, arabitol, inositol and traces of xylitol (). Raffinose, sorbitol and ribitol were the major components found in aqueous extract of the mycelium grown in glucose medium. It appeared that sorbitol and ribitol may be the byproduct of glucose utilization, whereas arabitol, inositol and xylitol represented storage carbohydrates.
Antioxidant activity
Free radicals that are generated in many bio-organic redox processes may induce oxidative damage in various components of the body (lipids, proteins and DNA) and have been implicated in aging and a number of life-limiting chronic diseases such as cancer, hypertension, cardiac infarction, atherosclerosis, rheumatism, cataracts and others. Efforts to counteract the damage caused by these species are gaining acceptance as a basis for novel therapeutic approaches. Thus, the field of preventive medicine is experiencing an upsurge of interest in medically useful antioxidants. We carried out experiments to explore the free-radical scavenging ability of the extracts of T. clypeatus, to develop potential antioxidants and therapeutic reagents. A large number of methods have been developed to evaluate the total antioxidant capacity of food and dietary supplements, herbal or fungal extracts or pure compounds (Dhalwal et al. Citation2005; Dehshiri et al. Citation2013; Wang et al. Citation2013). Nevertheless, few of them have been used widely due to the difficulty of measuring total antioxidant capacity owing to limitations associated with methodological issues and free radical sources (Prior et al. Citation2005; Schauss et al. Citation2006). In this study, the antioxidant activity of the ethanolic and aqueous extract of T. clypeatus was compared with BHA, BHT, catechin, curcumin, EDTA, ascorbic acid and α-tocopherol. The IC50 values of all the extracts obtained from different type of assay experiments strongly support that the extracts presented in this work possess excellent antioxidant activities, which are better than those of the standard antioxidants.
DPPH radical scavenging effect
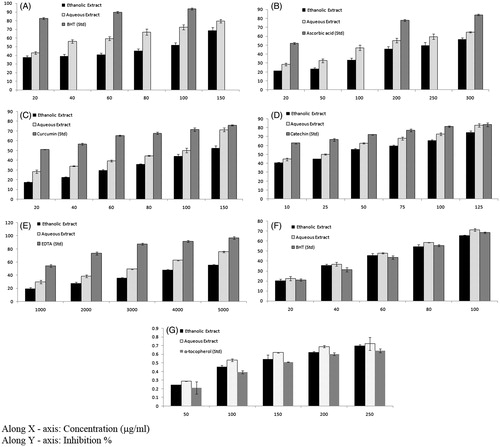
The DPPH radical is a stable free radical and due to the presence of an odd electron, it shows a strong absorption band at 517 nm in a visible spectrum. If this electron becomes paired off in the presence of a free-radical scavenger, this absorption vanishes resulting in decolorization stoichiometrically with respect to the number of electrons taken up. The DPPH radical scavenging activity of different extracts is given in . DPPH, a nitrogen-centred free radical with a characteristic absorbance at 517 nm, gets converted to 1,1, diphenyl 2- picryl hydrazine due to its rapid hydrogen accepting ability. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) has been used extensively as a free radical to evaluate reducing substances (Yamaguchi et al. Citation1998; Mohamed et al. Citation2013; Deng et al. Citation2014). Stable free radicals DPPH was effectively scavenged by T. clypeatus extracts, and the IC50 value of ethanol and aqueous extract was found to be 86.84 and 27.59 μg/mL, respectively, which showed that aqueous extract has been better scavenging activity than ethanol extract. A standard antioxidant BHT was used to compare the antioxidant potential, which exhibited 82.61% inhibition at a concentration of 20 μg/mL.
Figure 2. (A) DPPH radical-scavenging, (B) nitric oxide radical scavenging, (C) superoxide anion radical scavenging, (D) hydroxyl radical scavenging activities, (E) changes in the levels of chelating ability, (F) total antioxidant activity and (G) reducing ability of ethanol and aqueous extract of Termitomyces clypeatus.
NO radical scavenging effect
NO is a chemical mediator generated by endothelial cells, macrophages, neurons, etc., and which is involved in the regulation of various physiological processes (Lata & Ahuja Citation2003). Excess concentration of NO is associated with several diseases (Ialenti et al. Citation1993; Ross Citation1993). Oxygen reacts with the excess NO to generate nitrite and peroxynitrite anions, which act as free radicals (Robbins & Cotran Citation1979; Sainani et al. Citation1997). In the present study, the extracts compete with oxygen to react with NO and thus inhibit generation of the anions. illustrates the percentage inhibition of NO generation by the ethanol and aqueous extract of T. clypeatus. Ascorbic acid was used as a reference compound, which exhibited 52.26% inhibition at a concentration of 20 μg/mL. Ethanol and aqueous extract showed an IC50 value of 247.38 and 169.92 μg/mL, respectively, which clearly represent that the aqueous extract has been better scavenging activity than ethanol extract.
Superoxide anion radical scavenging effect
In the PMS/NADH-NBT system, superoxide radicals generated from a non-enzymatic reaction of PMS in the presence of NADH and molecular oxygen, reduces NBT to formazan at pH 7.8. demonstrated the superoxide scavenging effect of ethanolic and aqueous extract of T. clypeatus on the PMS/NADH-NBT system (Robak & Gryglewski Citation1988). The decrease of the absorbance at 560 nm with antioxidants clearly indicated the consumption of superoxide anion in the reaction mixture. Aqueous extract exhibited noticeably higher antioxidant activity when compared with the ethanol extract. The IC50 values of ethanol and aqueous extract of T. clypeatus were 133.08 and 91.55 μg/mL, respectively. Curcumin used as a reference showed 51.37% inhibition at a concentration of 20 μg/mL.
Hydroxyl radical scavenging effect
The hydroxyl radical is very reactive and can be generated in biological cells through the Fenton reaction. shows that the T. clypeatus displayed concentration-dependent scavenging activities against hydroxyl radicals generated in a Fenton reaction system.
The concentration of the extracts needed for 50% inhibition of hydroxyl radicals was found to be 40.67 and 21.94 μg/mL (). The aqueous extract showed better activity in comparison with ethanol extract, which is evident in Catechin, used as a standard, was highly effective in inhibiting the radicals, showing an inhibition of 66.58 ± 1.47% at a concentration of 25 μg/mL. High molecular weight and the proximity of many aromatic rings and hydroxyl groups are more important for the free-radical scavenging by specific functional groups (Hagerman et al. Citation1998) which may account for the above results.
Chelating abilities on ferrous ions
The chelating effects of various extracts on Fe2+ were determined by the formation of ferrozine–Fe2 + complexes. Chelating agents are able to capture ferrous ion before ferrozine, thus preventing the formation of ferrozine–Fe2+ complex. Ferrous ion chelating activity was calculated by measuring the absorbance of ferrozine–Fe2+ complex spectrophotometrically. The metal chelating capacity is important since it reduces the concentration of transition metals that may act as catalysts to generate the first few radicals to initiate the radical-mediated oxidative chain reactions in biological and/or in food systems. Ion chelating agents also may inhibit the Fenton reaction and hydrogen peroxide decomposition (Hinneburg et al. Citation2006). Chelating abilities of various extracts increased as the concentration is increased (). The chelating abilities of water extract of T. clypeatus on ferrous ions were stronger than that of ethanolic extracts. Here also the aqueous extracts demonstrated higher scavenging activities than ethanolic extracts. The concentration of the extracts needed for 50% inhibition was found to be 4486.66 and 3060 μg/mL. Ethylene diamine tetra acetic acid (EDTA) exhibited a high chelating ability of 54.18% at 1000 μg/mL.
Total antioxidant activity (ABTS radical cation decolorization assay)
Total antioxidant activity of T. clypeatus was assessed by measuring the reduction of the ABTS radical cation as the percentage of inhibition at 734 nm. The effect of various concentrations of extract (from 20 to 100 μg/mL) on ABTS+ radical is shown in . Termitomyces clypeatus exhibited effective antioxidant activity. The IC50 values of ethanol and aqueous extract of T. clypeatus were 70.57 and 64.36 μg/mL, respectively. The inhibition was found to be concentration dependent, and the antioxidant activity was better than the standard BHT. Their antioxidant powers were ranked in the order: aqueous extract > ethanol extract > BHT.
Reducing ability
shows the reductive capabilities of T. clypeatus and α-tocopherol. The reducing power of T. clypeatus increased concentration dependently, and it showed higher reducing power than α-tocopherol. The reducing ability was ranked in the order: aqueous extract > ethanol extract > BHT.
Cytotoxicity of fractions
Because the aqueous fractions had the strongest antioxidant activities compared with the ethanolic extracts of the fungus, an MTT assay was performed to determine the cytotoxic effects using eight human cancer cell lines, i.e., U373MG, MDA-MB-468, HepG2, HL-60, A549, U937 OAW-42 and Y-79. As shown in , exposure of cells to different concentrations of the AE (0.01, 0.1, 1, 10 and 100 μg/mL) affected significant cytotoxicity at concentrations up to 100 μg/mL. A strong reduction in cell viability was observed in a dose-dependent manner (). The concentrations that reduced growth by 50% (IC50) are summarized in . An important attribute of an efficient anticancer drug is cancer selectivity in its cytotoxic effect. The results of the initial MTT assay experiments showed that in all cases, aqueous extract was significantly toxic to the cancerous cell lines. The lowest IC50 values were found to be 25 ± 1.02 μg/mL against the human leukaemic monocyte lymphoma cell line (U937). Whereas the highest IC50 value was 50 ± 2.04 μg/mL against human ovarian cancer cell line (OAW-42). The aqueous extract showed a much higher activity, reflected by low IC50 values, corresponding to high antiproliferative effect against cancer cells (). As more knowledge becomes available for this mushroom, it is anticipated that T. clypeatus can soon be useful for health promotion. Our study represents the first report for the biological activity of T. clypeatus and was found to possess the highest activity in inducing cell death of several cancer cell lines.
Figure 3. Aqueous extract of Termitomyces clypeatus exhibit differential cytotoxicities towards various cancer cell lines. (A) MDA-MB-468, (B) HL-60, (C) Hep G2, (D) A549, (E) U-937, (F) Y-79, (G) U-373MG and (H) OAW-42.
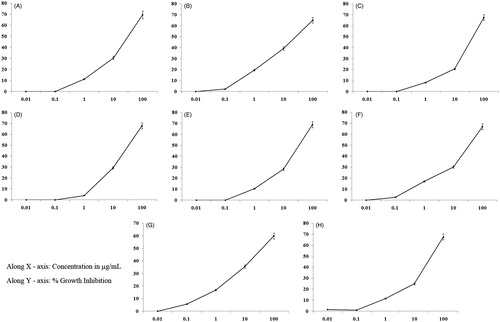
Table 2. List of components present in the aqueous fraction of T. clypeatus with their retention time in HPLC analyses.
Table 3. Growth inhibitory activity of aqueous extract of T. clypeatus on different cancer cell lines.
Acute toxicity study
In the acute toxicity assay, it was found that no mortality was observed up to doses 2000 mg/kg body weight, orally and were considered as safe and no lethality or any toxic reaction were found up to the end of the study period.
Antitumour activity
The antitumour activity of AETC against EAC tumour-bearing mice was assessed by the parameters such as tumour volume, packed cell volume, cell count (viable and non-viable), mean survival time and a percentage increase of life span. The antitumour activity of aqueous extract of T. clypeatus (AETC) on EAC-bearing mice is shown in . There is a significant increase in body weights in EAC-bearing mice (25.20 ± 0.43 g) in comparison with the normal group (20 ± 2 g). Treatment with AETC (200 and 400 mg/kg) significantly (p < 0.05) reduced the increase in body weight of EAC-bearing mice. There was a significant increase in survival time in treating with AETC at 200 and 400 mg/kg. A significant (p < 0.05) decrease of tumour volume and packed cell volume was observed in AETC-treated mice compared with the EAC control group. The number of viable cell count was decreased whereas the number of non-viable cell count was increased on treatment with AETC.
Table 4. The antitumour activity of AETC against EAC tumour-bearing mice.
Haematological parameters
Haematological parameters of AETC treated EAC bearing mice are presented in . The total WBC count was found to increase along with a reduction in the RBC count and haemoglobin content in tumour-bearing mice. A reduction in haemoglobin has been observed in an EAC control group with respect to normal mice, but in all the treated groups, the haematological parameters were significantly (p < 0.05) restored towards the normal.
Table 5. Haematological parameters of AETC-treated EAC-bearing mice.
Effect of AETC on lipid peroxidation and different antioxidant enzyme activity
Malondialdehyde (MDA) is the common marker of lipid peroxidation. MDA, the end product of lipid peroxidation, was reported to be higher in the cancer cell. The MDA content was significantly increased in EAC-treated mice (). On treatment with AETC significantly decreased the MDA content. The glutathione, SOD and catalase activity were decreased in EAC-treated mice (). All the parameters were restored significantly (p < 0.05) towards the normal on treatment with AETC.
Table 6. Effect of AETC on lipid peroxidation and different antioxidant enzyme activity.
Discussion
Our study represents the first functional report of the edible mushroom T. clypeatus with medicinal properties. The results of the current study showed that the aqueous extracts of T. clypeatus, contained the highest concentration of carbohydrates and exhibited potential antioxidant activity in comparison with ethanol extract through the scavenging of free radicals such as DPPH, NO, superoxide and hydroxyl radical; metal chelating, total antioxidant activity and reducing ability. By combining the knowledge of different antioxidant assays and assessment of oxidation parameters in the present study, it can be asserted that the investigated mushroom is a viable source of natural antioxidants. The current study also provided evidence of the cytotoxicity against a panel of cancer cell lines. The mushroom, T. clypeatus, has the potential as “nutraceuticals” for the preparation of functional foods (Ghorai et al. Citation2009). So the extracts of the fungus are the source of natural antioxidants, which can be accounted for the traditional uses in the prevention of cancer and health preservation. This study provides supportive data for future investigations that will lead to their use in cancer therapy. Purification of the active components (polysaccaharides) of the fungal extract and detailed pathway analysis is being studied to understand the basic mechanism of action.
The reliable criteria for judging the value of anticancer drugs are prolongation of lifespan, decrease of WBC from blood and decrease of tumour volume. The reduction in solid tumour volume showed that AETC plays a direct role in killing tumour cells and enhance the curative effect tumour chemotherapy. The level of lipid peroxidation, catalase and protein contents was summarized in tabular representation. Lipid peroxidation mediated by free radical considered being a primary mechanism of cell membrane destruction and cell damage. The present study showed that AETC significantly reduced the elevated levels of lipid peroxidation and thereby it may act as an antitumour agent. A decrease in tumour volume and viable tumour cell count as mentioned above finally reduced the tumour burden and enhanced the life span of EAC-bearing mice.
In cancer chemotherapy, the major problems are of myelosuppression and anaemia. The anaemia encountered in tumour-bearing mice are mainly due to reduction in RBC or haemoglobin percentage and this may occur either due to iron deficiency or due to haemolytic or myelopathic conditions.
Treatment with AETC brought back the haemoglobin content, RBC and WBC cell counts near normal values. This showed that AETC posses protective action on the haematopoietic system. Excessive production of free radicals resulted in oxidative stress, which leads to the damage of macromolecules such as lipids can induce lipid peroxidation in vivo. Increased lipid peroxidation would cause degeneration of tissues. Lipid peroxide formed in the primary site would be transferred through the circulation and provoke damage by propagating the process of lipid peroxidation. MDA, the end product of lipid peroxidation, was reported to be higher in carcinomatous tissues than in non-diseased organs.
Glutathione, a potent inhibitor of neoplastic process, plays an important role as an endogenous antioxidant system that is found particularly in high concentration in the liver and is known to have a key function in the protective process. AETC reduced the elevated levels of lipid peroxidation and increased glutathione content in EAC-bearing mice. These data indicate the aqueous extract of T. clypeatus exhibits significant antitumour activity, which might be due to the antioxidant effects on EAC-bearing hosts.
We propose that the aqueous extract of T. clypeatus has significant antitumour activity which can be inferred from the increased life span of EAC tumour-bearing mice. The present study demonstrates that the aqueous extract of T. clypeatus increased the life span of EAC tumour-bearing mice and decreased lipid peroxidation and thereby augmented the endogenous antioxidant enzymes in the liver. The data suggest that aqueous extract of T. clypeatus possesses anticancer properties valuable for application in food and drug products.
Acknowledgements
The authors are grateful to Department of Biotechnology (DBT), New Delhi, India, for providing a Post Doctoral fellowship to the Dr. Arijit Mondal.
Disclosure statement
No conflict of interest declared.
References
- Banik SP, Pal S, Ghorai S, Chowdhury S, Majumder R, Mukherjee S, Khowala S. 2012. In situ reversible aggregation of extracellular cellobiase in the filamentous fungus Termitomyces clypeatus. Biotechnol Bioprocess Eng. 17:925–936.
- Barros L, Calhelha RC, Vaz JA, Ferreira ICFR, Baptista P, Estevinho LM. 2007. Antimicrobial activity and bioactive compounds of Portuguese wild edible mushrooms ethanolic extracts. Euro Food Res Technol. 225:151–156.
- Blois MS. 1958. Antioxidant determinations by the use of a stable free radical. Nature. 181:1199–1200.
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254.
- Brown W, Anderson Ö. 1971. Preparation of xylodextrins and their separation by gel chromatography. J Chromatogr a. 57:255–263.
- Costantini S, Di Bernardo G, Cammarota M, Castello G, Colonna G. 2013. Gene expression signature of human HepG2 cell line. Gene. 518:335–345.
- D'Amour FE, Blood FR, Da B. 1965. Manual for laboratory work in mammalian physiology. Chicago: The University of Chicago Press.
- Deng Y, Yang G, Yue J, Qian B, Liu Z, Wang D, Zhong Y, Zhao Y. 2014. Influences of ripening stages and extracting solvents on the polyphenolic compounds, antimicrobial and antioxidant activities of blueberry leaf extracts. Food Control. 38:184–191.
- Dhalwal K, Deshpande YS, Purohit AP, Kadam SS. 2005. Evaluation of the antioxidant activity of Sida cordifolia. Pharm Biol. 43:754–761.
- Dehshiri MM, Aghamollaei H, Zarini M, Nabavi SM, Mirzaei M, Loizzo MR, Nabavi SF. 2013. Antioxidant activity of different parts of Tetrataenium lasiopetalum. Pharm Biol. 51:1081–1085.
- Eda H, Burnette BL, Shimada H, Hope HR, Monahan JB. 2011. Interleukin-1β-induced interleukin-6 production in A549 cells is mediated by both phosphatidylinositol 3-kinase and interleukin-1 receptor-associated kinase-4. Cell Biol Int. 35:355–358.
- Ferreira IC, Baptista P, Vilas-Boas M, Barros L. 2007. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: individual cap and stipe activity. Food Chem. 100:1511–1516.
- Ghorai S, Banik SP, Verma D, Chowdhury S, Mukherjee S, Khowala S. 2009. Fungal biotechnology in food and feed processing. Food Res Int. 42:577–587.
- Ghorai S, Mukherjee S, Khowala S. 2011. Improved production and properties of β-glucosidase influenced by 2-deoxy-d-glucose in the culture medium of Termitomyces clypeatus. Biotechnol Bioprocess Eng. 16:297–304.
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. 1982. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 126:131–138.
- Gupta M, Mazumder UK, Haldar P, Kandar CH, Manikandan L, Senthil G. 2010. Anticancer activity of Indigofera aspalathoides and Wedelia calendulaceae in Swiss albino mice. Iran J Pharm Res. 6:141–145.
- Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW, Riechel TL. 1998. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agric Food Chem. 46:1887–1892.
- Han Y, Wu J, Liu T, Hu Y, Zheng Q, Wang B, Lin H, Li X. 2016. Separation, characterization and anticancer activities of a sulfated polysaccharide from Undaria pinnatifida. Int J Biol Macromol. 83:42–49.
- Hashioka S, Klegeris A, Schwab C, McGeer PL. 2009. Interferon-γ-dependent cytotoxic activation of human astrocytes and astrocytoma cells. Neurobiol Aging. 30:1924–1935.
- Hinneburg I, Damien Dorman H, Hiltunen R. 2006. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem. 97:122–129.
- Ialenti A, Moncada S, Rosa M. 1993. Modulation of adjuvant arthritis by endogenous nitric oxide. Br J Pharmacol. 110:701–706.
- Kakkar P, Das B, Viswanathan P. 1984. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 21:130–132.
- Kanno C, Kashiwagi Y, Horie K, Inomata M, Yamamoto T, Kitanaka C, Yamashita H. 2009. Activin inhibits cell growth and induces differentiation in human retinoblastoma Y79 cells. Curr Eye Res. 34:652–659.
- Khowala S, Sengupta S. 1992. Secretion of β-glucoside by Termitomyces clypeatus: regulation by carbon catabolite products. Enzyme Microbial Technol. 14:144–149.
- Kostrzewa-Nowak D, Tarasiuk J. 2013. Bioreductive activation of mitoxantrone by NADPH cytochrome P450 reductase does not change its apoptotic stimuli properties in regard to sensitive and multidrug resistant leukaemia HL60 cells. Eur J Pharmacol. 721:141–150.
- Lata H, Ahuja G. 2003. Role of free radicals in health and disease (Review Article). Indian J Physiol Allied Sci. 57:124–132.
- Litchfield J, Wilcoxon F. 1949. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther. 96:99–113.
- Luck H. 1963. Methods of enzymatic analysis. New York: Academic Press.
- Maiti S, Bhutia SK, Mallick SK, Kumar A, Khadgi N, Maiti TK. 2008. Antiproliferative and immunostimulatory protein fraction from edible mushrooms. Environ Toxicol Pharmacol. 26:187–191.
- Marcocci L, Maguire JJ, Droylefaix MT, Packer L. 1994. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem Biophys Res Commun. 201:748–755.
- Mau JL, Chang CN, Huang SJ, Chen CC. 2004. Antioxidant properties of ethanolic extracts from Grifola frondosa, Morchella esculenta and Termitomyces albuminosus mycelia. Food Chem. 87:111–118.
- Menshova RV, Anastyuk SD, Ermakova SP, Shevchenko NM, Isakov VI, Zvyagintseva TN. 2015. Structure and anticancer activity in vitro of sulfated galactofucan from brown alga Alaria angusta. Carbohydr Polym. 132:118–125.
- Mizuno T. 1999. The extraction and development of antitumor-active polysaccharides from medicinal mushrooms in Japan (review). Int J Med Mushrooms. 1:9–30.
- Mohamed AA, Ali SI, El-Baz FK. 2013. Antioxidant and antibacterial activities of crude extracts and essential oils of Syzygium cumini Leaves. PLoS One. 8:e60269.
- Mulder TP, Manni JJ, Roelofs HM, Peters WH, Wiersma A. 1995. Glutathione S-transferases and glutathione in human head and neck cancer. Carcinogenesis. 16:619–624.
- Nishikimi M, Appaji Rao N, Yagi K. 1972. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 46:849–854.
- Ohkawa H, Ohishi N, Yagi K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 95:351–358.
- Okhuoya JA, Akpaja EO, Osemwegie OO, Oghenekaro AO, Ihayere CA. 2010. Nigerian mushrooms: underutilized non-wood forest resources. J Appl Sci Environ Manag. 14:43–54.
- Oyaizu M. 1986. Studies on products of browning reaction-antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr. 44:307–315.
- Oyetayo V. 2009. Free radical scavenging and antimicrobial properties of extracts of wild mushrooms. Braz J Microbiol. 40:380–386.
- Pal S, Banik SP, Ghorai S, Chowdhury S, Khowala S. 2010. Purification and characterization of a thermostable intra-cellular β-glucosidase with transglycosylation properties from filamentous fungus Termitomyces clypeatus. Bioresour Technol. 101:2412–2420.
- Pal S, Banik SP, Khowala S. 2013. Mustard stalk and straw: a new source for production of lignocellulolytic enzymes by the fungus Termitomyces clypeatus and as a substrate for saccharification. Ind Crop Prod. 41:283–288.
- Passmore J, Lukey P, Ress S. 2001. The human macrophage cell line U937 as an in vitro model for selective evaluation of mycobacterial antigen‐specific cytotoxic T‐cell function. Immunology. 102:146–156.
- Prior RL, Wu X, Schaich K. 2005. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 53:4290–4302.
- Qian ZG. 2014. Cellulase-assisted extraction of polysaccharides from Cucurbita moschata and their antibacterial activity. Carbohydr Polym. 101:432–434.
- Robak J, Gryglewski RJ. 1988. Flavonoids are scavengers of superoxide anions. Biochem Pharmacol. 37:837–841.
- Robbins SL, Cotran RS. 1979. Pathologic basis of disease. New York: Elsevier-Health Science Division.
- Ross R. 1993. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 362:801–803.
- Sainani G, Manika J, Sainani R. 1997. Oxidative stress: a key factor in pathogenesis of chronic diseases. Med Update. 1:1–4.
- Schauss AG, Wu X, Prior RL, Ou B, Huang D, Owens J, Agarwal A, Jensen GS, Hart AN, Shanbrom E. 2006. Antioxidant capacity and other bioactivities of the freeze-dried Amazonian palm berry, Euterpe oleraceae Mart. (acai). J Agric Food Chem. 54:8604–8610.
- Singh N, Rajini P. 2004. Free radical scavenging activity of an aqueous extract of potato peel. Food Chem. 85:611–616.
- Valko M, Rhodes C, Moncol J, Izakovic M, Mazur M. 2006. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem-Biol Interact. 160:1–40.
- Wang CZ, Yuan HH, Bao XL, Lan MB. 2013. In vitro antioxidant and cytotoxic properties of ethanol extract of Alpinia oxyphylla fruits. Pharm Biol. 51:1419–1425.
- Wenli Y, Yaping Z, Bo S. 2004. The radical scavenging activities of Radix puerariae isoflavonoids: a chemiluminescence study. Food Chem. 86:525–529.
- Wickens AP. 2001. Ageing and the free radical theory. Respir Physiol. 128:379–391.
- Wintrobe MM. 1962. Clinical hematology. Acad Med. 37:78.
- Wolfenden BS, Willson RL. 1982. Radical-cations as reference chromogens in kinetic studies of one-electron transfer reactions: pulse radiolysis studies of 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate). J Chem Soc. 2:805–812.
- Xu J, Chambers AF, Tuck AB, Rodenhiser DI. 2008. Molecular cytogenetic characterization of human breast cancer cell line MDA-MB-468 and its variant 468LN, which displays aggressive lymphatic metastasis. Cancer Genet Cytogen. 181:1–7.
- Yamaguchi T, Takamura H, Matoba T, Terao J. 1998. HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-diphenyl-2-picrylhydrazyl. Biosci Biotechnol Biochem. 62:1201–1204.
- Zaffaroni N, De Marco C, Villa R, Riboldi S, Daidone MG, Double JA. 2001. Cell growth inhibition, G2 M cell cycle arrest and apoptosis induced by the imidazoacridinone C1311 in human tumour cell lines. Eur J Cancer. 37:1953–1962.