Abstract
Context: Endophytic fungi, being a prolific source of bioactive secondary metabolites, are of great interest for natural product discovery.
Objective: Isolation and partial characterization of endophytic fungi inhabiting the leaves and woody parts of Taxus fuana Nan Li & R.R. Mill. (Taxaceae) and evaluation of biological activity.
Materials and methods: Endophytic fungal isolates were identified by molecular analysis of internal transcribed spacer (ITS) regions of 18S rDNA. Extracts of the endophytic fungi cultured on potato dextrose agar and modified medium were evaluated using cancer chemoprevention bioassays [inhibition of TNF-α-induced NFκB, aromatase and inducible nitric oxide synthase (iNOS); induction of quinone reductase 1 (QR1)] and growth inhibition with MCF-7 cells.
Results: Nine of 15 fungal isolates were identified as belonging to Epicoccum, Mucor, Penicillium, Chaetomium, Paraconiothriym, Plectania or Trichoderma. Five of the 15 extracts inhibited NFκB activity (IC50 values ranging between 0.18 and 17 μg/mL) and five inhibited iNOS (IC50 values ranging between 0.32 and 12.9 μg/mL). In the aromatase assay, only two isolates mediated inhibition (IC50 values 12.2 and 10.5 μg/mL). With QR1 induction, three extracts exhibited significant activity (concentrations to double activity values ranging between 0.20 and 5.5 μg/mL), and five extracts inhibited the growth of MCF-7 cells (IC50 values ranging from 0.56 to 17.5 μg/mL). Six active cultures were derived from woody parts of the plant material.
Conclusion: The endophytic fungi studied are capable of producing pharmacologically active natural compounds. In particular, isolates derived from the wood of Taxus fuana should be prioritized for the isolation and characterization of bioactive constituents.
Introduction
Natural products have long been hailed as candidates for designing drugs against various diseases. Over the years, many researchers have investigated the biological potential of ethno-botanical plants (Haq et al. Citation2013). Recent investigations have focused on endophytic micro-organisms that reside asymptomatically within the living tissues of higher plants (Joseph & Priya Citation2011). Endophytes have yielded a broad range of unique structures such as alkaloids, benzopyranones, quinones, flavonoids, phenolic acids and quinines (Pimentel et al. Citation2010). These secondary metabolites demonstrate various activities and are employed as antibiotics, immunosuppressants, antioxidants, antivirals, antidiabetics or anticancer agents (Gunatilaka Citation2006). In principle, endophytes provide a cost-effective and renewable source for such valuable natural products.
Paclitaxel (Taxol®) and its derivatives are forerunners of the first major group of anticancer agents produced by endophytes. Taxol interferes with the cell cycle by stabilizing microtubule formation and thus inhibits the proliferation of cancer cells (Cheewarat Citation2006). The isolation of taxol from the Yew prompted the screening of associated endophytes for the same chemical entity. These efforts turned fruitful with the discovery of a novel taxol-producing endophytic fungus, Taxomyces andreanae, from Taxus brevifolia (Stierle et al. Citation1993). Subsequently, endophytes have been investigated widely for anticancer agents (Strobel & Daisy Citation2003; Zhao et al. Citation2011).
Carcinogenesis is a multistage process involving multiple events including initiation, promotion and progression that ultimately culminate in tumourigenesis (Pezzuto et al. Citation2005). Therefore, any substance that can halt or reverse these stages is a potential chemopreventive agent (Kinghorn et al. Citation2004). Terrestrial plants have been widely evaluated for the discovery of cancer chemopreventive agents (Park & Pezzuto Citation2002; Kondratyuk & Pezzuto Citation2004). Included among these are plants from the Himalayan region of Pakistan (Haq et al. Citation2013), where there is a tremendous biodiversity of medicinal plants.
Taxus fuana Nan Li & R.R. Mill (Taxaceae) is an important medicinal plant found in the West Himalayan region of Pakistan. Endophytes associated with Taxus species have been studied for the isolation of potential chemopreventive agents which can target specific pathways in metastasis by inhibiting protein kinases and NFκB (Kondratyuk & Pezzuto Citation2004; Aly et al. Citation2010). However, to the best of our knowledge, there is no previous report describing the functional diversity and cancer chemopreventive potential of endophytes associated with Taxus fuana from this region. We have therefore performed such studies with indigenous endo-microflora to establish a cornerstone for isolating lead compounds for drug development.
Materials and methods
Collection of plant sample
Healthy and mature plants of Taxus were selected for sample collection from Nathiagali (District Hazara, Khyber Pakhtunkhwa, Pakistan). Leaves and woody parts (stem and bark) were collected randomly for study. The plant material was brought to the laboratory in sterile bags under cold conditions for further processing. The plant was initially identified at the Department of Plant Sciences, Quaid-i-Azam University, by Prof. Dr. Mir Ajab Khan, as Taxus baccata after comparison with a preserved voucher specimen no. 2978-ZB (accession no. 52035) in the Herbarium of Pakistan. Later, however, it was taxonomically identified as Taxus fuana as described by Shah et al. (Citation2008).
Isolation of endophytes
Isolation of the endophytes was carried out by the surface sterilization method (Petrini Citation1986). The material was thoroughly washed using distilled water followed by treatment with 70% ethanol for 2 min and 5% sodium hypochlorite for 5 min to accomplish surface sterilization. It was again rinsed with sterile distilled water and finally blotted with sterile blotting paper. The process of sterilization was evaluated by the imprinting method prior to plating (Schulz et al. Citation1993). Next, the samples were cut into 5–6 pieces (2–6 mm in size) and placed on water agar plates (distilled water, 1.5% agar) and potato dextrose agar (PDA Oxoid) plates supplemented with penicillin G (100 U/mL) and streptomycin (100 μg/mL). Plates were incubated at 28 °C for 2–4 weeks until growth was initiated (Hijri et al. Citation2002). The hyphal tips emerging from the plant part were selected, purified and maintained on PDA plates for further studies.
Taxonomic identification of fungal isolates
Taxonomic identification of selected endophytic fungi was conducted by analyzing the internal transcribed spacer (ITS) region of the 18S subunit of ribosomal DNA (rDNA) sequence using PCR. DNA was extracted from fungal mycelia by the CTAB method (Paul et al. Citation2007). A pair of universal primers ITS1 (5′-TCC GTA GGT GAA CCT GCG G-3′) and ITS4 (5′-TCC TCC GCT TAT TGA TAT GC-3′) was used to amplify the target genes. PCR was carried out in a programable MJ MiniTM gradient thermal cycler (Bio-Rad Laboratories Inc., Berkeley, CA). The reaction mixture (25 μL) contained 5 μL of DNA template, 3 μL of 25 mM MgCl2, 100 μM of each dNTP, 25 pM of each primer and 1 U of Taq DNA polymerase. All the primers, dNTPs and enzymes were purchased from Fermentas (Fermentas, Thermo Scientific, Waltham, MA). The amplification conditions involved: preheating at 95 °C for 5 min; followed by 35 cycles with a denaturation step at 95 °C for 30 s; annealing step at 55 °C for 1 min; and an extension step at 72 °C for 1 min, followed by final extension at 72 °C for 6 min (Ferrer et al. Citation2001). The identified sequences were deposited in the NCBI GenBank and accession numbers obtained.
Cultivation of fungi for production of metabolites
Selected isolates were cultivated on potato dextrose agar (PDA) purchased from OxoidTM (Oxoid, Basingstoke, UK) and modified taxol medium (TM) according to (Xu et al. Citation2006). The TM medium contained (g/L): sucrose, 40; phenylalanine, 0.01; peptone, 0.5; yeast extract, 0.8; (NH4)2SO4, 3.0; MgSO4·7H2O, 0.5; KH2PO4, 2.0; NaCl, 0.6; CH3COONa, 0.5; C6H5COONa, 0.1; agar, 15.0. A mycelial disc of 8 mm diameter, from a freshly grown colony of fungal isolate, was transferred aseptically to the center of the plates and incubated at 25 °C at 150 rpm for 21 d. After incubation, mycelia along with the agar was blended three-times with an equal volume of ethyl acetate and left overnight on a shaker. The organic solvent was pooled by filtration and concentrated to dryness using a rotary evaporator. A stock solution (5 mg/mL) was prepared in dimethyl sulphoxide (DMSO) for biological screening.
Biological evaluation of extracts
Ethyl acetate extracts obtained from 15 fungal strains were evaluated for biological potential using a panel of in vitro assays.
Inhibition of TNF-α-induced NFκB activity
This assay was performed using 293/NFκB-Luc HEK cells (Labvision Corp, Fremont, CA) as reported earlier (Haq et al. Citation2013). NFκB activity was measured with a luciferase kit (Promega Corporation, Madison, WI) using a LUMIstar Galaxy Luminometer (BMG Labtechnologies, Durham, NC) according to instructions of the manufacturer. Data were calculated as % inhibition relatively to DMSO control. Samples which showed more than 60% inhibition at the test concentration 20 μg/mL were tested for dose-dependence to determine IC50 values (Hoshino et al. Citation2010). Three NFκB inhibitors were used as positive controls: N-tosyl-l-phenylalanylchloromethyl ketone (TPCK), IC50 = 3.8 ± 0.6 μM; (E)-3-(4-methylphenylsulfonyl)-2-propenenenitrile (BAY-11), IC50 = 2.0 ± 0.33 μM; resveratrol, IC50 = 2.5 ± 0.3 μM. All chemicals were purchased from BD Biosciences, San Jose, CA. Sulphorhodamine B (SRB) cytotoxicity tests were performed in parallel to avoid false-positive results.
Inhibition of nitric oxide (NO) production in lipopolysaccharide (LPS)-activated murine macrophage RAW 264.7 cells
In this assay, potential to inhibit inducible nitric oxide synthase (iNOS) was assessed with lipopolysaccharide (LPS)-activated murine macrophage RAW 264.7 cells as described earlier (Haq et al. Citation2013). Samples showing more than 70% inhibition at a concentration 20 μg/mL were tested at three-fold serial dilutions to find the IC50 values. NG-Monomethyl-L-arginine, monoacetate salt (L-NMMA) was used as a positive control (IC50 = 19.7 μM; BD Biosciences, San Jose, CA). Sulphorhodamine B (SRB) cytotoxicity tests were performed in parallel to avoid false positive results.
Aromatase inhibition
Inhibition of aromatase was determined via dealkylation of dibenzylfluorescein as reported previously (Maiti et al. Citation2007). Fluorescence was measured at 485 nm (excitation) and 530 nm (emission). The experiment was performed in duplicate by using five concentrations of test substance to determine IC50 values. Naringenin (IC50 = 0.23 μM; BD Biosciences, San Jose, CA) was used as a positive control.
Quinone reductase 1 (QR1)
This assay was performed using Hepa 1c1c7 (murine hepatoma) cells as described earlier (Haq et al. Citation2013). Quinone reductase activity was measured as a function of the NADPH-dependent menadiol-mediated reduction of 3-(4,5-dimetylthiazo-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Generation of blue formazan was determined by measuring absorption at 595 nm (Song et al. Citation1999). Protein content was determined via crystal violet staining of identical plates. Specific activity was defined as nmol of formazan formed per mg protein per min. The induction ratio (IR) was determined at a fixed concentration of 20 μg/mL and defined as the activity obtained in the presence of the test substance divided by the activity in the absence of the test substance. If the IR was approximately 2 or greater, samples were tested in five-fold serial dilutions to find CD values, i.e., the concentration of test sample required to increase activity by 2-fold. 4′-Bromoflavone [concentration required to double activity (CD) = 0.01 μM; BD Biosciences, San Jose, CA] was used as a positive control. A total protein assay using crystal violet stain was performed simultaneously to test the cytotoxic effects of the samples (Haq et al. Citation2013).
DPPH-free radical scavenging
Free radical scavenging activity was determined using 2,2-diphenyl-1-picryl-hydrazyl (DPPH) as described by Lee et al. (Citation1998). Absorbance was measured at 515 nm using a microplate reader; the highest final concentration of the tested sample was 200 μg/mL. Samples showing more than 70% scavenging activity were further tested to determine IC50 values. Ascorbic acid (IC50 = 35.6 μM; BD Biosciences, San Jose, CA) and pure DMSO were used as positive and negative controls, respectively. The test was performed in triplicate and IC50 values were calculated.
where “Ac” is the absorbance of control and “As” is the absorbance of the test sample.
Growth inhibition with cultured MCF-7 cells
The cytotoxic potential of test substances with MCF-7 cells was determined with sulphorhodamine B (SRB) as described previously (Haq et al. Citation2013). Cells were seeded in 96-well plates (106 cells/mL), and various concentrations of samples dissolved in 10 μL of 10% DMSO were added to each well. A zero-day control was used in each case. The IC50 values were determined as the concentration of sample required to inhibit the 72 h growth of cells by 50%, relative to a control treated with solvent (0.5% DMSO) only. Paclitaxel (BD Biosciences, San Jose, CA: percentage purity <97%) was used as a positive control. Percent of cell survival was calculated using the formula:
(1)
where 'OD' is the optical density at 515 nm.
Statistical analysis
The data, in general, are presented as mean ± SEM of triplicate experiments. The least significant differences (LSD) between groups were determined by analysis of variance (ANOVA) and all pairwise comparison tests using statistical software (Statistix 8.1, SPSS Inc., Chicago, IL). *p < 0.05 and **p < 0.001 were set as significance criteria.
Results
A total of 15 endophytic fungi, 10 from wood and five from leaves, were isolated from Taxus fuana ( and ). The preliminary identification of the endophytic fungi was carried out on the basis of macro-morphological features and the in vitro growth pattern on potato dextrose agar. It was further confirmed by molecular analysis of 18S ribosomal DNA and isolates were assigned to different genera. A total of nine isolates were identified at the genus level by comparing percentage homology with closely related genera available at NCBI GenBank (). Most of the isolates belong to division Ascomycota, with the genus Epicoccum being the most frequent.
Figure 1. Isolation and macro-morphological characteristics of endophytic fungi isolated from the wood of Taxus fuana.
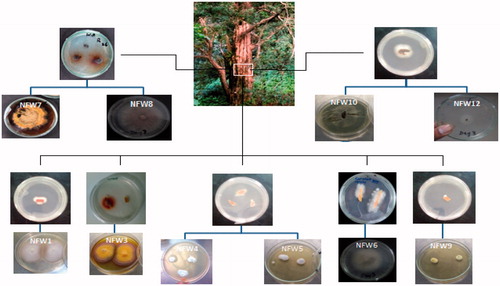
Figure 2. Isolation and macro-morphological characteristics of endophytic fungi isolated from the leaves of Taxus fuana.
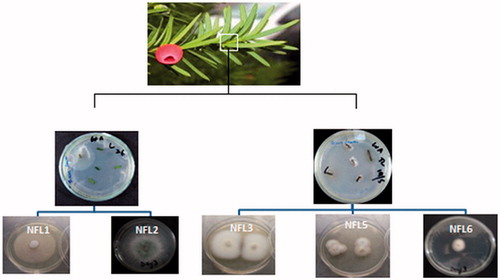
Table 1. Percent homology and accession numbers of the ITS region of 18S rDNA nucleotide sequences of endophytic fungi.
To evaluate the cancer chemopreventive potential of the selected endophytes, a panel of assays was utilized. In the TNF-α-induced NFκB inhibition assay, a total of five samples (NFW1, NFW3, NFW8, NFW9 and NFL1) demonstrated more than 60% inhibition at a concentration of 20 μg/mL, with IC50 values ranging from 0.18 to 17 μg/mL (). Among the leaf isolates, only one strain, NFL1, showed strong inhibition of 72.0% in TM medium; the IC50 value for the extract was 5.8 μg/mL.
Table 2. Biological activity mediated by extracts derived from endophytic fungi.
In the LPS-induced NO inhibition assay, five isolates (NFW1, NFW3, NFW6, NFW7 and NFL1) showed iNOS inhibition greater than 50% at a test concentration 20 μg/mL. The samples showing ≥70% inhibition and ≥70% cell survival at this concentration were selected for dose-dependence to determine IC50 values. Among positive strains, NFW1, NFW3 and NFL1 showed the highest activity with percentage inhibition of 99.6, 69.7 and 84.2% with corresponding IC50 values of 0.32, 7.8 and 4.4 μg/mL, respectively ().
In the present study, we used the MCF-7 breast cancer cell line to evaluate cytotoxic potential of fungal endophytes. Five samples, i.e., crude extracts of NFW1 (both media), NFW8, NFW9 and NFL1, demonstrated cytotoxicity against MCF-7 cells with percentage survival in the range of 0.00–45.7 and corresponding IC50 values of 0.56–17.5 μg/mL. The crude extract of the wood isolate NFW1 demonstrated strong cytotoxicity [% survival = 0.00 (TM extract) and 16.6% (PDA extract)] with IC50 values of 0.56 and 6.82 μg/mL when grown in TM and PDA media, respectively. One of the leaf isolates, NFL1, also showed cytotoxicity towards the MCF-7 cell line with 45.7% survival in TM media and a corresponding IC50 value of 11.3 μg/mL.
In the aromatase inhibition assay, two isolates, NFW3 and NFW9, demonstrated 73.3 and 76.4% inhibition with corresponding IC50 values 12.2 and 10.5 μg/mL, respectively. Similarly, in the quinone reductase induction assay, it was found that three of the isolates, namely NFW3, NFW7 and NFL1, exhibited significant activity showing induction ratios (IR) of 2.6–6.9 with concentration to double activity (CD) values in the range of 0.2–5.5 μg/mL (). NFW6 was weakly active with a CD value of 20.4 ± 1.4 μg/mL.
In the DPPH assay for free radical scavenging potential, four samples (NFW3, NFW6, NFW7 and NFW9) showed ≥70% scavenging activity at a test concentration of 200 μg/mL (). The corresponding IC50 values ranged from 11.7 to 53.9 μg/mL. The most potent activity was observed with NFW3 (an IC50 value of 11.7 μg/mL). Other strains showed low levels of activity, in the range of 3-34% scavenging at a test concentration of 200 μg/mL.
Discussion
Endophytic fungi are considered a valuable source for isolating novel bioactive compounds. In the present study, we isolated 15 endophytic fungi from the medicinal plant Taxus fuana and nine were identified. Most of the isolates belonged to division Ascomycota. Among the wood isolates, NFW3 and NFW7 were identified as the genus Epicoccum. Epicoccum species are often isolated as a major crop endophyte (Martini et al. Citation2009; Stuart et al. Citation2010; Fávaro et al. Citation2012). As reported previously, Epicoccum has the capability of producing tetramic acid derivatives (epiconigine and epicoccamide) and diketopiprazine, and macrolides (Wright et al. Citation2003; Wangun et al. Citation2007).
The strain NFW5 was identified as Tritirachium sp. Recently, an endophytic Tritirachium species was also isolated from Quercus pannosa and Rhododendron spp. collected from the Baima Snow Mountain, Southwest China (Li et al. Citation2012). Macrosphelides macrolide polyketides, exhibiting antitumour activity, have been reported from Tritirachium fungus (Ivanova et al. Citation2007). However, none of the previous studies report it as an endophyte associated with the Taxus plant species. Consistent with earlier reports from Taxus chinensis (Zhou et al. Citation2009; Tayung & Jha Citation2010), we also identified the NFW6 isolate as a strain of Mucor hiemalis. NFW8 was identified as Chaetomium which resides within various plants such as Curcuma wenyujin, Viguiera robusta and Taxus globosa (Momesso et al. Citation2008; Wang et al. Citation2010; Soca-Chafre et al. Citation2011). The genus Chaetomium has great potential to produce bioactive metabolites including chaetoviridins, which exhibit tumour inhibition and antifungal activities (Park et al. Citation2005; Borges et al. Citation2011). NFW9 was identified as Penicillium polonicum. Various Penicillium species have been reported to produce anthraquinones, alkaloids, deketopiprazines and steroids possessing antitumour, antioxidant and antimicrobial potential (Nicoletti et al. Citation2008).
Among the leaf isolates, NFL1 and NFL2 were identified as Plectania and Trichoderma spp., respectively, which have been reported as endophytes from Taxus plants (Liu et al. Citation2009; Zhao et al. Citation2010). The Trichoderma genus is also well known for its bioactive metabolites trichothecens, camptothecin (quinoline alkaloid), anthraquinones and lactones (Lu et al. Citation2012; Pu et al. Citation2013).
NFL6 was identified as Paraconiothyrium sp., which correlates with the findings of other researchers (Han et al. Citation2012; Khan et al. Citation2012). Previous reports indicate that Paraconiothyrium, an endophyte of the marine sponge Ectyplasia perox, can produce meroterpenoids (epoxyphomalins) exhibiting potent in vitro growth inhibitory activity against human tumour cell lines (Mohamed et al. Citation2009, Citation2010).
Fungi are considered a rich source of cancer chemopreventive and cytotoxic compounds. Metabolites from endophytes are used to target specific pathways, which consequently inhibit or regulate carcinogenesis and the cell cycle. Based on such a targeted approach, NFκB inhibition is a plausible mechanism for the treatment or prevention of cancer (Schupp et al. Citation2009; Luqman & Pezzuto Citation2010). Some fungal metabolites reported for potential to inhibit NFκB include trichodion, tricyclic acid, panepoxydone, hispidin derivatives, chaetoglobosin and mycoepoxydiene (Erkel Citation2000; Wijeratne et al. Citation2003; Erkel et al. Citation2007; Dou et al. Citation2011; Wu et al. Citation2011; Wang et al. Citation2012). In our study, six of the isolates presented more than 60% NFκB inhibition, suggesting their potential to produce cancer chemopreventive compounds.
Similarly, inducible nitric oxide synthase (iNOS) is consistently associated with chronic inflammation and tumourigenesis. During early tumour development, there is up-regulation of iNOS, suggesting its inhibition as a cancer chemopreventive strategy (Park & Pezzuto Citation2002; Nomelini et al. Citation2008). In the LPS-induced NO inhibition assay, five isolates showed more than 50% inhibition with IC50 values ranging from 0.32 to 12.9 μg/mL. These results are consistent with the iNOS inhibitory potential of fungal metabolites such as radicicol, sporogen, S14-95, curvularin, azaphilones and lactone derivatives (Jeon et al. Citation2000; Yao et al. Citation2003; Quang et al. Citation2006; Yang et al. Citation2010; Lee et al. Citation2011).
Fungal metabolites known to exhibit cytotoxic activity include taxol, norsesquiterpene peroxides, cycloheptapeptides, lactones and chlorinated anthraquinones (Wang & Tang Citation2011; Chen et al. Citation2012; Huang et al. Citation2012). In previous work, one of these strains, NFW1 crude extract, showed overlapping peak distribution at a similar retention time indicating the presence of taxol and related moieties (Jadoon et al. Citation2015). This was further confirmed in that NFW1 extract showed taxol peaks with molecular ions m/z 854.82 (M + H)+, 870.80 (M + NH4)+ and 876.82 nm (M + Na)+ in the LC-ESI-MS spectrum (Jadoon et al. Citation2015). During the current study, we tested the extracts and could not identify taxol using a Varian 500-MS ion trap mass spectrometer with an electrospray source (ESI). It is likely that fermentation conditions could be optimized to elicit taxol production, and work of this type is ongoing in Pakistan.
In any case, fungal isolates NFW1, NFW8, NFW9 and NFL1 mediated cytotoxicity against MCF-7 cells with percentage survival of 0.00–45.7% and corresponding IC50 values of 0.56–17.5 μg/mL. These values are below the limit established by the National Cancer Institute (IC50 value< 20 μg/mL) (Lee & Houghton Citation2005).
Aromatase is a cytochrome P450 enzyme complex which converts androgens to estrogens (Jongen et al. Citation2005). Estrogens, being involved in important physiological processes, play a key role in diseases like endometrial and breast cancers (Maiti et al. Citation2007). Aromatase inhibitors block the production of estrogens and may be effective against estrogen receptor positive breast cancer. Although some agents are already used in the clinic, the search for more efficient aromatase inhibitors continues (Balunas & Kinghorn Citation2010). Fungal metabolites with azol rings act as inhibitors of different P450 enzymes including aromatase (Zhang et al. Citation2002). Various other fungal metabolites, such as monomeric xanthones, benzophenone, depsidones and diaryl ethers, also inhibit P450 enzymes (Krick et al. Citation2007; Chomcheon et al. Citation2009; Sureram et al. Citation2012). In this work, isolates NFW3 and NFW9 showed aromatase inhibition of 73.3 and 76.4% with IC50 values of 12.2 and 10.5 μg/mL, respectively. The activity is rather modest but prior to conducting any additional work specificity for aromatase (CYP19) should be assessed, and activity should be reaffirmed in an assay that is not dependent on a fluorescent signal.
One of the strategies for protecting cells from cancer initiation events involves increasing phase II enzymes which can deactivate reactive species. Quinone reductase is an important phase II enzyme and plays an important role in the detoxification of certain reactive chemical species. It is widely distributed in mammalian cells and regarded as a convenient biomarker (Cuendet et al. Citation2006). Therefore, we studied the effect of endophytes on quinone reductase 1 (QR1) induction using cultured Hepa 1c1c7 (murine hepatoma) cells. Three of the isolates, namely NFW3, NFW7 and NFL1, exhibited significant induction levels, showing induction ratios (IR) of 2.6–6.9 and concentration to double activity (CD) values of 0.20–5.5 μg/mL, respectively. These findings are comparable with the quinone reductase induction potential of two xanthone derivatives from the marine algicolous fungus Monodictys putredinis showing CD values 22.1 and 24.8 μM, respectively (Pontius et al. Citation2008).
The discovery of novel and safe antioxidants from natural products to combat or prevent diseases is a continuing process. Some compounds suppressing superoxide generation and showing antioxidant activity are associated with cancer chemoprevention (Krick et al. Citation2007). To determine the free radical scavenging potential of our samples, the DPPH assay was performed. Four fungal extracts, NFW3, 6, 7 and 9, showed significant scavenging activity. The most potent activity was observed with NFW3 (an IC50 value of 11.7 μg/mL). Antioxidant potential has been reported previously with organic extracts of other fungal endophytes such as Phomopsis, Xylaria and Colletotrichum (Joseph & Priya Citation2011; Tianpanich et al. Citation2011; Ravindran et al. Citation2012; Wu et al. Citation2013). In addition, Nitya et al. (Citation2011) reported DPPH scavenging activities with methanolic extracts of Fusarium, Aspergillus, Penicillium and Mucor, with IC50 values ranging between 200 and 325 μg/mL.
Conclusion
It is well established that endophytes are capable of producing remarkable cancer chemotherapeutic agents (Tayung & Jha Citation2010; Cragg & Pezzuto Citation2015). This investigation of biological activities mediated by endophytic fungi isolated from Taxus fuana indicates their diverse potential for the generation of bioactive metabolites. Most of the wood isolates (NFW1, NFW3 and NFW9) displayed significant cancer chemopreventive activity in more than one assay which suggests the production of pharmaceutically relevant biochemical entities, especially when cultured in TM mineral salt medium. This supports the value of these isolates for future studies to isolate and identify active principle candidates for targeted drug development.
Funding information
Funding was provided by the Higher Education Commission of Pakistan under the “International Research Support Initiative Program.”
Acknowledgements
The authors acknowledge Prof. Dr. Mir Ajab Khan and Dr. Mushtaq Ahmad of the Department of Plant Sciences, Quaid-i-Azam University, Islamabad, for their help with plant collection and identification.
Disclosure statement
The authors report that they have no conflicts of interests.
References
- Aly A, Debbab A, Edrada-Ebel R, Proksch P. 2010. Protein kinase inhibitors and other cytotoxic metabolites from the fungal endophyte Stemphylium botryosum isolated from Chenopodium album. Mycosphere. 1:153–162.
- Balunas MJ, Kinghorn AD. 2010. Natural compounds with aromatase inhibitory activity: an update. Planta Med. 76:1087–1093.
- Borges WS, Mancilla G, Guimaraes DO, Durán-Patrón R, Collado IG, Pupo MT. 2011. Azaphilones from the endophyte Chaetomium globosum. J Nat Prod. 74:1182–1187.
- Cheewarat S. 2006. Investigation of bioactive compounds from Phomposis spp., endophytic fungi of Casearia greviaefolia and Stephania hernandifolia [Thesis]. Mahidol University.
- Chen Z, Song Y, Chen Y, Huang H, Zhang W, Ju J. 2012. Cyclic heptapeptides, cordyheptapeptides C-E, from the marine-derived fungus Acremonium persicinum SCSIO 115 and their cytotoxic activities. J Nat Prod. 75:1215–1219.
- Chomcheon P, Wiyakrutta S, Sriubolmas N, Ngamrojanavanich N, Kengtong S, Mahidol C, Ruchirawat S, Kittakoop P. 2009. Aromatase inhibitory, radical scavenging, and antioxidant activities of depsidones and diaryl ethers from the endophytic fungus Corynespora cassiicola L36. Phytochemistry. 70:407–413.
- Cragg GM, Pezzuto JM. 2015. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med Princ Pract. doi:10.1159/000443404.
- Cuendet M, Oteham CP, Moon RC, Pezzuto JM. 2006. Quinone reductase induction as a biomarker for cancer chemoprevention. J Nat Prod. 69:460–463.
- Dou H, Song Y, Liu X, Gong W, Li E, Tan R, Hou Y. 2011. Chaetoglobosin Fex from the marine-derived endophytic fungus inhibits induction of inflammatory mediators via Toll-like receptor 4 signaling in macrophages. Biol Pharm Bull. 34:1864–1873.
- Erkel G. 2000. Trichodion, a new inhibitor of inflammatory signal transduction pathways from a Trichosporiella species. FEBS Lett. 477:219–223.
- Erkel G, Wisser G, Anke T. 2007. Influence of the fungal NF-kappaB inhibitor panepoxydone on inflammatory gene expression in MonoMac6 cells. Int Immunopharmacol. 7:612–624.
- Fávaro LCdL, Sebastianes FLdS, Araújo WL. 2012. Epicoccum nigrum P16, a sugarcane endophyte, produces antifungal compounds and induces root growth. PLoS One. 7:e36826.
- Ferrer C, Colom F, Frasés S, Mulet E, Abad JL, Alió JL. 2001. Detection and identification of fungal pathogens by PCR and by ITS2 and 5.8S ribosomal DNA typing in ocular infections. J Clin Microbiol. 39:2873–2879.
- Gunatilaka AL. 2006. Natural products from plant-associated microorganisms: distribution, structural diversity, bioactivity, and implications of their occurrence. J Nat Prod. 69:509–526.
- Han M, Liu T, Cai X, Chen K, Liu C, Kemp B, Xue Y, Gu Y. 2012. A new endophytic Paraconiothyrium brasiliens LT161 shows potential in producing antifungal metabolites against phytopathogens. Afr J Microbiol Res. 6:7572–7578.
- Haq I-u, Mirza B, Kondratyuk TP, Park EJ, Burns BE, Marler LE, Pezzuto JM. 2013. Preliminary evaluation for cancer chemopreventive and cytotoxic potential of naturally growing ethnobotanically selected plants of Pakistan. Pharm Biol. 51:316–328.
- Hijri M, Redecker D, Petetot JAM-C, Boigt K, Wostemeryer J, Sander IR. 2002. Identification and isolation of two ascomycete fungi from spores of the arbuscular mycorrhizal fungus Scutellospora castanea. Appl Environ Microbiol. 68:4567–4573.
- Hoshino J, Park E-J, Kondratyuk TP, Marler L, Pezzuto JM, van Breemen RB, Mo S, Li Y, Cushman M. 2010. Selective synthesis and biological evaluation of sulfate-conjugated resveratrol metabolites. J Med Chem. 53:5033–5043.
- Huang H, Wang F, Luo M, Chen Y, Song Y, Zhang W, Zhang S, Ju J. 2012. Halogenated anthraquinones from the marine-derived fungus Aspergillus sp. SCSIO F063. J Nat Prod. 75:1346–1352.
- Ivanova V, Kolarova M, Aleksieva K, Graefe U, Schlegel B. 2007. Diphenylether and macrotriolides occurring in a fungal isolate from the Antarctic lichen Neuropogon. Prep Biochem Biotechnol. 37:39–45.
- Jadoon M, Fatima N, Khan I, Mirza B, Haq UI, Qazi MA, Ahmed S. 2015. Biological evaluation and preliminary screening of endophytes of Taxus fuana for taxol production. Intl J Biosci. 7:38–46.
- Jeon YJ, Kim YK, Lee M, Park SM, Han SB, Kim HM. 2000. Radicicol suppresses expression of inducible nitric-oxide synthase by blocking p38 kinase and nuclear factor-kappaB/Rel in lipopolysaccharide-stimulated macrophages. J Pharmacol Exp Ther. 294:548–554.
- Jongen V, Thijssen J, Hollema H, Donker GH, Santema JG, Van der Zee AG, Heineman MJ. 2005. Is aromatase cytochrome P450 involved in the pathogenesis of endometrioid endometrial cancer? Int J Gynecol Cancer. 15:529–536.
- Joseph B, Priya RM. 2011. Bioactive compounds from endophytes and their potential in pharmaceutical effect: a review. Am J Biochem Mol Biol. 1:291–309.
- Khan AL, Hamayun M, Hussain J, Kang SM, Lee IJ. 2012. The newly isolated endophytic fungus Paraconiothyrium sp. LK1 produces ascotoxin. Molecules. 17:1103–1112.
- Kinghorn AD, Su B-N, Jang DS, Chang LC, Lee D, Gu JQ, Carcache-Blanco EJ, Pawlus AD, Lee SK, Park EJ, et al. 2004. Natural inhibitors of carcinogenesis. Planta Med. 70:691–705.
- Kondratyuk TP, Pezzuto JM. 2004. Natural product polyphenols of relevance to human health. Pharm Biol. 42:46–63.
- Krick A, Kehraus S, Gerhäuser C, Klimo K, Nieger M, Maier A, Fiebig HH, Atodiresei I, Raabe G, Fleischhauer J, et al. 2007. Potential cancer chemopreventive in vitro activities of monomeric xanthone derivatives from the marine algicolous fungus Monodictys putredinis. J Nat Prod. 70:353–360.
- Lee C, Houghton P. 2005. Cytotoxicity of plants from Malaysia and Thailand used traditionally to treat cancer. J Ethnopharmacol. 100:237–243.
- Lee D-S, Jeong G-S, Li B, Lee SU, OH H, Kim YC. 2011. Asperlin from the marine-derived fungus Aspergillus sp. SF-5044 exerts anti-inflammatory effects through heme oxygenase-1 expression in murine macrophages. J Pharmacol Sci. 116:283–295.
- Lee SK, Mbwambo Z, Chung H, Luyengi L, Gamez EJ, Mehta RG, Kinghorn AD, Pezzuto JM. 1998. Evaluation of the antioxidant potential of natural products. Comb Chem High Throughput Screen. 1:35–46.
- Li H-Y, Shen M, Zhou Z-P, Li T, Wei W-I, Lin L-B. 2012. Diversity and cold adaptation of endophytic fungi from five dominant plant species collected from the Baima Snow Mountain, Southwest China. Fungal Divers. 54:79–86.
- Liu K, Ding X, Deng B, Chen W. 2009. Isolation and characterization of endophytic Taxol-producing fungi from Taxus chinensis. J Ind Microbiol Biotechnol. 36:1171–1177.
- Lu Y, Chen C, Chen H, Zhang J, Chen W. 2012. Isolation and identification of endophytic fungi from Actinidia macrosperma and investigation of their bioactivities. Evid Based Complement Alternat Med. 2012: Article ID: 382742.
- Luqman S, Pezzuto JM. 2010. NFkappaB: a promising target for natural products in cancer chemoprevention. Phytother Res. 24:949–963.
- Maiti A, Cuendet M, Croy VL, Endringer DC, Pezzuto JM, Cushman M. 2007. Synthesis and biological evaluation of (±)-abyssinone II and its analogues as aromatase inhibitors for chemoprevention of breast cancer. J Med Chem. 50:2799–2806.
- Martini M, Musetti R, Grisan S, Polizzotto R, Borselli S, Pavan F, Osler R. 2009. DNA-dependent detection of the grapevine fungal endophytes Aureobasidium pullulans and Epicoccum nigrum. Plant Dis. 93:993–998.
- Mohamed IE, Gross H, Pontius A, Kehraus S, Krick A, Kelter G, Maier A, Fiebig HH, König GM. 2009. Epoxyphomalin A and B, prenylated polyketides with potent cytotoxicity from the marine-derived fungus Phoma sp. Org Lett. 11:5014–5017.
- Mohamed IE, Kehraus S, Krick A, König GM, Kelter G, Maier A, Fiebig HH, Kalesse M, Malek NP, Gross H. 2010. Mode of action of epoxyphomalins A and B and characterization of related metabolites from the marine-derived fungus Paraconiothyrium sp. J Nat Prod. 73:2053–2056.
- Momesso LS, Kawano CY, Ribeiro PH, Nomizo A, Goldman GH, Pupo MT. 2008. Chaetoglobosinas produzidas por Chaetomium globosum, fungo endofítico associado a Viguiera robusta Gardn.(Asteraceae). Quim Nova. 31:1680–1685.
- Nicoletti R, Ciavatta ML, Buommino E, Tufano MA. 2008. Antitumor extrolites produced by Penicillium species. Int J Biomed Pharm Sci. 2:1–23.
- Nitya K, Murthy K, Pushpalatha CJG, Joshi C. 2011. Antioxidant activity and phytochemical analysis of endophytic fungi isolated from Lobelia nicotianifolia. J Chem Pharm Res. 3:218–225.
- Nomelini RS, Ribeiro LCdA, Tavares-Murta BM, Adad SJ, Murta EFC. 2008. Production of nitric oxide and expression of inducible nitric oxide synthase in ovarian cystic tumors. Mediators Inflamm. 2008. Article ID: 186584, 7 pages.
- Park EJ, Pezzuto JM. 2002. Botanicals in cancer chemoprevention. Cancer Metastasis Rev. 21:231–255.
- Park JH, Choi GJ, Jang KS, Lim HK, Kim HT, Cho KY, Kim JC. 2005. Antifungal activity against plant pathogenic fungi of chaetoviridins isolated from Chaetomium globosum. FEMS Microbiol Lett. 252:309–313.
- Paul NC, Kim WK, Woo SK, Park MS, Yu SH. 2007. Fungal endophytes in roots of Aralia species and their antifungal activity. Plant Pathol J. 23:287–294.
- Petrini O. 1986. Taxonomy of endophytic fungi of aerial plant tissues. In: Fokkema NJ, vanden Heuvel J, editors. Microbiology of the phyllosphere. Cambridge: Cambridge University Press. p. 175–187.
- Pezzuto J, Kosmeder J II, Park E-J, Lee S, Cuendet M, Gills J, Bhat K, Grubjesic S, Park H-S, Mata-Greenwood E, et al. 2005. Characterization of natural product chemopreventive agents. In: Kelloff G, Hawk E, Sigman C, editors. Cancer chemoprevention. Humana Press. p. 3–37.
- Pimentel MR, Molina G, Dionísio AP, Marostica Junior MR, Pastore GM. 2010. The use of endophytes to obtain bioactive compounds and their application in biotransformation process. Biotechnol Res Int. 2011:1–11.
- Pontius A, Krick A, Mesry R, Kehraus S, Foegen SE, Müller M, Klimo K, Gerhäuser C, König GM. 2008. Monodictyochromes A and B, dimeric xanthone derivatives from the marine algicolous fungus Monodictys putredinis. J Nat Prod. 71:1793–1799.
- Pu X, Qu X, Chen F, Bao J, Zhang G, Luo Y. 2013. Camptothecin-producing endophytic fungus Trichoderma atroviride LY357: isolation, identification, and fermentation conditions optimization for camptothecin production. Appl Microbiol Biotechnol. 97:9365–9375.
- Quang DN, Harinantenaina L, Nishizawa T, Hashimoto T, Kochi C, Soma GI, Asakawa Y. 2006. Inhibition of nitric oxide production in RAW 264.7 cells by azaphilones from xylariaceous fungi. Biol Pharm Bull. 29:34–37.
- Ravindran C, Naveenan T, Varatharajan GR, Rajasabapathy R, Meena RM. 2012. Antioxidants in mangrove plants and endophytic fungal associations. Bot Mar. 55:269–279.
- Schulz B, Wanke U, Draeger S, Aust HJ. 1993. Endophytes from herbaceous plants and shrubs: effectiveness of surface sterilization methods. Mycol Res. 97:1447–1450.
- Schupp PJ, Kohlert-Schupp C, Whitefield S, Engemann A, Rohde S, Hemscheidt T, Pezzuto JM, Kondratyuk TP, Park EJ, Marler L, et al. 2009. Cancer chemopreventive and anticancer evaluation of extracts and fractions from marine macro-and microorganisms collected from Twilight Zone waters around Guam. Nat Prod Commun. 4:1717–1728.
- Shah A, Li D-Z, Moller M, Gao L-M, Hollingsworth ML, Gibby M. 2008. Delimitation of Taxus fuana Nan Li RR Mill (Taxaceae) based on morphological and molecular data. Taxon. 57:211–222.
- Soca-Chafre G, Rivera-Orduña FN, Hidalgo-Lara ME, Hernandez-Rodriguez C, Marsch R, Flores-Cotera LB. 2011. Molecular phylogeny and paclitaxel screening of fungal endophytes from Taxus globosa. Fungal Biol. 115:143–156.
- Song LL, Kosmeder JW, Lee SK, Gerhäuser C, Lantvit D, Moon RC, Moriarty RM, Pezzuto JM. 1999. Cancer chemopreventive activity mediated by4′-bromoflavone, a potent inducer of phase II detoxification enzymes. Cancer Res. 59:578–585.
- Stierle A, Strobel G, Stierle D. 1993. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science. 260:214–216.
- Strobel G, Daisy B. 2003. Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev. 67:491–502.
- Stuart RM, Romão AS, Pizzirani-Kleiner AA, Azevedo JL, Araújo WL. 2010. Culturable endophytic filamentous fungi from leaves of transgenic imidazolinone-tolerant sugarcane and its non-transgenic isolines. Arch Microbiol. 192:307–313.
- Sureram S, Wiyakrutta S, Ngamrojanavanich N, Mahidol C, Ruchirawat S, Kittakoop P. 2012. Depsidones, aromatase inhibitors and radical scavenging agents from the marine-derived fungus Aspergillus unguis CRI282-03. Planta Med. 78:582–588.
- Tayung K, Jha D. 2010. Antimicrobial endophytic fungal assemblages inhabiting bark of Taxus baccata L. of Indo-Burma mega biodiversity hotspot. Indian J Microbiol. 50:74–81.
- Tianpanich K, Prachya S, Wiyakrutta S, Mahidol C, Ruchirawat S, Kittakoop P. 2011. Radical scavenging and antioxidant activities of isocoumarins and a phthalide from the endophytic fungus Colletotrichum sp. J Nat Prod. 74:79–81.
- Wang J-M, Ding G-Z, Fang L, Dai JG, Yu SS, Wang YH, Chen XG, Ma SG, Qu J, Xu S, et al. 2010. Thiodiketopiperazines produced by the endophytic fungus Epicoccum nigrum. J Nat Prod. 73:1240–1249.
- Wang J, Zhao B, Yi Y, Zhang W, Wu X, Zhang L, Shen Y. 2012. Mycoepoxydiene, a fungal polyketide inhibits MCF-7 cells through simultaneously targeting p53 and NF-κB pathways. Biochem Pharmacol. 84:891–899.
- Wang Y, Tang KX. 2011. A new endophytic taxol- and baccatin III-producing fungus isolated from Taxus chinensis var. mairei. Afr J Biotechnol. 72:16379–16386.
- Wangun HVK, Dahse HM, Hertweck C. 2007. Epicoccamides B–D, glycosylated tetramic acid derivatives from an Epicoccum sp. associated with the tree fungus Pholiota squarrosa. J Nat Prod. 70:1800–1803.
- Wijeratne EK, Turbyville TJ, Zhang Z, Bigelow D, Pierson LS 3rd, VanEtten HD, Whitesell L, Canfield LM, Gunatilaka AA. 2003. Cytotoxic constituents of Aspergillus terreus from the rhizosphere of Opuntia versicolor of the Sonoran desert. J Nat Prod. 66:1567–1573.
- Wright AD, Osterhage C, König GM. 2003. Epicoccamide, a novel secondary metabolite from a jellyfish-derived culture of Epicoccum purpurascens. Org Biomol Chem. 1:507–510.
- Wu C-S, Lin Z-M, Wang L-N, Guo DX, Wang SQ, Liu YQ, Yuan HQ, Lou HX. 2011. Phenolic compounds with NF-κB inhibitory effects from the fungus Phellinus baumii. Bioorg Med Chem Lett. 21:3261–3267.
- Wu L-S, Hu C-L, Han T, Zheng CJ, Ma XQ, Rahman K, Qin LP. 2013. Cytotoxic metabolites from Perenniporia tephropora, an endophytic fungus from Taxus chinensis var. mairei. Appl Microbiol Biotechnol. 97:305–315.
- Xu F, Tao W, Cheng L, Guo L. 2006. Strain improvement and optimization of the media of Taxol-producing fungus Fusarium maire. Biochem Eng J. 31:67–73.
- Yang S-S, Wang G-J, Cheng K-F, Chen CH, Ju YM, Tsau YJ, Lee TH. 2010. Bioactive γ-lactones from the fermented broth of Neosartorya sp. Planta Med. 76:1701–1705.
- Yao Y, Hausding M, Erkel G, Anke T, Förstermann U, Kleinert H. 2003. Sporogen, S14-95, and S-curvularin, three inhibitors of human inducible nitric oxide synthase expression isolated from fungi. Mol Pharmacol. 63:383–391.
- Zhang W, Ramamoorthy Y, Kilicarslan T, Nolte H, Tyndale RF, Sellers EM. 2002. Inhibition of cytochromes P450 by antifungal imidazole derivatives. Drug Metab Dispos. 30:314–318.
- Zhao J, Shan T, Mou Y, Zhou L. 2011. Plant-derived bioactive compounds produced by endophytic fungi. Mini Rev Med Chem. 11:159–168.
- Zhao J, Zhou L, Wang J, Shan T, Zhong L, Liu X, Gao X. 2010. Endophytic fungi for producing bioactive compounds originally from their host plants. Curr Res Technol Educ Trop Appl Microbiol Microbial Biotechnol. 1:567–576.
- Zhou X, Zheng W, Zhu H. 2009. Identification of a taxol-producing endophytic fungus EFY-36. Afr J Biotechnol. 8:2623–2625.
