Abstract
Context: Hertia cheirifolia L. (Asteraceae) is traditionally used in Northern Africa to treat various inflammatory infections. However, few studies on this plant have been reported.
Objective: The anti-inflammatory activity of methanol extract of H. cheirifolia leaves was investigated using different experimental models.
Materials and methods: Phytochemical analysis was performed to determine phenolic compounds. Acute toxicity of the extract (2000 mg/kg) was examined in Swiss albino mice for 14 days, before croton oil-induced ear oedema in mice, carrageenan-induced paw oedema in Swiss albino rats, cotton pellet-induced granuloma in rats and carrageenan-induced air pouch in mice were conducted. The IL-1β and TNF-α release from concanavalin A-stimulated monocytes was measured by ELISA.
Results: Methanol extract of H. cheirifolia is rich in polyphenols and flavonoids. Cinnamic acid and rutin represent the major constituents. Methanol extract up to 2000 mg/kg did not produce any toxic effects. Topical application of 2 mg/ear of the extract produced 78.7% of inhibition on ear swilling. Oral pre-treatment of rats with 200 and 400 mg/kg of the extract inhibited paw oedema by 70% and 89%, respectively. At 200 mg/kg, granuloma dry and wet weights were reduced by 41.85% and 61.72%, respectively. Moreover, the treatment with methanol extract at 1 mg/kg exerted 62.7% of inhibition on leucocytes migrated into the ear pouch. TNF-α and IL-1β release was reduced by 69% and 78%, respectively, with 1 μg/mL of the extract.
Conclusion: Methanol extract of H. cheirifolia possesses a strong anti-inflammatory activity and may be considered an interesting source of effective anti-inflammatory compounds.
Introduction
Inflammation is a mechanism of great benefit for maintaining homeostasis in the body. The inflammatory response may be appropriate, physiologic and necessary in the presence of an infection and cellular damage or stress. Conversely, it may be inappropriate, altered homeostasis, pathologic and damaging when it is reacting out of proportion causing undesired effects (Miller et al. Citation2009; Abdelmagid et al. Citation2015) and contribute to diseases. In fact, inflammation is implicated in osteoarthritis, heart disease, Alzheimer’s, age-related macular degeneration, chronic obstructive pulmonary disease, multiple sclerosis, stroke and cancer (McCarty Citation1999; Azeem et al. Citation2010; Medzhitov Citation2010; Kumar et al. Citation2011). Inflammation is frequently associated with the increase in vascular permeability and mediator release (Vane & Botting Citation1998), increase of protein denaturation and membrane alteration (Umapathy et al. Citation2010). Further, leucocyte infiltration, oedema and granuloma formation represent typical features of inflammation (Gorzalczany et al. Citation2011). Moreover, the host response has been considered to be mediated mainly by B and T lymphocytes, neutrophils and monocytes/macrophages. These cells are triggered to produce inflammatory mediators, including cytokines, chemokines, arachidonic acid metabolites and proteolytic enzymes, which collectively contribute to tissue degradation by activation of several distinct host degradative pathways (Birkedal-Hansen Citation1993; Hernandez et al. Citation2011).
Steroidal and non-steroidal anti-inflammatory drugs are known to treat inflammation and pain. However, their prolonged use often leads to serious side-effects such as gastrointestinal tract dyspepsia, peptic ulceration, haemorrhage and perforation leading to death in some patients (Griffin Citation1998). Many medicines of plant origin have been used since long time without any adverse effects, and new medicinal plants are introduced to develop analgesic and anti-inflammatory drugs.
Hertia cheirifolia L. (Asteraceae) is widely distributed in the North of Africa (Pottier-Alapetite Citation1981; Beniston & Beniston Citation1984). It is originally from Algeria and Tunisia (Le Houérou Citation1995) and used by traditional healers for the treatment of spasm, inflammation, diarrhea and haemorrhoid (Iserin Citation2001). Phytochemical analysis showed the presence of sesquiterpenoids and steroids (Aclinou et al. Citation1991; Ammar et al. Citation2009). However, few studies on biological activities of H. cheirifolia have been reported. Therefore, the present study was designed to investigate the anti-inflammatory properties of the methanol extract of H. cheirifolia leaves by using different experimental models.
Materials and methods
Plant material
Hertia cheirifolia was collected in June 2010 from Sétif area in Algeria. The plant was identified and authenticated taxonomically by Dr. N. Boulaacheb, University of Sétif 1, Algeria. A voucher specimen (No. H.C. 2010-1) was preserved for future reference at the local Herbarium of Botany, Department of Botany, University of Sétif 1. Leaves were air-dried at room temperature and then reduced to powder.
Animals
Swiss Albino rats weighing 150–180 g and Swiss Albino mice weighing 20–25 g of either sex were obtained from Pasteur Institute of Algiers, Algeria. All animals were kept to acclimatize under the laboratory conditions for 1 week and were provided with standard rodent diet and water ad libitum. Animals were randomly selected for different experimental groups (6 animals/group) and fasted overnight prior to the experiments. All procedures were performed in accordance with European Union Guidelines for Animals Experimentation (2007/526/EC).
Preparation of H. cheirifolia leaf extract
Methanol extract of H. cheirifolia leaves was prepared by maceration of 100 g of powdered plant material with 80% methanol at room temperature for 48 h with frequent agitation. After filtration, the filtrate was concentrated under reduced pressure at 40 °C. The residue was lyophilized using a lyophilizator (PHYWE chrisa) to give a brown powder (yield: 19.3%).
Determination of total polyphenol content
The amount of total phenolics in extracts was determined according to a modified method of Li et al. (Citation2007). Methanol extract of H. cheirifolia (2 mg/mL) was mixed with 200 μL Folin-Ciocalteu reagent and 1200 μL of distilled water. Four minutes later, 600 μL of 7.5% Na2CO3 was added. The mixture was shaken for 2 h at room temperature and the absorbance was measured at 765 nm. All tests were performed in triplicate. Gallic acid was used as a standard. The concentration of total phenolic compounds in methanol extract was determined as mg of gallic acid equivalent per 1 g of extract (mg GAE/g extract).
Determination of total flavonoid content
Total flavonoid content in the extract was determined according to Bahorun et al. (Citation1996). Briefly, 1 mL of 2% AlCl3 in ethanol was added to 1 mL of methanol extract (2 mg/mL). After 10 min of incubation at room temperature, the absorbance was measured at 430 nm. Total flavonoid content was expressed as mg of quercetin equivalent per 1 g of extract (mg QE/g extract).
HPLC-TOF/MS analysis
HPLC-TOF/MS analysis of H. cheirifolia was carried out as described elsewhere (Abay et al. Citation2015). This HPLC method was developed and validated to analyze phenolic acids and flavonoids in the plant extracts. Agilent Technology of 1260 Infinity HPLC System was coupled with 6210 Time of Flight (TOF) LC/MS detector and ZORBAX SB-C18 (4.6 mm ×100 mm, 3.5 μm) column. Mobile phases A and B were ultra-pure water with 0.1% formic acid and acetonitrile, respectively. Flow rate was 0.6 mL/min and column temperature was 35 °C. Injection volume was 10 μL. The solvent program was as follows: 0–1 min 10% B; 1–20 min 50% B; 20–23 min 80% B; 23–25 min 10% B; 25–30 min 10% B. Ionization mode of HPLC-TOF/MS instrument was negative and operated with a nitrogen gas temperature of 325 °C, nitrogen gas flow of 10.0 L/min, nebulizer of 40 psi, capillary voltage of 4000 V and finally, fragmentor voltage of 175 V. For sample analysis, dried crude extracts (200 ppm) were dissolved in methanol at room temperature. Samples were filtered passing through a PTFE (0.45 μm) filter by an injector to remove particulates.
Acute toxicity
The acute oral toxicity was evaluated following the Organization of Economic Co-operation and Development guideline (OECD Citation2001) for chemical testing. Briefly, mice were divided into two groups of five of either sex. The treated group was orally given the methanol extract in a single dose of 2000 mg/kg body weight, while the control group received only water vehicle. Animals were monitored for apparent signs of toxicity for 14 days.
Croton oil-induced ear oedema in mice
To evaluate the effect of H. cheirifolia methanol extract on acute inflammation, croton oil-induced ear oedema was performed according to Manga et al. (Citation2004). Cutaneous inflammation was induced in the inner surface of the right ear of mice (6 mice/group) by application of 15 μL of acetone containing 80 μg of croton oil as irritant. Treated animals received topically 2 mg/ear of methanol extract of H. cheirifolia leaves or 0.5 mg/ear of indomethacin, used as a reference drug. The thickness of ears was measured before and 6 h after induction of inflammation using a dial calliper. The oedema was expressed as an increase in the ear thickness due to croton oil application.
Carrageenan-induced paw oedema in rats
Paw oedema was induced by injecting 0.1 mL of 1% λ-carrageenan into the subplantar region of the right hind paw of rats (Winter et al. Citation1962). One hour before carrageenan injection, rats received orally 200 and 400 mg/kg of H. cheirifolia methanol extract or 100 mg/kg aspirin (suspended in CMC 1%). Rats of control group were injected with 0.1 mL λ-carrageenan and received orally only the vehicle before the injection. The oedema was assessed by measuring the injected paw initially (V0) and 1, 2, 3, 4, 5, and 6 h after carrageenan injection (Vt), using a plethysmometer (UGO Basile, Varese, Italy). Inflammation was calculated as the increase in volume of the paw after treatment subtracted of the basal volume. Results were expressed as a percentage of inhibition of oedema, calculated according to the following equation:
Cotton pellet-induced granuloma in rats
The effect of H. cheirifolia methanol extract on cotton pellet-induced granuloma in rats was conducted as described by Ismail et al. (Citation1997) in three groups of six animals each. Granulomatous lesions were induced by surgically inserting 15 mg of sterile cotton pellet subcutaneously in both axilla regions of each rat, following a single incision which was thereafter closed by interrupted sutures. After implantation of cotton pellets, the plant extract (200 mg/kg) was orally administered once daily throughout the experimental period of 7 days. Dexamethasone (2.5 mg/kg) was also given daily to standard group, while the control group received only the same volume of distilled water. On the eighth day after implantation, the cotton pellets were dissected out under ether anaesthesia, cleaned of extraneous tissues, weighed and dried at 50 °C to a constant weight. The mean weight of the cotton pellets of the control group as well as of the test groups was calculated. The increase in dry weight of the pellets was taken as the measure of the granuloma formation.
Air pouch induced in mice
The air pouches were raised on the dorsum by subcutaneous injection of 3 mL of sterile air, as previously described (Colville-Nash & Lawrence Citation2003). After 3 days, the pouches were re-inflated with 1.5 mL of sterile air. Mice were injected with 0.1 mL of methanol extract (1 mg/pouch), indomethacin (0.15 mg/pouch) or sterile saline solution (control) into the pouch 1 h prior to the injection of 0.1 mL λ-carrageenan (1%). Four hours after the treatment, the mice were sacrificed by cervical dislocation. The pouches were flushed by 0.1 mL of sterile saline solution, opened and then the exudates were collected. Aliquots were diluted 1:2 with Trypan blue (0.01 w/v% in PBS), and the polymorphonuclear leukocytes were counted in a standard hemocytometer chamber.
Peripheral blood mononuclear cells’ isolation
Peripheral blood mononuclear cells (PBMCs) were isolated according to Amro et al. (Citation2013). Briefly, venous blood was collected from healthy volunteers into heparinized vacutainer tubes. The heparinized blood was diluted 1:1 with RPMI-1640 and layered on a Ficol-Hypaque gradient (Lymphoprep, Accurate Chemical Corp., Westbury, NY). The Ficol-Hypaque density gradient was centrifuged at 1250 rpm for 15 min at room temperature, and the buffy coat containing PBMCs was collected and washed twice in RPMI-1640.
TNF-α and IL-1β determination by enzyme-linked immunosorbant assay (ELISA)
To elucidate a possible mechanism for the anti-inflammatory action, the effect of H. cheirifolia methanol extract on the production of TNF-α and IL-1β by PBMCs was examined. Cells (2 × 105 cells/mL) were seeded in a 24-well plate and activated for 30 min with 5 μg/mL mitogen concanavalin A (Con A), after the treatment with different concentrations of H. cheirifolia methanol extract (1, 10, 50 and 100 μg/mL). Cultures were incubated in a humidified atmosphere of 37 °C and 5% CO2 overnight. After centrifugation, supernatants were then harvested for analysis by ELISA assay according to the manufacturer’s protocol (eBioscience, San Diego, CA) to determine TNF-α and IL-1β concentrations. Untreated cells with the extract were used as a control. All incubation steps were performed at room temperature. The optical density at 450 nm, corrected by the reference wavelength 570 nm, was measured with microplate reader (Biotech). All cytokine assays were calibrated against the World Health Organization international standards by the kit manufacturer. The lower limit of detection for the assay of TNF-α is 4 pg/mL.
Statistical analysis
Results are expressed as mean ± SEM. Differences between the control and the treatments in these experiments were tested for significance using one-way ANOVA followed by Tukey’s multiple comparison tests. The differences were considered statistically significant at p < 0.05.
Results
Determination of total poly phenol and flavonoid contents
The phytochemical analysis showed that methanol extract from H. cheirifolia is rich in polyphenols. The polyphenol content was 89.4 ± 6.7 mg GAE/g of extract, while the amount of flavonoids was 4.0 ± 1.3 mg QE/g of extract.
HPLC-TOF/MS analysis
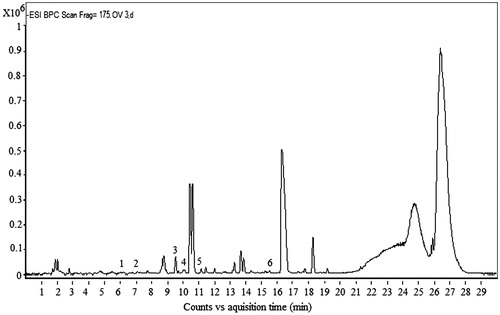
Results of HPLC-TOF/MS analysis revealed the presence of phenolic acids and flavonoids in H. cheirifolia methanol leaf extract (). The conditions used led to a good separation of the peaks which could be identified in the chromatogram (). The leaves of H. cheirifolia contain the highest amount of cinnamic acid (RT = 15.68), ferulic acid (RT = 10.74) and p-coumaric acid (RT = 9.99). Among the tested flavonoids, rutin was detected as a major compound in the methanol extract of H. cheirifolia leaves.
Table 1. Compounds determined by HPLC-TOF/MS in H. cheirifolia methanol extract.
Acute toxicity of H. cheirifolia methanol extract
Results showed that methanol extract from H. cheirifolia leaves is safe and has no toxicity effects. Indeed, this extract did not produce any mortality and behavioural change even at 2000 mg/kg body weight during 2 weeks of observation.
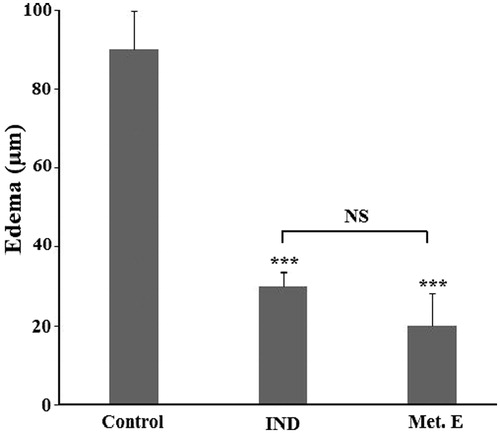
Effect of H. cheirifolia methanol extract on croton oil-induced ear oedema
The topical application of 2 mg/ear of H. cheirifolia methanol extract inhibited significantly (p < 0.001) the development of ear oedema by 78.7%. This inhibition is close to that produced by indomethacin, used as a standard anti-inflammatory agent ().
Figure 2. Effect of H. cheirifolia methanol extract on croton oil-induced ear oedema. Mice were treated with 2 mg/ear of methanol extract (Met. E) or 0.5 mg/ear of indomethacin (IND). Control group received sterile saline solution only. Oedema is expressed as mean thickness of ears before and 6 h after croton oil application. Values are expressed as means ± SEM (n = 6). ***p < 0.001; NS: not significant versus control.
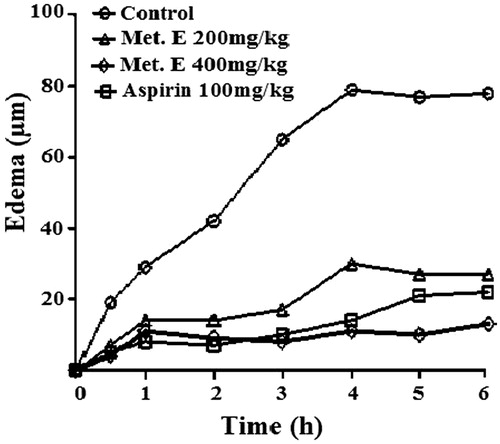
Effect H. cheirifolia methanol extract on carrageenan-induced paw oedema
Sub-plantar injection of 0.1 mL of λ-carrageenan induced a progressive swelling of the rat paw, reaching a maximal volume in the control group at 4 h. The oral treatment of rats by 200 and 400 mg/kg of H. cheirifolia methanol leaf extract inhibited the oedema formation during all measurement time. The inhibition of the oedema reached the maximum values 70% and 89%, respectively, as compared to the non-treated group. Similar result was observed with aspirin, used as a standard non-steroidal anti-inflammatory drug ().
Figure 3. Time course of carrageenan-induced rat paw oedema. The oedema was induced by sub-plantar injection of 0.1 mL of carrageenan 1% in rat pre-treated orally with 200 and 400 mg/kg of methanol extract (Met. E), 100 mg/kg body weight of aspirin or vehicle (control). Each value represents the percentage increase in volume of the injected paw at different times after injection of carrageenan compared with the control group set to 100%. Values are means ± SEM of six animals for each group.
Effect of H. cheirifolia methanol extract on cotton pellet granuloma
Methanol extract of H. cheirifolia exerted a significant (p < 0.001) protection on granuloma formation by reducing the weight of the cotton pellet. Indeed, at 200 mg/kg the granuloma tissue dry weight was reduced by 41.85%, while granuloma tissue wet weight was reduced by 61.72%. This inhibition was better than that obtained with dexamethasone, used as a standard anti-inflammatory agent ().
Table 2. Effect of H. cheirifolia methanol extract on granuloma tissue formation.
Effect of H. cheirifolia methanol extract on air pouch
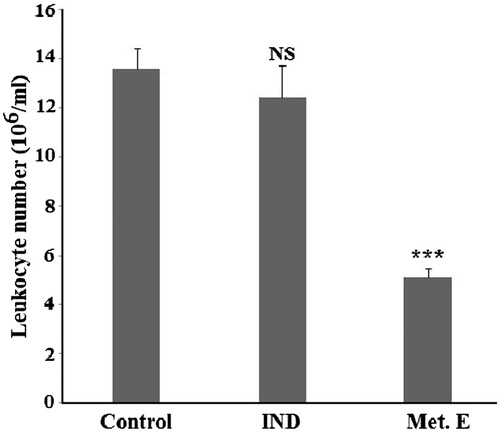
In air pouch model, the mice of the control group developed after 4 h an inflammation with infiltration of 13.6 ± 0.7 × 106 cells/mL into air pouch exudate. Treatment with 1 mg/pouch of methanol extract of H. cheirifolia induced a significant (p < 0.001) reduction in the number of infiltrating leukocytes (5.07 ± 0.42 × 106 cells/mL of exudates), compared to the control group. This value corresponds to an inhibition of 62.72%, which is better than that obtained with 0.15 mg/pouch of indomethacin ().
Figure 4. Effect of H. cheirifolia methanol extract on leukocytes infiltrated into air pouch exudate. The pouch inflammation was induced by 0.1 mL of carrageenan (1%). One hour after the induction of inflammation, mice were treated by 1 mg/pouch of the extract or 0.15 mg/pouch of indomethacin. The comparison was made with respect to the control group (without treatment). Values are means ± SEM (n = 6). ***p <0.001; NS: not significant versus control.
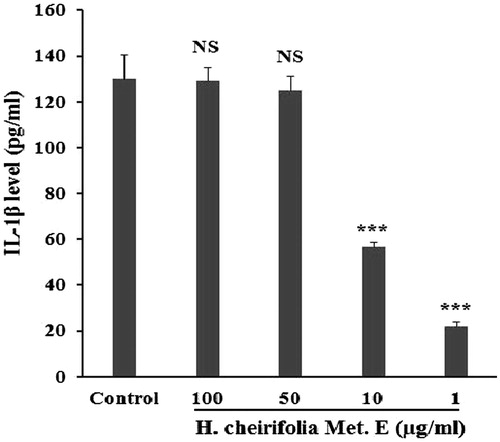
Effect of H. cheirifolia methanol extract on TNF-α and IL-1β production
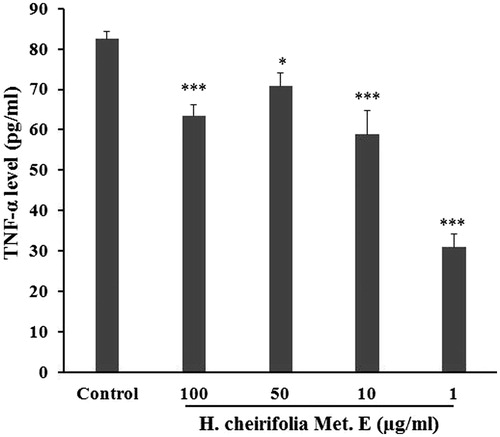
Concanavalin A stimulated PBMCs (control) showed a significant increase in TNF-α release (82.46 pg/mL) and IL-1β (130 pg/mL) compared to non-stimulated cells. The release of TNF-α and IL-1β from Con A-stimulated PBMCs was significantly reduced with all tested concentrations of H. cheirifolia methanol extract ( and ). The maximum reduction of TNF-α and IL-1β levels was obtained with 1 μg/mL of the extract. The non-stimulated PBMCs pre-treated with different concentrations of the extract showed no response when tested for TNF-α and IL-1β levels.
Figure 5. Effect of H. cheirifolia methanol extract (Met. E) on TNF-α produced by Con A stimulated PBMCs. Cells (2 × 105 cells/mL) were seeded in a 24-well plate and activated for 30 min with 5 μg/mL Con A after the treatment with different concentrations of H. cheirifolia methanol extract (100, 50, 10 and 1 μg/mL). Cultures were incubated in a humidified atmosphere at 37 °C and 5% CO2 overnight. After centrifugation, supernatants were then harvested for analysis by an ELISA assay. Untreated cells with the extract were used as the control. Data present the mean concentration of triplicates ± SEM. ***p < 0.001, *p < 0.05; NS: not significant versus control.
Discussion
Medicinal plants are a source of wide variety of bioactive compounds and have been used as a crude material or as pure compounds for treating various disease conditions (Dar et al. Citation2012). In this context, the anti-inflammatory properties of H. cheirifolia were studied in the pathophysiological events induced by various agents.
According to our findings, H. cheirifolia can be considered to be safe and non-toxic, as the oral administration of the methanol extract of this plant did not produce any mortality and abnormal behaviour in tested animals.
Oedema, leukocyte infiltration and granuloma formation represent typical feature of inflammation (Gorzalczany et al. Citation2011). Moreover, the inhibition of major cytokines like TNF-α and IL-1β could be the best solution for preventing inflammation related diseases.
The overall results showed that H. cheirifolia methanol extract exerted a significant anti-inflammatory activity in different models of inflammation. In croton oil-induced ear oedema in mice, croton oil contains 12-O-tetracanoilphorbol-13-acetate (TPA) and other phorbol esters as main irritant agents. The TPA is able to activate protein kinase C, which activates other enzymatic cascades in turn such as cyclooxygenase 2 and inducible nitric oxide synthase (Aquila et al. Citation2009). This cascade of events stimulates vascular permeability, vasodilation, polymorphonuclear leukocytes migration, histamine and serotonin release and moderate synthesis of inflammatory eicosanoids by cyclooxygenase and 5-lipoxygenase enzymes (Wang et al. Citation2001; Murakawa et al. Citation2006). The effect of methanol extract on croton oil-induced ear oedema is probably attributed to lipophilic methanol-soluble substances that are able to penetrate through the skin barrier (Okoli et al. Citation2007). Likely, candidates for this anti-inflammatory are flavonoids and polyphenols (Zhong et al. Citation2012). Indeed, phytochemical analysis of methanol extract showed that H. cheirifolia contains phenolic compounds and rutin, as unique and major flavonoid constituent. Selloum et al. (Citation2003) revealed that rutin possesses anti-inflammatory properties by inhibiting paw oedema, neutrophil chemotaxis and degranulation.
Carrageenan-induced paw oedema experiment model was conducted in order to determine the anti-inflammation activity of H. cheirifolia methanol extract in acute-phase of inflammation. In this model, methanol extract elicited a significant reduction of oedema formation at all assessment times. The subcutaneous injection of carrageenan into the rat paw produces plasma exudation associated with a neutrophil extravasation and increase arachidonic acid product release (Paschapur et al. Citation2009). Therefore, methonolic extract of H. cheirifolia may act by inhibiting the release these inflammatory mediators. In fact, Romier-Crouzet et al. (Citation2009) reported the inhibition of inflammatory mediators by polyphenolic plant extracts.
The cotton pellet-induced granuloma is an established and widely used method for studying the efficacy of drugs against the proliferative phase of inflammation in which fluid extravasation, leukocyte migration (Radhika et al. Citation2005), tissue degeneration and fibrosis occur and granuloma develops during the period of several days (Sokeng et al. Citation2013). In this model, the methanol extract of H. cheirifolia inhibited the development of granulomatous tissue as compared to control group proving its activity in the proliferative phase of inflammation.
In order to assess the efficacy of H. cheirifolia methanol extract against proliferative phase of inflammation, carrageenan-induced air-pouch was carried out. In this acute inflammatory model, the proliferation of macrophages, neutrophils, and fibroplasts are the sources of inflammation (Li et al. Citation2008). The present study revealed that methanol extract of H. cheirifolia inhibited neutrophil recruitment into the cavity of the air pouch. This result indicates that the studied extract may alter the action of endogenous factors that are involved in the migration of neutrophils into inflated site. It has been shown that IL-1 and TNF-α stimulates neutrophil migration and fibroblast activation (Henry & Garner Citation2003; Kondo & Ishida Citation2010). Therefore, the measurement of IL-1 and TNF-α level may be useful in monitoring of disease progression and inflammation (Smith et al. Citation2004). This study revealed that the release of TNF-α from Con A-stimulated PBMCs was reduced significantly with all studied concentrations of the plant extract. The IL-1β was also inhibited by 10 and 1 μg/mL. This effect could be due to the presence of the bioactive metabolites in the extract. Indeed, Murase et al. (Citation1999) and Calixto et al. (Citation2003) reported that plant constituents inhibited the arachidonic acid pathway, nitric oxide, ICAM-1, VCAM-1 and E-selectin in the vascular endothelial cells and suggested that this inhibition is due to the inhibition of IL-1B, TNF-α and NF-κB. Plant extract has the potential to act as an effective inflammation inhibitor (Rupasinghe et al. Citation2015). Chlorogenic acid and rutin were polyphenols found in the highest concentrations in plant extract and have been implicated in inhibiting inflammatory transcription factors (Palikova et al. Citation2009; Zdarilova et al. Citation2010). In addition, Hwang et al. (Citation2014) demonstrated that chlorogenic acid and ferulic acid, two constituents present in the studied extract, attenuated pro-inflammatory cytokines including IL-1 and TNF-α in a dose-dependent manner. Therefore, the anti-inflammatory activity of H. cheirifolia methanol extract might be due to the presence of bioactive constituents such as chlorogenic acid, 4-hydroxy benzoic acid, ferulic acid, p-coumaric acid, cinnamic acid or rutin determined by HPLC-TOF/MS in this study.
Conclusions
Methanol extract of H. cheirifolia possesses clear anti-inflammatory effects against both exudative and cellular phases of inflammation. Further investigations are required to understand the mechanisms of action underlying the effects of the extract and their active compounds.
Funding information
Financial support for this work was provided by grants from Algerian Ministry of high education.
Disclosure statement
The authors declare no conflicts of interest.
References
- Abay G, Altun M, Koldaş S, Tüfekçi AR, Demirtas I. 2015. Determination of antiproliferative activities of volatile contents and HPLC profiles of Dicranum scoparium (Dicranaceae, Bryophyta). Comb Chem High Throughput Screen. 18:453–563.
- Abdelmagid SM, Barbe MF, Safadi FF. 2015. Role of inflammation in the aging bones. Life Sci. 123:25–34.
- Aclinou P, Benkouider A, Massiot G, Le Men-Olivier L. 1991. Eremophilenolides from Hertia cheirifolia. Phytochemistry. 30:2083–2084.
- Ammar S, Edziri H, Mahjoub MA, Chatter R, Bouraoui A, Mighri Z. 2009. Spasmolytic and anti-inflammatory effects of constituents from Hertia cheirifolia. Phytomedicine. 16:1156–1161.
- Amro BI, Haddadin RN, Tawaha K, Mohammad M, Mashallah S, M Assaf A. 2013. In vitro antimicrobial and anti-inflammatory activity of Jordanian plant extracts: a potential target therapy for Acne vulgaris. Afr J Pharm Pharmacol. 7:2087–2099.
- Aquila S, Giner RM, Recio MC, Spegazzini ED, Ríos JL. 2009. Anti-inflammatory activity of flavonoids from Cayaponia tayuya roots. J Ethnopharmacol. 121:333–337.
- Azeem AK, Dilip C, Prasanth SS, Shahima VJH, Sajeev K, Naseera C. 2010. Anti-inflammatory activity of the glandular extracts of Thunnus alalunga. Asia Pac J Med. 3:4–412.
- Bahorun T, Gressier B, Trotin F, Brunet C, Dine T, Luyckx M, Vasseur J, Cazin M, Cazin JC, Pinkas M. 1996. Oxygen species scavenging activity of phenolic extracts from hawthorn fresh plant organs and pharmaceutical preparations. Arzneimittelforschung. 46:1086–1089.
- Beniston NT, Beniston WS. (1984). Fleurs d’Algerie. Alger: ENL Publisher and Distributor.
- Birkedal-Hansen H. 1993. Role of cytokines and inflammatory mediators in tissue destruction. J Periodont Res. 28:500–510.
- Calixto JB, Otuki MF, Santos AR, et al. 2003. Anti-inflammatory compounds of plant origin. Part I action on arachidonic acid pathway, nitric oxide and nuclear factor κB (NF-κB). Planta Med. 69:973–983.
- Colville-Nash P, Lawrence T. 2003. Air-pouch models of inflammation and modifications for the study of granuloma-mediated cartilage degradation. Methods Mol Biol. 225:181–189.
- Dar SA, Yousuf AR, Ganai FA, Sharma P, Kumar N, Singh R. 2012. Bioassay guided isolation and identification of anti-inflammatory and anti-microbial compounds from Urtica dioica L. (Urticaceae) leaves. Afr J Biotechnol. 11:12910–12920.
- Gorzalczany S, Lopez P, Acevedo C, Ferraro G. 2011. Anti-inflammatory effect of Lithrea molleoides extracts and isolated active compounds. J Ethnopharmacol. 133:994–998.
- Griffin MR. 1998. Epidemiology of nonsteroidal anti-inflammatory drug-associated gastrointestinal injury. Am J Med. 104:23–29.
- Henry G, Garner WL. 2003. Inflammatory mediators in wound healing. Surg Clin North Am. 83:483–487.
- Hernandez M, Dutzan N, García-Sesnich J, Abusleme L, Dezerega A, Silva N, González FE, Vernal R, Sorsa T, Gamonal J. 2011. Host-pathogen interactions in progressive chronic periodontitis. J Dent Res. 90:1164–1170.
- Hwang H, Kim YW, Park Y, Hwang SJ, Kim YW, Park Y, Lee HJ, Kim KW. 2014. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide stimulated RAW 264.7 cells. Inflamm Res. 63:81–90.
- Iserin P. 2001. Encyclopédie des plantes médicinales. Paris: Larousse-Bordas.
- Ismail TS, Gopalakrishnan S, Begum VH, Elango V. 1997. Anti-inflammatory activity of Salacia oblonga Wall. and Azima tetracantha Lam. J Ethnopharmacol. 56:145–152.
- Kondo T, Ishida Y. 2010. Molecular pathology of wound healing. Forensic Sci Int. 203:93–98.
- Kumar V, Bhat ZA, Kumar D, Bohra P, Sheela S. 2011. In-vitro antiinflammatory activity of leaf extracts of Basella alba linn. Var. alba. Int J Drug Dev Res. 3:124–127.
- Le-Houérou HN. 1995. Bioclimatologie et biogéographie des steppes arides du Nord de l'Afrique: Diversité biologique, développement durable et désertisation. Montpellier: CIHEAM-IAM Publisher and Distributor.
- Li HB, Cheng KW, Wong CC, Fan KW, Chen F, Jiang Y. 2007. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 102:771–776.
- Li H, Lu X, Zhang S, Lu M, Liu H. 2008. Anti-inflammatory activity of polysaccharide from Pholiota nameko. Biochem Mosc. 73:669–675.
- Manga HM, Brkic D, Marie DEP, Quetin-Leclercq J. 2004. In vivo anti-inflammatory activity of Alchornea cordifolia (Schumach. & Thonn.) Mull. Arg. (Euphorbiaceae). J Ethnopharmacol. 92:209–214.
- McCarty MF. 1999. Interleukin-6 as a central mediator of cardiovascular risk associated with chronic inflammation, smoking, diabetes, and visceral obesity: down-regulation with essential fatty acids, ethanol and pentoxifylline. Med Hypotheses. 52:465–477.
- Medzhitov R. 2010. Inflammation 2010: new adventures of an old flame. Cell. 140:771–776.
- Miller AH, Maletic V, Raison CL. 2009. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 65:732–741.
- Murakawa M, Yamaoka K, Tanaka Y, Fukuda Y. 2006. Involvement of tumor necrosis factor (TNF)-alpha in phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced skin edema in mice. Biochem Pharmacol. 71:1331–1336.
- Murase T, Kume N, Hase T, Shibuya Y, Nishizawa Y, Tokimitsu I, Kita T. 1999. Gallates inhibit cytokine-induced nuclear translocation of NF-κB and expression of leucocytes adhesion molecules in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 19:1412–1420.
- OECD: Organisation for Economic Co-operation and Development. 2001. Guideline for testing of chemicals, 420, Acute oral toxicity-fixed dose method. Paris.
- Okoli CO, Akah PA, Nwafor SV, Anisiobi AI, Ibegbunam IN, Erojikwe O. 2007. Anti-inflammatory activity of hexane leaf extract of Aspilia africana C.D. Adams. J Ethnopharmacol. 109:219–225.
- Palikova I, Valentova K, Oborna I, Ulrichova J. 2009. Protectivity of blue honeysuckle extract against oxidative human endothelial cells and rat hepatocyte damage. J Agric Food Chem. 57:6584–6589.
- Paschapur MS, Patil MB, Kumar R, Patil SR. 2009. Evaluation of anti-inflammatory activity of ethanolic extract of Borassus flabellifer L. male flowers (inflorescences) in experimental animals. J Med Plants Res. 3:49–54.
- Pottier-Alapetite G. 1981. Flore de la Tunisie: angiospermes, dicotylédones. Tunisie: Tunis IORT Publisher and Distributor.
- Radhika P, Rao PR, Archana J, Rao NK. 2005. Anti-inflammatory activity of a new sphingosine derivative and cembrenoid diterpene (Lobohedleolide) isolated from marine soft corals of Sinularia crassa TIXIER-DURIVAULT and Lobophytum species of the Andaman and Nicobar Islands. Biol Pharm Bull. 28:1311–1313.
- Romier-Crouzet B, Van De Walle J, During A, Joly A, Rousseau C, Henry O, Larondelle Y, Schneider YJ. 2009. Inhibition of inflammatory mediators by polyphenolic plant extracts in human intestinal Caco-2 cells. Food Chem Toxicol. 47:1221–1230.
- Rupasinghe VHP, Boehm MMA, Sekhon-Loodu S, Parmar I, Bors B, Jamieson AR. 2015. Anti-inflammatory activity of Haskap cultivars is polyphenols-dependent. Biomolecules. 5:1079–1098.
- Selloum L, Bouriche H, Tigrine C, Boudoukha C. 2003. Anti-inflammatory effect of rutin on rat paw oedema, and on neutrophils chemotaxis and degranulation. Exp Toxicol Pathol. 54:313–318.
- Smith AR, Shenvi SV, Widlansky M, Suh JH, Hagen TM. 2004. Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr Med Chem. 11:1135–1146.
- Sokeng SD, Koube J, Dongmo F, Snnhaffouo S, Barnabé Lucien NYN, Germain ST. 2013. Acute and chronic anti-inflammatory effects of the aqueous extract of Acacia nilotica (L.) Del. (Fabaceae) pods. Acad J Med Plants. 1:1–5.
- Umapathy E, Ndebia EJ, Meeme A, Adam B, Menziwa P, Nkeh-Chungag NB, Iputo JE. 2010. An experimental evaluation of Albuca setosa aqueous extract on membrane stabilization, protein denaturation and white blood cell migration during acute inflammation. J Med Plants Res. 4:789–795.
- Vane JR, Botting RM. 1998. Anti-inflammatory drugs and their mechanism of action. Inflamm Res. 47:78–87.
- Wang HQ, Kim MP, Tiano HF, Langenbach R, Smart RC. 2001. Protein kinase C-alpha coordinately regulates cytosolic phospholipase A2 activity and the expression of ciclooxygenase-2 through different mechanism in mouse keratinocytes. Mol Pharmacol. 59:860–6.
- Winter CA, Risley EA, Nuss GW. 1962. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med. 111:544–547.
- Zdarilova A, Svobodova AR, Chytilova K, Simánek V, Ulrichová J. 2010. Polyphenolic fraction of Lonicera caerulea L. fruits reduced oxidative stress and inflammatory markers induced by lipopolysaccharide in gingival fibroblasts. Food Chem Toxicol. 48:1555–1561.
- Zhong Y, Chiou YS, Pan MH, Shahidi F. 2012. Anti-inflammatory activity of lipophilic epigallocatechin gallate (EGCG) derivatives in LPS-stimulated murine macrophages. Food Chem. 134:742–748.