Abstract
Background: Cryptococcus neoformans is the etiologic agent of opportunistic systemic fungal infection cryptococcosis, which affects individuals with compromised immune systems. Thus, natural products research has become important, since monoterpenes such as carvacrol, a promising molecule in the search antifungal agents, have shown significant biological activity.
Objective: The study aimed to investigate the antifungal activity and mode of action of carvacrol against strains of C. neoformans.
Methods: The minimum inhibitory concentration (MIC) was determined by microdilution method. Minimum fungicidal concentration (MFC) was performed by seeding technique on solid media. Studying the mode of action was performed using broth microdilution.
Results: The MIC ranged from 25 to 81 μg/mL and the MFC ranged from 25 to 102 μg/mL. Carvacrol bonded to exogenous ergosterol and cholesterol.
Discussion: The results suggest that carvacrol has antifungal activity against C. neoformans and its mode of action is related to fungal membrane instability.
Conclusions: The phytoconstituent carvacrol may eventually become a drug; however, further studies are needed to elucidate its mechanism.
Introduction
Cryptococcosis is a fungal infection that most commonly affects people with compromised immune systems. The etiological agent is Cryptococcus neoformans. The fungus appears as yeast surrounded by a capsule (Queiroz et al. Citation2008). Infection occurs by inhalation of infectious particles and can reach various parts of the body including the lungs, yet also displays tropism for the central nervous system, causing meningitis (Contin et al. Citation2011). According to Park et al. (Citation2009), roughly one million cryptococcal meningitis cases are diagnosed worldwide and the disease is responsible for over 600 thousand deaths.
Treatment is performed with fungistatic/or fungicidal drugs (Nobre et al. Citation2002). Amphotericin B and flucytosine are used in combination, when treating meningitis caused by Cryptococcus spp. (Chen & Sorrell Citation2007). However, a high incidence of renal toxicity is one of the effects seen with the use of amphotericin B (Sara et al. Citation1990).
Limitations in the use of antifungals have provoked research for treatment alternatives. Phytochemicals isolated from essential oils are effective alternatives (Bakkali et al. Citation2008), helping to obtain new drugs that are used in the treatment of these infections and other diseases (Lima et al. Citation2005; Bezerra et al. Citation2013).
To this end, new compounds such as carvacrol, a phytoconstituent present in essential oils with biological properties, for example; oregano (Origanum vulgare), thyme (Tymus vulgaris), lipia (Lippia graveolens) rosemary, and pepper-rosmarin (Lipia sidoides) are under test (Pozzatti et al. Citation2006; Freitas et al. Citation2013). Carvacrol is a phenolic compound, and the presence of a hydroxyl group and a delocalized electron system may be responsible for its biological activity (Ultee et al. Citation2002).
The objective of this study was to determine carvacrol’s minimum inhibitory concentration (MIC), minimum fungicidal concentration (MFC) and to evaluate the interference of ergosterol and cholesterol (at MIC), in order to elucidate the possible mechanism of activity.
Material and methods
Test substances
The substances carvacrol, ergosterol, cholesterol and amphotericin B (used to perform the tests) were purchased from Sigma Aldrich® (Steinheim, Germany). Further, Sabouraud dextrose agar (SDA) and Sabouraud dextrose broth were purchased from Difco Laboratories Ltd (Le Pont de Claix, France).
Fungal strains
Various strains of Cryptococcus neoformans (LM-0310, FCF-119, ICB-59, FGF-102, ICB-2601, JM-10, LM-5, LM-120, LM-39, LM-22), belonging to the Mico-Tech of the Federal University of Paraíba, Mycology Laboratory, were used and provided by Professor Dr. Edeltrudes de Oliveira Lima.
Inoculum
The strains were maintained in SDA culture medium for 72 h at 37 °C. To prepare the inoculum, the fungal colonies were suspended in 5 mL of sterile saline (0.85%). The suspensions were then shaken with the aid of a vortex and adjusted to a 0.5 McFarland scale, which corresponds to 1–5 × 106 CFU/mL (Cleeland & Squires Citation1991; Hadacek & Greger Citation2000).
Determination of MIC and MFC
The MIC was determined by the microdilution method in 96 well plates for each of the strains. A 100 μL of Sabouraud dextrose broth and carvacrol were distributed in all the wells of the plate. Through serial dilution at a ratio of two, concentrations from 1024 to 1 μg/mL were obtained, made in each plate itself, from column 1 to 11. The last column (12) was reserved for micro-organism growth control (Sabouraud dextrose broth, without the tested product). Control with a standard synthetic antifungal (amphotericin B) was realized. Then 10 μL of the fungal suspension was added to each well. The assay was performed in triplicate and subjected to incubation at 37 °C for 72 h. The readings were made by observing turbidity of the medium; indicating growth of the microorganism. Thus, the MIC was determined as the lowest concentration which could inhibit fungal growth (Cleeland & Squires Citation1991; Eloff Citation1998; Hadacek & Greger Citation2000; CLSI Citation2002).
To determine the MFC, 10 μL of the volume of each well with no microorganism growth (MIC, 2xMIC and 4xMIC) was seeded onto plates containing Sabouraud dextrose agar. The plates were then incubated at 37 °C temperature for 72 h. The reading was performed by counting the number of colony forming units (CFU), and the MFC was considered as the lowest concentration able to inhibit fungal growth up to a limit of three CFUs (Klepser et al. Citation1998; Ernst et al. Citation1999; Cantón et al. Citation2003; Costa et al. Citation2008).
Ergosterol and cholesterol assay
The assay was performed in accordance with Escalante et al. (Citation2008). In this trial, two strains of C. neoformans LM-22 and FGF-102 were used. The carvacrol MIC determination was made through the microdilution method, comparing strains in the absence and presence of ergosterol and cholesterol in concentrations of 100, 200 and 400 μg/ml. As a positive control, amphotericin B was used, and the negative control consisted of culture medium and inoculum.
Results
The results of tests determining the minimum inhibitory concentration are shown in . The MIC of carvacrol ranged from 25 to 81 μg/mL. There was an increase in the positive control (culture medium and inoculum), and none in the sterility control (culture medium); these same were also used to validate the method of this study (Cavalcanti et al. Citation2001).
Table 1. MIC results for amphotericin B and carvacrol against C. neoformans strains.
Amphotericin B (AB) was used for MIC determination as positive control. The MIC values ranged from 0.5 to 1 μg/ml (). All strains were sensitive to AB, given that Nguyen and Yu (Citation1998) and Lozano-Chiu et al. (Citation1998) have defined resistance to AB as being MIC values equal to or greater than 2 μg/mL.
After determining the MIC of carvacrol, minimum fungicidal concentration (MFC) was determined according to the method of Ernst et al. (Citation1999). The results are shown in .
Table 2. MFC results of carvacrol against C. neoformans strains.
The carvacrol MFC was considered as the lowest concentration that resulted in growth of three colonies (Ernst et al. Citation1999). We used the control of each strain to facilitate growth on solid medium. The MFC ranged from 25–102 mg/mL, for all strains, MFC was equal to MIC, except for the ICB-59 and LM-309 strains where MFC was 2× MIC.
To investigate the mode of action, carvacrol was tested in the presence (and absence) of ergosterol according to the methodology of Escalante et al. (Citation2008), and also in the presence and absence of cholesterol (Lima et al. Citation2013), a sterol present in mammalian cells (Zacchino Citation2001). Ergosterol (sterol) is the lipid component of fungal membranes considered most important for permeability and fluidity (Thevissen et al. Citation2003). It may be observed that the MIC of carvacrol in concentrations of 100, 200, 400 μg/ml increased in the presence of ergosterol and cholesterol ( and ).
Table 3. Study of the effect of ergosterol on MIC against carvacrol C. neoformans LM-22, and C. neoformans FGF-102.
Table 4. Study of the effect of cholesterol on carvacrol’s MIC against C. neoformans LM-22 and C. neoformans FGF-102.
In the presence of ergosterol, the MIC increased by four times the concentration of 100 and 200 μg/mL, and eight times at the concentration of 400 μg/mL when tested against the LM-22 strain (). In the same test, when carried out against the FGF-102 strain, the MIC increased by eight times at all concentrations (100, 200, 400 μg/mL) when tested in the presence of ergosterol (). The MIC was determined by calculating the geometric mean.
In , it is observed that the carvacrol MIC changed in the presence of cholesterol. The strain LM-22 MIC increased to six times at concentrations of 100 and 200 μg/mL, the concentration of 400 μg/mL increased MIC by 10-fold (). Also in this test, against the FCF-102 strain we observed that the MIC increased by eight times when used at concentrations of 100 and 200 μg/mL, and the concentration of 400 μg/mL MIC increased the MIC by 14 times in the presence of cholesterol. The MIC was determined by calculating the geometric mean.
In this study, testing was of ergosterol also performed with AB as a positive control. This drug acts by binding to ergosterol, leading to increased permeability and ultimately, to cell death (Moreira Citation2005).
In the presence of ergosterol the AB MIC against the LM-22 strain, increased 10 times for the concentration of 100 μg/mL, and at concentrations of 200 and 400 μg/mL it increased 14-fold (). Also in this test, the AB MIC for the FCF-102 strain increased 10 times for the concentration of 100 μg/mL, and at concentrations of 200 and 400 μg/ml increased 12 times (). The MIC was determined by calculating the geometric mean.
Table 5. Study of the effect of ergosterol on the MIC of amphotericin B against C. neoformans LM-22 and C. neoformans FGF-102.
The effect of cholesterol on the MIC of AB was twice that of the antifungal’s MIC value for FGF-22 and LM-109 at all concentrations (). It is noteworthy that in conjunction with these experiments were held assays of the positive and negative controls which indicated that the strains were viable, and the medium sterile. The MIC was determined by calculating the geometric mean.
Table 6. Study of the effect of cholesterol on the MIC of amphotericin B against C. neoformans LM-22 and C. neoformans FGF-102.
Discussion
The MIC results obtained were lower and different than the results obtained by Lima et al. (Citation2013), where, in evaluating the antifungal activity of carvacrol against the Candida albicans strain, MICs of 128 and 256 μg/mL were obtained. We show that this phytoconstituent has antifungal effect. Another study by Kaloustion et al. (Citation2008) demonstrated that carvacrol had an MIC of 400 μg/mL for E. coli ATCC 10536, and S. aureus ATCC 65288 by broth dilution method.
Studies with essential oils rich in carvacrol demonstrate antimicrobial activity. Thus, Pozzatti et al. (Citation2010) reported that the essential oil of Mexican oregano with carvacrol (58%) presented an MIC ≤800 μg/mL against species of the genus Candida. In another study, Carmo et al. (Citation2008) determined the antifungal activity of oregano essential oil against Aspergillus species and found an MIC of between 20 and 80 μL/mL.
Comparison of this study with the findings of other authors, shows agreement, though certain factors may interfere with MIC values; such as culture conditions (incubation time, temperature and oxygen%), culture medium, concentration of the tested substances, and dispersion and emulsion of the agents used in oil-water emulsions (Hammer et al. Citation1999; Rios & Recio Citation2005). These factors may contribute to the difference in results and hamper comparison with other studies.
Lima et al. (Citation2013) determined the MFC of carvacrol against strains of Candida albicans and obtained values between 256 and 512 μg/mL. In this study, the MFC of carvacrol was between 25 and 102 μg/mL; this being different from the value obtained by Lima et al. (Citation2013). In another study by Manohar et al. (Citation2001), the MFC of carvacrol against a standard strain of C. albicans (ATCC-48274) was 500 μg/mL. The concentrations of MIC and MFC determined in this study were low and could completely inhibit the strains, which shows that at the evaluated concentrations, carvacrol has fungicidal action. It is believed to carvacrol fungal growth inhibition occurs thru alteration of the cell wall/membrane functions (Lee et al. Citation1999).
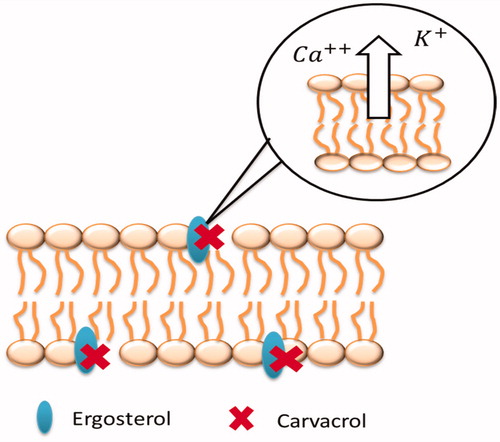
Based on the results, the MIC of carvacrol increased proportionally to the concentration of ergosterol in the medium. This result suggests that the carvacrol may act by binding to exogenous ergosterol. Since ergosterol is a sterol present in fungal cells, carvacrol-ergosterol binding would result in increased permeability, causing the output of Ca2+, and K+ ions, radicals, and proteins (Bakkali et al. Citation2008) and thus destabilization of the membrane and inhibition of fungal growth, as shown in . Cell membrane integrity is essential for survival of fungi as it is responsible for the cell function (Gray et al. Citation2012).
Hammer et al. (Citation2004) evaluated the antifungal activity of M. alternifolia essential oil (rich in terpenoids) on Candida albicans, C. glabrata and Saccharomyces cerevisiae, finding that the essential oil at concentrations of 0.25 and 1.0% changed fluidity and permeability of the fungal membrane.
Gill and Holley (Citation2006) investigated cell membrane rupturing in strains of L. monocytogenes, E. coli O157H7, and Lb. sake when exposed to bactericidal concentrations of aromatic compounds (carvacrol, eugenol and cinnamaldehyde). The results indicated that carvacrol caused rupture of the cytoplasmic membrane and increased its permeability.
However, carvacrol interacted with cholesterol; this result showed that the MICs were higher in the presence of cholesterol, as compared to medium containing ergosterol. The result suggests that the phytoconstituent might be toxic to human cells, since cholesterol is a component of animal cell plasma membranes.
Cyclic hydrocarbons, such as terpenes, cyclic alkanes, may be toxic. Such action is attributed to interactions between the lipophilic portions of these groups with the hydrophobic part of the membrane structure (Sikkema et al. Citation1995), the lipophilic portions of these essential oils tend to accumulate within the cytoplasmic membrane (Pozzatti et al. Citation2006).
The lipophilicity of a physicochemical characteristic of a compound depends on other characteristics as well, such as polarity, solubility and density (Leo et al. Citation1976). For information on the lipophilic character of carvacrol, Ultee et al. (Citation2002) evaluated its partitioning behaviour in a mixture of octanol and water. Carvacrol had a preference for the octanol phase (being more hydrophobic) which resulted in a log Po/w 3.64. Given this information, carvacrol may have affinity to fat tissues, and concentrating there, may cause harmful effects (Ultee et al. Citation2002).
Wright and Shadnia (Citation2008) found the co-existence of two toxicity mechanisms for phenols, one being nonspecific, and dependent on the lipophilicity, and the other, being the phenolic ability to form radicals by interacting with reactive oxygen species, generated in cells by oxidative stress. Thus, a toxicological study of the phytoconstituent is necessary to ensure its safety.
Yet, a study by Chami et al. (Citation2004) evaluated carvacrol and eugenol activity against oral candidiasis in immune compromised mice. No acute toxicity at the doses used (160 and 380 mg/kg) was found. Categorizing these oils as promising agents for the treatment of oral candidiasis became possible. In another study, carried out by Cleff et al. (Citation2008) evaluated the toxicity of 3% O. vulgare essential oil, orally and topically in rats. When used for 30 d, it caused no changes in organ mass, or in erythrocyte or leukocyte count; a demonstration of low toxicity.
Today, liposomes are well used in the development of technologies. These vesicles are of amphoteric nature which allow encapsulation of various hydrophilic and/or lipophilic molecules. When used as chemotherapeutic agents they provide greater efficacy, and reduced toxicity and dosage of the substance (Machado et al. Citation2007). Thus, liposomes might be a good alternative to serve the carvacrol and achieve efficient therapeutic application.
AB is a polyenic antibiotic and used for systemic antifungal therapy; it acts quickly and causes lethal damage to most endemic and opportunistic mycosis agents. Primary AB therapy indications are cases of cryptococcosis, aspergillosis, and disseminated Candida spp. infections due to triazole resistance (Ghannoum & Rice Citation1999).
AB molecules self-associate in high concentrations to form dimers or larger aggregates (Larabi et al. Citation2004). A study by Huang et al. (Citation2002) found that AB as a monomer or as an aggregate may form channels in membranes containing ergosterol, but only the aggregated forms channels in membranes containing cholesterol.
Though AB has a greater affinity for ergosterol, many of its attributed toxic effects are the result of its ability to bind to cholesterol (Bolard et al. Citation1993; Huang et al. Citation2002). A higher incidence in renal toxicity is seen when using this antifungal agent, the main characteristics are: reduction of glomerular filtration, hypokalemia and hypomagnesemia (Sara et al. Citation1990). In a comparative study between amphotericin B and caspofungin, 24.8% of patients who were treated with AB (0.6–1.0 mg/kg/day) showed nephrotoxicity confirmed by laboratory findings (Mora-Duarte et al. Citation2002).
Another limiting factor in the treatment of fungal infections is fungal resistance to the action of the available drugs. Dalazen et al. (Citation2011) demonstrated a high rate of AB resistance (96.6%) in clinical isolates of Candida spp. In the face of these considerations, the phytoconstituent carvacrol shows promise as an alternative for the treatment of fungal infections. Despite the interaction of carvacrol with cholesterol, toxicity studies to prove possible effects are necessary since the techniques developed in this study do not replace toxicity studies.
One way to optimize the use of carvacrol is by association with conventional drugs. These associations have been widely studied in in vitro methods; the “checkerboard” technique being widely used (Greco et al. Citation1995; Odds Citation2003). A carvacrol association study with AB to assess whether there is a synergistic effect is needed. Rosato et al. (Citation2008) demonstrated the potential synergistic activity of essential oils from Melaleuca alternifolia, Origanum vulgare, Pelargonium graveolens and AB against species of Candida sp. It is noteworthy that the pharmacologically active compound present in the essential oil of oregano is carvacrol at 3–17% (Farmacopea Ufficiale Della Republica Italiana Citation1998).
The antifungal of the future should have a broad spectrum of activity, being safe and non- toxic to the host cells (Carrillo-Muñoz et al. Citation2006). Thus, in view of the results, the phytoconstituent carvacrol may eventually become a drug; however, further studies are needed to elucidate its mechanism and toxicity.
Conclusions
Under the conditions evaluated, the phytoconstituent carvacrol showed antifungal activity against Cryptococcus neoformans strains. It may act by binding to exogenous ergosterol and presented interaction with cholesterol, a sterol present in mammalian cells.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Bakkali F, Averbeck S, Averbeck D, Idaomar M. 2008. Biological effects of essential oils – a review. Food Chem Toxicol. 46:446–475.
- Bezerra LMD, Ferreira GLS, Silva ICG, Castro RD. 2013. Atividade antibacteriana in vitro de fitoconstituintes sobre micorganismos do biofilme dentário. Revista Brasileira De Ciências Da Saúde. 17:79–84.
- Bolard J, Joly V, Yeni P. 1993. Mechanism of action of amphotericin B at the cellular level. It's modulation by delivery system. J Liposome Res. 3:409–427.
- Cantón E, Pemán J, Viudes UM, Viudes A, Quindós G, Gobernado M, Espinel-Ingroff A. 2003. Minimum fungicidal concentrations of amphotericin B for bloodstream Candida species. Diagn Microbiol Infect Dis. 45:203–206.
- Carmo ES, Lima EO, Souza EL. 2008. The potential of Origanum vulgare L. (Lamiaceae) essential oil in inhibiting the growth of some food-related Aspergillus species. Braz J Microbiol. 39:362–367.
- Carrillo-Muñoz AJ, Giusiano G, Ezkurra PA, Quindós G. 2006. Antifungal agents: mode of action in yeast cells. Rev Esp Quimioter. 19:130–139.
- Cavalcanti YM, Almeida LFD, Padilha WWN. 2001. Atividade antifúngica de três óleos essenciais sobre cepas de Candida. Revista Odontológica Do Brasil Central. 20:68–73.
- Chami N, Chami F, Bennis S, Troiillas J, Remmal A. 2004. A. Antifungal treatment witch carvacrol and eugenol of oral candidiasis in immunosuppressed rats. Braz J Infect Dis. 8:217–226.
- Chen SC, Sorrell TC. 2007. Antifungal agents. Med J Aust. 187:404–409.
- Cleeland R, Squires E. (1991). Evaluation of new antimicrobials “in vitro” and in experimental animal infections. In: Lorian V. M. D., editor. Antibiotics in laboratory medicine. 3rd ed. Baltimore: Williams & Wilkins. p. 739–788.
- Cleff MB, Meinerz AR, Sallis ES, Antunes TA, Mattei A, Rodrigues MR, Meireles MCA, Mello JRB. 2008. Toxicidade pré-clínica em doses repetidas do óleo essencial do Origanum vulgare L. (Óregano) em ratas wistar. Lat Am J Pharm. 27:704–709.
- CLSI 2002. Clinical and Laboratory Standards Institute, formely NCCLS (National Committee for Clinical Laboratory Standards), Method M27-A2, 2nd ed. Wayne Ed.; v. 22, p. 1–29.
- Contin JT, Quaresma GS, Silva EF, Linardi VR. 2011. Ocorrência de Cryptococcus neoformans em fezes de pombos na cidade de Caratinga, MG – Brasil. Revista Médica De Minas Gerais. 21:19–24.
- Costa EV, Texeira SD, Marques FA, Teixeira SD, Marques FA, Duarte MC, Delarmelina C, Pinheiro ML, Trigo JR, Sales MBG. 2008. Chemical composition and antimicrobial activity of the essential oils of the Amazon Guatteriopsis species. Phytochemistry. 69:1895–1899.
- Dalazen D, Zanrosso D, Wanderley L, Silva NL, Fuentefria AM. 2011. Comparação do perfil de suscetibilidade entre isolados clínicos de Candida spp. orais e vulvovaginais no Sul do Brasil. J Bras Patol Med Lab. 47:33–38.
- Eloff JN. 1998. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Medica. 64:711–713.
- Ernst EJ, Klepser ME, Ernst ME, Messer SA, Pfaller MA. 1999. In vitro pharmacodynamic properties of MK-0991 determined by time-kill methods. Diagn Microbiol Infect Dis. 33:75–80.
- Escalante A, Gattuso M, Pérez P, Zacchino S. 2008. Evidence for the mechanism of action of the antifungal phytolaccoside B isolated from Phytolacca tetramera Hauman. J Nat Prod. 71:1720–1725.
- Farmacopea Ufficiale Della Republica Italiana. 1998. X Edizione. Roma: Instituto Poligrafico e Zecco dello Stato, V.I, p. 206–210.
- Freitas MA, Andrade JC, Guedes GMM, Tintino SR, Souza CES, Leite NF, Gondim CNFL, Morais-Braga MFB, Matias EFF, Coutinho HDM. 2013. Avaliação in vitro da atividade antimicrobiana do carvacrol através dos métodos de contato direto e gasoso. Biosci J. 29:781–786.
- Gill AO, Holley RA. 2006. Description of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei membranes by plants oil aromatics. Int J Food Microbiol. 108:1–9.
- Ghannoum MA, Rice LB. 1999. Antifugal agents: mode of action, mechanisms of resistance and correlation of these mechanisms with bacterial resistence. Clin Microbiol Rev. 12:501–517.
- Greco WR, Bravo G, Parsons JC. 1995. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev. 47:331–385.
- Gray KC, Palacios DS, Dailey I, Endo MM, Uno BE, Wilcock BC, Burke MD. 2012. Amphotericin primarily kills yeast by simply binding ergosterol. Proc Natl Acad Sci USA 109:2234–2239.
- Hadacek F, Greger H. 2000. Testing of antifungal natural products: methodologies, comparatibility of results and assay choice. Phytochem Anal. 11:137–147.
- Hammer KA, Carson CF, Riley TV. 1999. Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol. 86:985–990.
- Hammer KA, Carson CF, Riley TV. 2004. Antifungal effects of Melaleuca alternifolia (tea tree) oil and its components on Candida albicans, Candida glabrata and Saccharomyces cerevisiae. J Antimicrob Chemother. 53:1081–1085.
- Huang W, Zhang Z, Han X, Tang J, Wang J, Dong S, Wang E. 2002. Ion channel behavior of amphotericin B in sterol-free and cholesterol- or ergosterol-containing supported phosphatidylcholine bilayer model membranes investigated by electrochemistry and spectroscopy. Biophys J. 83:3245–3255.
- Kaloustion J, Chevalier J, Mikail C, Martino M, Abou L, Vergenes MF. 2008. Étude de six huiles essentielles: composition chimique et activité antibactérienne. Phytotherapié. 6:160–164.
- Klepser ME, Ernst EJ, Lewis RE, Ernst ME, Pfaller MA. 1998. Influence of test conditions on antifungal time-kill curve results: proposal of standardized methods. Antimicrob Agents Chemother. 42:1207–1212.
- Larabi M, Gulik A, Dedieu JP, Legrand P, Barratt G, Cheron M. 2004. New lipid formulation of amphotericin B: spectral and microscopic analysis. Biochimica et Biophysica Acta – Biomembranes. 1664:172–181.
- Lee SH, Lee JR, Lunde CS, Kubo I. 1999. In vitro antifungal susceptibilities of Candida albicans and other fungal pathogens to polygodial, a sesquiterpene dialdehyde. Planta Medica. 65:204–208.
- Leo A, Hansh C, Jow PYC. 1976. Dependence of hydrophobicity of apolar molecules on their molecular volume. J Med Chem. 19:611–615.
- Lima IO, Oliveira RAG, Lima EO. 2005. Inhibitory effect of some phytochemicals in the growth of yeasts potentially causing opportunistic infections. Revista Brasileira De Ciências Farmacêuticas. 41:199–203.
- Lima IO, Pereira FO, Oliveira WA. 2013. Antifungal activity and mode of action of carvacrol against Candida albicans strains. J Essent Oil Res. 25:138–142.
- Lozano-Chiu M, Paetznick VL, Gannoum MA, Rex JH. 1998. Detection of resistance to amphotericin B among Cryptococcus neoformans clinical isolates: performances of three different media assessed by using E-test and National Committee for Clinical Laboratory Standards M27-A methodology. J Clin Microbiol. 36:2817–2822.
- Machado LC, Gnoatto SA, Kluppel MLW. 2007. Lipossomas aplicados em farmacologia. Estudos Biologia. 29:215–224.
- Manohar V, Ingram C, Gray J, Talpur NA, Echard BW, Bagchi D, Preuss HG. 2001. Antifungal activities of origanum oil against Candida albicans. Mol Cell Biochem. 228:111–117.
- Mora-Duarte J, Betts R, Rotstein C, Colombo AL, Thompson-Moya L, Smietana J, Lupinacci R, Sable C, Kartsonis N, Perfect J. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med. 347:2020–2029.
- Moreira MEL. 2005. Controvérsias a respeito da sepse fúngica no pré-termo extremo: profilaxia e esquemas terapêuticos. Jornal Pediatria. 81:S52–S57.
- Nguyen MH, Yu CY. 1998. In vitro comparative efficacy of vorconazole nd itrconzole against fluconazole-susceptible and resistant Cryptococcus neoformans isolates. Antimicrob Agents Chemother. 42:471–472.
- Nobre MO, Nascente PS, Meireles MC, Ferreiro L. 2002. Drogas antifúngicas para pequenos e grandes animais. Ciência Rural. 32:175–184.
- Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 52:1
- Park BJ, Wannemuehler KA, Marston BJ. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 23:525–530.
- Pozzatti P, Muller LG, Spader TB, Alves SH. 2006. Atividade antimicrobiana de óleos essenciais extraídos de condimentos. Revista Saúde. 32:53–58.
- Pozzatti P, Loreto ES, Lopes PG, Athayde ML, Santurio JM, Alves SH. 2010. Comparison of the susceptibilities of clinical isolates of Candida albicans and Candida dubliniensis to essential oils. Mycoses. 53:12–15.
- Queiroz PJAF, Sousa FDN, Lage RA, Izael MA, Santos AG. 2008. Criptococose – Uma revisão bibliográfica. Acta Veterinária Brasílica. 2:32–38.
- Rios JL, Recio MC. 2005. Medicinal plants and antimicrobial activity. J Ethnopharmacol. 100:80–84.
- Rosato A, Vitali C, Gallo D, Ballenzano L, Malamaci R. 2008. The inhibition of Candida species by selected essential oils and their synergism with amphotericin B. Phytomedicine. 15:635–638.
- Sara R, Takahashi K, Branch RA, Badr KF. 1990. Mechanisms of amphotericin B-induced reduction of the glomerular filtration rate: a micropuncture study . J Pharmacol Exp Ther. 253:34–37.
- Sikkema J, Bont JAM, Poolman B. 1995. Mechanisms of Membrane Toxicity of Hydrocarbons. Microbiol Rev. 59:201–222.
- Thevissen K, Kathelijne KA, Ferket I, Ferket KK, François IE, Camue BP. 2003. Interactions of antifungal plant defensins with fungal membrane components. Peptides. 24:1705–1712.
- Ultee A, Bennik MHJ, Moezelaar R. 2002. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl Environ Microb. 68:1561–1568.
- Wright JS, Shadnia H. 2008. Computational modeling of substituent effects on phenol toxicity. Chem Res Toxicol. 21:1426–1432.
- Zacchino S. (2001). Estratégias para a descoberta de novos agentes antifúngicos. In: Yunes RA, Calixto JB, editors. Plantas medicinais sob a ótica da moderna química medicinal. Chapecó: Argos. p. 435–479.