Abstract
Context: An ethnobotanical survey of Cordia rothii Roem. & Schult. (Boraginaceae) reveals it as a medicinal plant.
Objective: Antimicrobial and antioxidant potential evaluation and identification of chemical constituents via GC-MS of C. rothii roots fractions. To the best of our knowledge, this is the first systematic investigation of the roots exploiting GC-MS.
Materials and methods: Extraction and fractionation of C. rothii roots furnished various fractions using solvents of varying polarity, i.e., n-hexane, chloroform, ethyl acetate, acetone and methanol. In vitro antimicrobial and antioxidant screening was performed using disk diffusion and DPPH methods, respectively. MIC of active fractions was also determined using disk diffusion method. GC-MS was used to identify constituents which may be responsible for these activities.
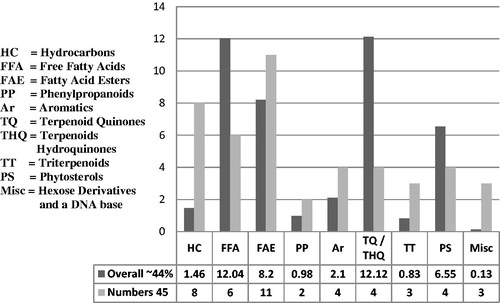
Results: Among various fractions from C. rothii roots, fraction KA-C showed strong antibacterial activity against 17 microorganisms tested, with MIC ranging from 250–31.25 μg/mL. Fractions KA-A, KM and KM-A exhibited significant antioxidant potential with EC50 46.875 μg/mL, while fractions KEA-PE, KM-PE and KM-M were good with EC50 93.750 μg/mL. Forty-five phytochemicals were identified in GC-MS studies including eight hydrocarbons, six free fatty acids, 11 fatty acids esters, two phenylpropanoids, four aromatics, four terpenoid quinones/hydroquinones, three triterpenes, four phytosterols, two hexose metabolites and a DNA base. Of these, 32 constituents have been reported for the first time from C. rothii, 24 from genus Cordia and 15 from Boraginaceae.
Discussion and conclusion: Strong antibacterial and antioxidant potential of C. rothii roots may be due to the contribution of phytoconstituents identified through GC-MS studies.
Introduction
Cordia (Boraginaceae) is well known for its phytochemical and pharmacological diversity (Matias et al. Citation2015). Cordia rothii Roem. & Schult., locally called gondani, is phytochemically and pharmacologically a less investigated species and has been selected for this study due to its immense medicinal properties such as antidote, antiseptic, antidiarrhoeal, astringent, abortifacient, anti-inflammatory, antileprotic (Chauhan & Chavan Citation2009), wound healer (Hajabhai et al. Citation2012) and immunomodulation (Firdous et al. Citation2014).
Increased antimicrobial resistance is the main cause of spread of many infectious diseases. Efforts have been made to overcome the pathological effects of these etiological agents (Doğan et al. Citation2013). Since plant-derived drugs offer minimum or no side effects, search for such therapeutics has become the goal of phytochemists and microbiologists to reduce the suffering of mankind. According to an analysis, 50% of the marketed drugs during 1981 to 2006 originated from natural products e.g., morphine, quinine, artemisinin, etc. (Kingston Citation2011). Free radical species are responsible for various diseases including cardiovascular, diabetes mellitus and neurodegenerative disorders (Valko et al. Citation2007). Literature survey of C. rothii reveals its use in heart ailments, diabetes (Chauhan & Chavan Citation2009) and CNS depression (Asolkar et al. Citation1992). In this study, antimicrobial potential of C. rothii roots fractions against some pathogenic microbes and antioxidant activity against free radical species is tested. Chemical composition of these fractions is also determined by GC-MS.
Materials and methods
Collection of plant material
C. rothii roots collected in July 2010 from the University of Karachi Campus were identified by Prof. Dr. Surraya Khatoon, Department of Botany, University of Karachi. A voucher specimen (No. 85853) has been deposited in the herbarium of the same department.
Extraction procedure
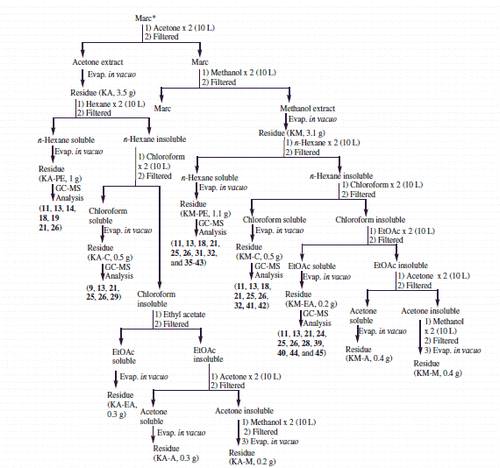
Classical extraction procedure was adopted to obtain various fractions using solvents of varying polarity. Freshly collected, powdered roots (4.5 kg) from C. rothii were defatted by soaking in n-hexane (2 × 10 L) at room temperature for two days furnishing fraction 2 A. The marc left was then sequentially percolated in solvents of increasing polarity; chloroform, ethyl acetate, acetone and methanol to afford chloroform (4A), ethyl acetate (6A), acetone (KA) and methanol soluble (KM) fractions, respectively. Detailed fractionation is elaborated in Schemes 1 and 2. A total of 19 fractions thus obtained were screened for their antimicrobial and antioxidant potential. Out of 19, 12 non- to moderate polar fractions (2A, 4A, KC-PE, KC-C, 6A, KEA-PE, KEA-C, KA-PE, KA-C, KM-PE, KM-C and KM-EA) were subjected to GC-FID and GC-MS analyses on the basis of their resolved TLC profile. The protocol already reported in earlier communications (Azmat et al. Citation2010; Muhammad et al. Citation2015) was used with little variations.
Scheme 1. Extraction, and fractionation and identified compounds f rom C. rothii roots. *Continued in Scheme 2.
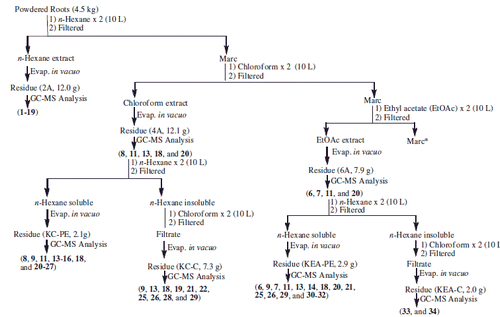
Scheme 2. Extraction and fractionation and identified compounds from C. rothii roots. *Continued from Scheme 1.
Gas chromatography-mass spectrometric (GC-MS) analyses of C. rothii roots
Initial profiling of fractions was performed using gas chromatography-flame ionization detection (GC-FID) on a Shimadzu GC-17 A (Shimadzu Corp., Kyoto, Japan) equipped with a SPB-5® capillary column (30 m ×0.25 mm ID and 0.25 μm df). Carrier gas was helium with a flow rate of 1 mL/min. The oven was kept initially at 70 °C for 5 min, programmed to 260 °C at a rate of 5 °C/min and kept isothermal for 20 min. Temperature and splitting ratio of injector was set to 280 °C and 1:30, respectively. For GC-MS, Hewlett-Packard 5890 (Bunker Lake Blvd, Ramsey, MN) gas chromatograph coupled to Jeol JMS-600 mass spectrometer and equipped with ZB-5MS® capillary column (30 m × 0.25 mm ID and 0.25 μm df) was used, keeping analytical conditions similar as described for GC-FID. EI ion source was kept at 250 °C and 70 eV.
For the identification of compounds, electronic mass spectral survey (NIST Citation2005) and EIMS data reported in literature were compared (Moir & Thomson Citation1973; Manners & Jurd Citation1977) with the experimental mass spectra. The order of elution of compounds was confirmed through reported retention indices (RI) data (Kováts Citation1958; Van den dool & Kratz Citation1963). Area normalization method was used to quantify constituents.
Bacterial and fungal strains
Pure bacterial cultures (15 Gram-positive and 13 Gram-negative, ) and 14 fungal strains were obtained from Department of Microbiology, University of Karachi. The fungal strains included six saprophytic fungi; Aspergillus flavus, Aspergillus niger, Fusarium sp., Penicillium sp., Rhizopus sp., Helminthosporium, three non-filamentous fungi; Candida albicans, Candida albicans ATCC 0383, Saccharomyces cerevisiae and five dermatophytes; Microsporum canis, Microsporum gypseum, Trichophyton rubrum, Trichophyton mentagrophytes and Trichophyton tonsurans.
Table 1. In vitro antibacterial activity of C. rothii roots against Gram-positive and Gram-negative bacteria.
Antimicrobial assay
Roots extracts were screened for in vitro antibacterial and antifungal (data not shown as extracts were weakly antifungal) bioassays using disk diffusion method (Bauer et al. Citation1966). Then 50 mg/mL stock solution was used to prepare sterile disk of extracts, and 10 μL containing 500 μg sample was loaded onto the disks. Finally prepared disks were incubated for 24 h at 37 °C. Dimethylsulfoxide (DMSO) was used as control. The samples exhibiting strong antibacterial activity ≥15 mm (zone of inhibition/concentration in μg) in preliminary screening were evaluated for their MIC. Samples were serially diluted to get different concentrations (31.25–250 μg/disk) for determining MIC ().
Table 2. Minimum inhibitory concentration (μg/disc) of C. rothii roots.
Antioxidant assay using DPPH protocol
The method for antioxidant bioassay was adopted from Lee et al. (Citation1998). Ethanol was used to make 300 μM solution of 1,1-diphenyl-2-picrylhydrazyl (DPPH). Reaction mixture containing 10 μL test sample in DMSO and 90 μL DPPH was incubated for 30 min at 37 °C, and absorbance was measured at 515 nm. Comparison between the DMSO-treated control (ascorbic acid) and test sample provided the % inhibition. The EC50 concentration of test sample was calculated in μg/mL ().
Table 3. Antioxidant potential of C. rothii roots.
Results
C. rothii roots were extracted sequentially with organic solvents of increasing polarity furnishing 19 fractions. All these fractions (Schemes 1 and 2) were screened for their antimicrobial potential against 15 Gram-positive, 13 Gram-negative bacteria and 14 fungal strains. KA-C exhibited broad spectrum antibacterial activity against all the Gram-positive and Gram-negative bacteria tested. Strong antibacterial activity was observed against B. thuringiensis, M. luteus, MRSA, S. aureus AB188, S. saprophyticus, E. coli ATCC 8739 and K. pneumoniae (MIC 31.25–62.50 μg/disk). Good antibacterial activity was observed against C. diphtheriae, C. hofmannii, C. xerosis, M. luteus ATCC 9341, S. epidermidis, S. pyogenes, S. faecalis, P. mirabilis and P. aeruginosa (MIC 125–250 μg/disk) (). Similarly, KM-C exhibited significant antibacterial activity against M. luteus ATCC 9341 and S. saprophyticus (MIC 31.25–62.50 μg/disk) and good antibacterial activity against C. hofmannii and C. xerosis (MIC 125–250 μg/disk) (). KA-PE and KM-EA showed good antibacterial activity against S. epidermidis (MIC 250 μg/disk) while KA-PE strongly inhibited S. faecalis (MIC 31.25 μg/disk). With the exception of KA-A, KA-M, KM and KM-A, all samples exhibited little to moderate antibacterial activity. KA-PE and KM-EA exhibited no activity (). No antifungal activity was observed in these fractions, except KEA-PE, which exhibited some activity against Fusarium specie and S. cerevisiae.
The above-mentioned fractions were also screened for their antioxidant potential. KA-A, KM and KM-A exhibited significant antioxidant activity (EC50 46.875 μg/mL), whereas KEA-PE, KM-PE and KM-M showed good antioxidant potential (EC50 93.750 μg/mL). Most of the samples exhibited antioxidant activity with inhibitory effect above 50%. Fractions 2A, 4A, 6A, KEA-C, KA, KA-C, KA-M, KM-C and KM-EA exhibited little antioxidant activity (EC50 187.5–375 μg/mL) ().
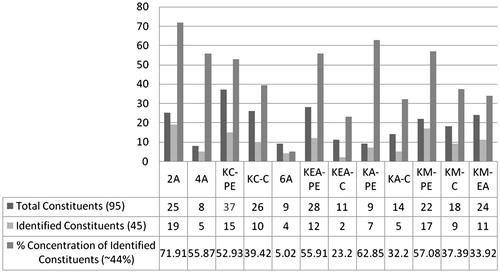
Analyses of the 12 non-polar to moderately polar fractions of C. rothii roots (Schemes 1, 2 and ) through GC-MS resulted in identification of 45 out of 95 metabolites (constituting ∼44%). To the best of our knowledge 32 of these constituents (comprising 71.11% of composition) are reported for the first time from C. rothii, 24 from genus Cordia and 15 from Boraginaceae. Non-polar fraction 2 A provided the most promising results. Out of 25, 19 phytoconstituents, quantifying 71.91% of the sample have been identified. These included seven hydrocarbons (HC); 1–4, 6, 8 and 10; two free fatty acids (FFA); 13 and 18; eight fatty acid esters (FAE); 5, 7, 11, 12 and 14–17; a terpenoid quinone (TQ); 9; and a phytosterol (PS); 19. Twelve of these 1, 2, 4, 5, 7, 11, 12, 14–17 and 19 are being reported for the first time from C. rothii. KM-PE also furnished promising results in GC-MS analyses, with 17 identifications out of 22 constituents and 57.08% concentration. These metabolites included a terpenoid hydroquinone (THQ); 21, two PS; 25 and 26, three triterpenoids (TT); 31, 32 and 43, two hexose metabolites; 36 and 37; three FFA; 38, 40 and 41; a FAE; 42, a phenylpropanoid (PP); 39 and a DNA base; 35. Eleven of these constituents (21, 25, 31, 32, 35–39, 42 and 43) are being reported for the first time from C. rothii. GC-MS studies on fractions KC-PE and KM-EA resulted in identification of 15 and 11 metabolites from a total of 37 and 24 metabolites, constituting 52.93 and 33.92% of fractions, respectively. These fractions resulted in identification of a HC; 20, two FAE; 22 and 23, a THQ; 24; a PS; 27, a PP; 28 and two aromatics (Ar); 44 and 45. The rest of the identified metabolites reappeared. This is the first report of phytoconstituents 22, 23, 24 and 28 from C. rothii. A THQ; 29 and a FFA; 30 were identified in KEA-PE while two aromatics; 33 and 34 in KEA-C along with other constituents. Three of these metabolites, 29, 33 and 34 are being reported for the first time from C. rothii.
Table 4. Composition (%) of phytochemicals in GC-MS studies on C. rothii roots.
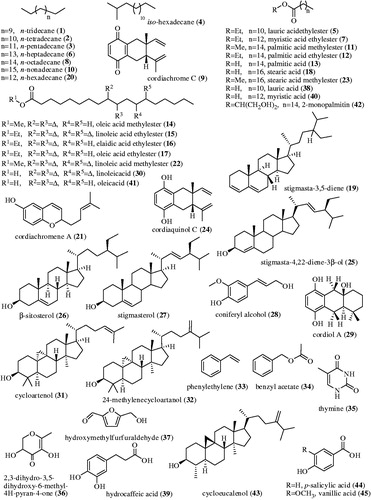
summarizes number of total and identified phytoconstituents along with the % composition while shows the diversity in the compounds identified. The structures of chemical constituents identified through GC-MS studies are given in . A table in supplementary data justifies their natural occurrence.
Discussion
Plants of genus Cordia are highly reputed for their diverse pharmacology and phytochemistry (Matias et al. Citation2015). However, C. rothii is relatively less explored specie. Fractionation using organic solvents in increasing order of polarity affects antimicrobial property since change in solvents result in the extraction of different metabolites. Plant based antimicrobials not only show promising results but are also reported to have reduced side effects. The synergism arises due to the complex structural variety of natural products as individual or in mixture whose interaction(s) with pathogens or toxins do not affect other healthy cells or molecules (Di Pasqua et al. Citation2005; Negi et al. Citation2012). It is also true for antagonism. Studies showed that lipophilic or hydrophilic properties of compounds are associated with antimicrobial properties, e.g., the proposed order of antimicrobial activity against various phytochemicals is phenols > aldehydes > ketones > alcohols > esters > hydrocarbons. Increasing carbon chain length also imparts good effect on antibacterial activity (Bendiabdellah et al. Citation2013).
Hydrocarbons are also reported to exhibit antimicrobial property (Bassolé & Juliani Citation2012). Essential oil from Minuartia meyeri containing hydrocarbons as major constituents has exhibited antibacterial activity (Yayli et al. Citation2006). Role of fatty acids as antimicrobial agents in food additives (Freese et al. Citation1973) and the antifungal and antibacterial properties of their esters are also well known (Zhang et al. Citation2013). All the FFAs identified in this study are reported to exhibit antimicrobial properties (Agoramoorthy et al. Citation2007). Although the exact mechanism of antibacterial activity of FFA is unknown, but it may be suggested that hydrophobic part is responsible for the activity by interacting with the hydrophobic parts of bacterial cell membrane. Moreover, elongated carbon chain length, presence of double bond and its position also affects antibacterial activity (Furtado et al. Citation2014). Organic compounds having aromatic and phenolic moiety may attribute increased antimicrobial activity to the compound (Patnaik et al. Citation2008). Essential oils, having phenols or quinones as major constituents, exhibited potent antimicrobial activity (Shigeharu et al. Citation2006). Sesquiterpenoid quinones and hydroquinones also exhibited antimicrobial potential (Cariello et al. Citation1982; Takahashi et al. Citation2008). Structure–activity relationship (SAR) studies indicate that side chain of PS is responsible for its antibacterial activity (Tanaka et al. Citation2013).
Fractions from this study did not exhibit antifungal potential. In general, samples exhibiting antibacterial activity usually do not show antifungal behaviour or it may be due to the antagonistic effect of the other components present in the samples.
Antimicrobial and antioxidant activities of phenolic compounds have been evaluated in many earlier studies (Osoro et al. Citation2013). It is now a well-established fact that antioxidant and antimicrobial potential of plant extracts is characterized by their phenolic content (Proestos et al. Citation2005). Although several citations are available for the correlation between antioxidant potential and phenolic content of plants (Guruvaiah et al. Citation2012) but this might not always be true. Antioxidant potential is correlated with the applied method, concentration, nature and physicochemical properties of the sample under investigation (Stamenic et al. Citation2014).
Antioxidant behaviour of FFA has been evaluated earlier (Zhao et al. Citation2013). Fatty acid content and antioxidant property can be associated with many biological benefits. An increased amount of unsaturated FFA imparts positive effect on antioxidant potential of seaweeds (Huang & Wang Citation2004). Antioxidant activity of benzoic and cinnamic acid derivatives is in the order of p-hydroxy < p-hydroxymethoxy < dihydroxy < p-hydroxydimethoxy (Natella et al. Citation1999). p-Hydroxybenzoic acid and its derivatives exhibit diverse range of biological activities including antimicrobial and antioxidant (Manuja et al. Citation2013). Aromatic compounds with methoxy group exhibits high antioxidant activity (Narayanan et al. Citation2012). Such compounds have been identified in this study.
Various environmental factors generate oxidative stress in plants. Consequently, formation of phenolic metabolites with antioxidant properties is induced to overcome this stress (Grace Citation2005). Free radical species are reduced by hydroxyl group of an aromatic system as a result of which resonance stabilized phenoxyl radical is produced (Calil et al. Citation2012). Phenolic acids and their derivatives scavenge reactive oxygen species showing antioxidant behaviour. Compounds having one, two or three free hydroxyl groups on aromatic ring exhibit low, medium and high antioxidant activity, respectively (Katti & Ranjekar Citation2014). In another contrast study maximum antioxidant activity was exhibited by monohydroxybenzoic acid derivatives and position of carboxylic acid and hydroxyl group is found responsible for the antioxidant activity of a compound. Highest antioxidant activity was observed in compounds having -OH group oriented ortho- or para- to the -COOH group. The antioxidant activity decreases if the hydroxyl group is blocked whereas no pronounced effect was observed in the case of blocked carboxylic acid group (Velika & Kron Citation2012). Structures of metabolites 39, 44 and 45 are noteworthy for their probable activity. Phenylpropanoids play important role as antioxidants (Kurkin Citation2013), which is related to the hydroxyl moiety of aromatic ring (Park et al. Citation2003). SAR study shows that number of -OH groups and the nature of alkyl spacer between aromatic ring and carboxylic group play important role in antioxidant behaviour of a compound (Siquet et al. Citation2006). Sesquiterpenoid hydroquinones and sesquiterpenoid quinones are also reported as antioxidant agents with hydroquinones being stronger as compared to quinones (Belisario et al. Citation1992). Thus, 21, 24 and 29 can also be the potential contributors in antioxidant potential of C. rothii. Phytosterols are reported as the antioxidant constituents of plants (Conforti et al. Citation2007).
Conclusion
C. rothii roots showed significant antimicrobial and antioxidant potential. Thirty-two out of 45 phytoconstituents identified through GC-MS studies are here reported for the first time. These fractions were evaluated in mixture, therefore, it is suggested that these activities could be due to the synergistic effect of the components identified. Further, inhibitory effects against a broad range of bacterial strains reveal the diversified antimicrobial potential of the plant due to the extraction of secondary metabolites of varying nature, in different organic solvents.
Compounds 9 (Shigeharu et al. Citation2006), 13 (Agoramoorthy et al. Citation2007), 26 (Yadav et al. Citation2014) and 21 (Benslimane et al. Citation1988) being antimicrobial agents may be the potential contributors of fraction KA-C. Similarly 13 and 18 (Agoramoorthy et al. Citation2007), 21 (Benslimane et al. Citation1988), 26 (Yadav et al. Citation2014), 41 (Agoramoorthy et al. Citation2007) and 42 (Zheng et al. Citation2012), reported as antimicrobial agents may be responsible for the significant antimicrobial potential of fraction KM-C.
The most active antibacterial fractions (KA-C and KM-C), exhibited little or no antioxidant activity while fractions exhibiting significant antioxidant activity; KA-A, KA-M, KM and KM-A did not exhibit antibacterial activity. Fractions exhibiting good antioxidant activity KEA-PE, KM-PE, KM-M, showed variable activity against Gram-positive and Gram-negative bacteria tested and fractions exhibiting good antibacterial activity (KA-PE and KM-EA) showed little or no antioxidant activity at all ().
Polar fractions (KA-PE, KA-C, KA-A, KM, KM-PE, KM-C, KM-A and KM-M) having varying classes of natural products with different polar functionalities were found to be more active, which is in accordance with various earlier reports. However, reports where chloroform extract was proved to be more active than methanol extract are also available. Further studies on identification and characterization of natural constituents from the bioactive fractions are in progress. These may lead to unveil templates for bioactive lead compounds.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Agoramoorthy G, Chandrasekaran M, Venkatesalu V, Hsu MJ. 2007. Antibacterial and antifungal activities of fatty acid methyl esters of the blind-your-eye mangrove from India. Braz J Microbiol. 38:739–742.
- Asolkar LV, Kakkar KK, Chakre OJ. 1992. Second Supplement to Glossary of Indian Medicinal Plants with Active Principles, Part–I (A–K) (1965–1981), National Institute of Science Communications (CSIR), Dr. KS Krishnan Marg, New Delhi-110 012, 231.
- Azmat S, Ifzal R, Rasheed M, Mohammad FV, Ahmad VU. 2010. GC-MS analysis of n-hexane extract from seeds and leaves of Phoenix dactilifera L. J Chem Soc Pak. 32:672–676.
- Bassolé IHN, Juliani HR. 2012. Essential oils in combination and their antimicrobial properties. Molecules. 17:3989–4006.
- Bauer AW, Kirby WMM, Sherris JC, Turck M. 1966. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 45:493–496.
- Belisario MA, Maturo M, Pecce R, De Rosa S, Villani GRD. 1992. Effect of avarol and avarone on in vitro- induced microsomal lipid peroxidation. Toxicology. 72:221–233.
- Bendiabdellah A, Dib MA, Meliani N, Muselli A, Nassim D, Tabti B, Costa J. 2013. Antibacterial activity of Daucus crinitus essential oils along the vegetative life of the plant. J Chem (NY). 2013:1–7.
- Benslimane AF, Pouchus YF, Le Boterff J, Verbist JF, Roussakis C, Monniot F. 1988. Cytotoxic and antibacterial substances of ascidian Aplidium antillense. J Nat Prod. 51:582–583.
- Calil NO, De Carvalho GSG, da Silva AF, da Silva AD, Raposo NRB. 2012. Antioxidant activity of synthetic resveratrol analogs: a structure-activity insight. Lett Drug Des Discov. 9:676–679.
- Cariello L, Zanetti L, Cuomo V, Vanzanella F. 1982. Antimicrobial activity of avarol, a sesquiterpenoid hydroquinone from the marine sponge, Dysidea avara. Comp Biochem Physiol B Biochem Mol Biol. 71:281–283.
- Chauhan MG, Chavan SS. 2009. Pharmacognosy and biological activity of Cordia rothii Roem. & Schult. bark. Ind J Tradit Know. 8:598–601.
- Conforti F, Statti GA, Menichini F. 2007. Chemical and biological variability of hot pepper fruits (Capsicum annuum var. acuminatum L.) in relation to maturity stage. Food Chem. 102:1096–1104.
- Di Pasqua R, De Feo V, Villani F, Mauriello G. 2005. In vitro antimicrobial activity of essential oils from Mediterranean Apiaceae, Verbenaceae and Lamiaceae against food borne pathogens and spoilage bacteria. Ann Microbiol. 55:139–143.
- Doğan HH, Duman R, Özkalp B, Aydin S. 2013. Antimicrobial activities of some mushrooms in Turkey. Pharm Biol. 51:707–711.
- Firdous S, Khan K, Zikr-Ur-Rehman S, Ali Z, Soomro S, Ahmad VU, Rasheed M, Mesaik MA, Faizi S. 2014. Isolation of phytochemicals from Cordia rothii (Boraginaceae) and evaluation of their immunomodulatory properties. Rec Nat Prod. 8:51–55.
- Freese E, Sheu CW, Galliers E. 1973. Function of lipophilic acids as antimicrobial food additives. Nature. 241:321–325.
- Furtado FB, De Aquino FJT, Nascimento EA, Martins CM, de Morais SAL, Chang R, Cunha LCS, Leandro LF, Martins CHG, et al. 2014. Seasonal variation of the chemical composition and antimicrobial and cytotoxic activities of the essential oils from Inga Laurina (Sw.) wild. Molecules. 19:4560–4577.
- Grace SC. 2005. Phenolics as antioxidants. In: Smirnoff N, editor. Antioxidants and reactive oxygen species in plants. Oxford, UK: Blackwell Publishing Ltd. p. 141–168.
- Guruvaiah P, Arunachalam A, Velan LPT. 2012. Evaluation of phytochemical constituents and antioxidant activities of successive solvent extracts of leaves of Indigofera caerulea Roxb using various in vitro antioxidant assay systems. Asian Pac J Trop Dis. 2:S118–S123.
- Hajabhai ZA, Pandya SS, Rabari HA. 2012. Wound healing activity of leaf extracts of Cordia rothii Roem & Schult. Int J Pharm Life Sci. 3:2048–2054.
- Huang H-L, Wang B-G. 2004. Antioxidant capacity and lipophilic content of seaweeds collected from the Qingdao coastline. J Agr Food Chem. 52:4993–4997.
- Katti SA, Ranjekar SS. 2014. Evaluation of antioxidant capacities of some phenolic acids by DPPH method. Int J Res Pharm Chem. 4:101–104.
- Kingston DGI. 2011. Modern natural products drug discovery and its relevance to biodiversity conservation. J Nat Prod. 74:496–511.
- Kováts E. 1958. Gas-chromatographische charakterisierung organischer verbindungen teil 1: retention indices aliphatischer halogenide, alkohole, aldehyde und ketone. Helv Chim Acta. 41:1915–1932.
- Kurkin VA. 2013. Phenylpropanoids as the biologically active compounds of the medicinal plants and phytopharmaceuticals. Adv Biol Chem. 3:26–28.
- Lee SK, Zakaria H, Chung H, Luyengi L, Games EJC, Mehta RJ, Kinghorn D, Pezzuto JM. 1998. Evaluation of the antioxidant potential of natural products. Comb Chem High Throughput Screen. 1:35–46.
- Manners GD, Jurd L. 1977. The hydroquinone terpenoids of Cordia alliodora. J Chem Soc Perkin Trans 1. 4:405–410.
- Manuja R, Sachdeva S, Jain A, Chaudhary J. 2013. A comprehensive review on biological activities of p-hydroxy benzoic acid and its derivatives. Int J Pharm Sci Rev Res. 22:109–115.
- Matias EFF, Alves EF, Silva MKN, Carvalho VRA, Coutinho HDM, da Costa JGM. 2015. The genus Cordia: botanists, ethno, chemical and pharmacological aspects. Rev Bras Farmacogn. 25:542–552.
- Moir M, Thomson RH. 1973. Naturally occurring quinones. Part XXII. Terpenoid quinones in Cordia spp. J Chem Soc Perkin Trans 1. 13:1352–1357.
- Muhammad MT, Lubna, Fayyaz N, Samina T, Razaq U, Versiani MA, Ahmed A, Faizi S, Rasheed M. 2015. Antibacterial activity of flower of Melia azedarach Linn. and identification of its metabolites. J Korean Soc Appl Biol Chem. 58:219–227.
- Narayanan BL, Rajkumar LAP, Arulanandham A, Babu NS, Gayathri T, Raju A. 2012. Antioxidant and anti-inflammatory activity of synthesized 3(substituted) chromen-2-one. Int J Pharm Sci Res. 3:474–478.
- Natella F, Nardini M, Di Felice M, Scaccin C. 1999. Benzoic and cinnamic acid derivatives as antioxidants: structure-activity relation. J Agr Food Chem. 47:1453–1459.
- Negi BS, Dave BP, Agarwal YK. 2012. Evaluation of antimicrobial activity of Bauhinia purpurea leaves under in vitro conditions. Indian J Microbiol. 52:360–365.
- NIST. 2005. Mass Spectral Search Program for the NIST/EPA/NIH Mass Spectral Library. Version 2.0 d, build Dec. 2.
- Osoro EK, He YZ, Ndagijimana A, Imbenzi PS. 2013. A review on phenolic compounds in Illicium species and their pharmacological effects. Der Pharmacia Sinica. 4:17–30.
- Park YM, Yoon MY, Kim KW, Cho NY, Lim HW, Lee JY, Lee JH, Kim YJ, Kim JC, Sim SS. 2003. Effects of phenylpropanoid compounds on melanin production in B16 melanoma cells. Yakhak Hoechi. 47:398–403.
- Patnaik T, Dey RK, Gouda P. 2008. Antimicrobial activity of friedelan-3β-ol and trans-N-caffeoyltyramine isolated from the root of Vitis trifolia. Asian J Chem. 20:417–421.
- Proestos C, Boziaris IS, Nychas G-JE, Komatis M. 2005. Analysis of flavonoids and phenolic acids in Greek aromatic plants: investigation of their antioxidant capacity and antimicrobial activity. Food Chem. 95:664–671.
- Shigeharu I, Katsuhisa U, Shigeru A. 2006. Correlation of chemical composition and antimicrobial activity of seventy essential oils. Aroma Res. 7:265–273.
- Siquet C, Paiva-Martins F, Lima JLFC, Reis S, Borges F. 2006. Antioxidant profile of dihydroxy- and trihydroxy- phenolic acids-A structure activity relationship study. Free Radic Res. 40:433–442.
- Stamenic M, Vulic J, Djilas S, Misic D, Tadic V, Petrovic S, Zizovic I. 2014. Free-radical scavenging activity and antibacterial impact of Greek oregano isolates obtained by SFE. Food Chem. 165:307–315.
- Takahashi Y, Kubota T, Ito J, Mikami Y, Fromont J, Kobayashi J. 2008. Nakijiquinones G-I, new sesquiterpenoid quinones from marine sponge. Bioorg Med Chem. 16:7561–7564.
- Tanaka A, Shimizu K, Kondo R. 2013. Antibacterial compounds from shoot skins of moso bamboo (Phyllostachys pubescens). J Wood Sci. 59:155–159.
- Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. 2007. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 39:44–84.
- Van den Dool H, Kratz PD. 1963. A generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. J Chromatogr A. 11:463–471.
- Velika B, Kron I. 2012. Antioxidant properties of benzoic acid derivatives against superoxide radical. Free Radicals Antioxid. 2:62–67.
- Yadav A, Bhardwaj R, Sharma RA. 2014. Isolation, quantification and antimicrobial activities of phytosterols from different parts of Cassia pumila lamk. Int J Pharm. 4:86–92.
- Yayli N, Gülec C, Üçüncü O, Yasar A, Ülker S, Coskunçelebi K, Terzioglu S. 2006. Composition and antimicrobial activities of volatile components of Minuartia meyeri. Turk J Chem. 30:71–76.
- Zhang X, Yang M, Song F, Zhang H, Feng F. 2013. Antimicrobial activity of selected fatty acids and their derivatives. Zhejiang Daxue Xue Bao Nong Ye Yu Sheng Ming Ke Xue Ban. 39:155–160.
- Zhao X, Shen J, Chang KJ, Kim SH. 2013. Analysis of fatty acids and phytosterols in ethanol extracts of Nelumbo nucifera seeds and rhizomes by GC-MS. J Agr Food Chem. 61:6841–6847.
- Zheng J, Chen Y, Yao F, Chen W, Shi G. 2012. Chemical composition and antioxidant/antimicrobial activities in supercritical carbon dioxide fluid extract of Gloiopeltis tenax. Mar Drugs. 10:2634–2647.